Abstract
With the development of immune checkpoint inhibitors, the efficacy of immunotherapy as a cancer treatment that is effective against multiple tumor types has been established, and this modality came to be considered as the fourth pillar of cancer therapy. The clinical success of immunotherapy greatly changed the field of oncology by highlighting the importance of the immune system in cancer control and elimination. It has now become clear that research into, and the clinical application of, the immune response are important for effective cancer treatment. Moreover, it has become apparent that conventional cancer treatments, such as radiotherapy and chemotherapy, can modulate the cross-talk between the tumor and the immune system, and their efficacy depends, in part, on the ability to elicit antitumor immune response. The ability of radiotherapy to induce an immune response has become relevant in the immunotherapy age. Radiotherapy has been redefined as a partner for cancer immunotherapy, based on evidence indicating the potential synergistic effect of the combination of these therapeutic modalities. This review outlines the major findings reported to date on the immune response induced by radiotherapy and discusses the role of radiotherapy in combination with immunotherapy. Furthermore, we introduce research aimed at the clinical application of combination therapy and discuss its potential in clinical practice and future issues.
Keywords: radiotherapy, DNA damage response, immunogenic cell death, immunotherapy, immune checkpoint inhibitors
Radiotherapy is under investigation as an optimal partner of cancer immunotherapy due to its immune stimulatory effects and potential for enhancing the efficacy of immunotherapy.
Introduction
In the age of immunotherapy, which is represented by immune checkpoint inhibitors (ICIs), the importance of the immune system for the elimination of cancer has become clear. Cancer cells acquire the ability to escape immune control through a process called ‘immunoediting’ before they develop into clinical cancer (1). Furthermore, it has been reported that a similar immune escape process contributes to the mechanism of resistance to immunotherapy (2), suggesting that clinically evident tumor is potentially refractory to immunotherapy. Therefore, if a tumor-specific immune response can be effectively generated both locally and systemically in patients with cancer, it may be possible to overcome this immunosuppressive scenario. In this context, a new role is emerging for radiotherapy in overcoming immunosuppression in the tumor microenvironment.
Radiotherapy is one of the three pillars of cancer therapy, together with surgery and chemotherapy, and has been widely used for the treatment of various types of cancer, both in a curative and palliative manner. To date, its therapeutic effect has been considered to be mainly local and to occur via the direct or indirect DNA damage of irradiated cancer cells. However, accumulating preclinical and clinical evidence now indicates that radiotherapy has systemic antitumor effects that are exerted through changes in the immune environment. In fact, the systemic immune response induced by radiotherapy is thought to be responsible for the so-called abscopal effect, which consists in the shrinkage of tumors outside the irradiation field (3,4). The abscopal effect has a long history, with its first report dating back to 1953 (5). This was a very rare phenomenon in the setting of radiotherapy alone (6). However, in the age of immunotherapy, abscopal effects have been increasingly reported in patients treated with radiotherapy due to progression on immune checkpoint blockade therapy (7). Thus, radiotherapy is under investigation as a modality that can enhance the efficacy of immunotherapy. The potential of radiotherapy used with immunotherapy to foster systemic tumor regression in advanced stage cancers with distant metastases has opened a new filed of investigation into the mechanisms of the underlying immune response induced by radiotherapy. Importantly, it should be noted that a prospective randomized clinical trial of immunotherapy alone versus the combination of radiotherapy with the same immunotherapy is required to assess accurately the abscopal effect of local radiotherapy plus immunotherapy (4). The shrinkage of tumors outside the irradiation field in a single-arm combination of radiotherapy and immunotherapy should not be interpreted as an abscopal effect, if the tumor type treated is known to be responsive to the immunotherapy used.
Recent advances in therapeutic technology enable the delivery of more accurate radiotherapy, in addition to conventional treatments using X-rays (3D conformal radiotherapy). This technological advancement allows the reduction of the irradiation dose to the normal tissue around the target (i.e. the primary lesion or metastatic lesion), reducing unwanted side effects. In the current radiotherapy setting, it is possible to select the treatment modality, including intensity-modulated radiotherapy (IMRT), stereotactic body radiotherapy (SBRT) and particle beam therapy (heavy ion radiotherapy, proton therapy), on a case-by-case basis considering the target site, disease stage and patient background. This advancement in treatment technology has allowed the safe administration of high doses of radiation, thus improving local control rates while enhancing the importance of controlling tumors outside the irradiation field for the long-term survival of patients.
The application of ICIs, which are currently attracting the greatest amount of attention in oncology field, achieved high efficacy (such as complete response) in a restricted number of patients (8,9). Therefore, the combination of radiotherapy and immunotherapy may be an effective complementary strategy, because radiotherapy can assist immunotherapy by enhancing immune activation both systemically and locally, whereas immunotherapy can enhance the immune response induced by local radiotherapy. In this review, we discuss the data supporting this combinatorial therapeutic strategy and the role of radiotherapy in the age of the rapid expansion of cancer immunotherapy.
Immune responses induced by the DNA damage of irradiation
Conventionally, the therapeutic mechanism underlying the effects of radiotherapy has been considered to be the induction of direct or indirect DNA damage, which leads to cancer cell death. However, it is now recognized that the induction of an antitumor immune response by radiotherapy also contributes to its antitumor effects (10). Furthermore, evidence accumulated over recent years has revealed that this radiation-induced DNA damage itself triggers the recognition of cancer cells by the immune system.
The cyclic GMP–AMP (cGAMP) synthase (cGAS)—stimulator of interferon genes (STING) pathway plays a crucial role in the DNA damage-induced immune response. Cytoplasmic double-strand DNA of nuclear and mitochondrial origin increases as the result of radiation-induced DNA damage and binds to cGAS, followed by the catalysis of the synthesis of cGAMP, which is a secondary messenger that binds to and activates the adaptor protein STING (11–14). The activated cGAS/STING pathway eventually leads to the generation of the type-I interferon (IFN-I) mRNA via IRF3/NF-κB-dependent transcriptional activation and the induction of IFN-stimulated genes (15–17). The IFN-I generated by the cGAS/STING pathway induces dendritic cell migration to the tumor and cross-priming of T cells, which are required for the antitumor effect of radiotherapy (18). Activation of the cGAS/STING pathway is regulated by TREX1, which is an abundant intracellular 3′ → 5′ exonuclease that degrades cytoplasmic single- or double-stranded DNA (19). TREX1 regulates the immune response by suppressing IFN-I production; its deficiency leads to autoimmune diseases in humans and mice (20). Importantly, the balance between cGAS/STING pathway activation and DNA damage-initiated TREX1 induction after irradiation is affected by the radiation dose. Preclinical data suggest that hypofractionated radiation given as a few doses in the range of 8–12 Gy per fraction activates the cGAS/STING pathway more effectively than higher single doses of 20 Gy or more, at least in part due to the upregulation of TREX1 (13). TREX1-mediated degradation of cytosolic DNA hinders the activation of the cGAS/STING pathway in the cancer cells themselves as well as in dendritic cells that uptake extracellular vesicles produced by the irradiated cancer cells (21). The role of TREX1 in response to radiotherapy used in combination with immunotherapy in the clinic remains to be elucidated. However, in a recent phase I study of multi-site radiotherapy and pembrolizumab in which serial biopsies of the irradiated tumors were evaluated, increased TREX1 post-radiation was associated with poor tumor response (22). The mechanisms regulating the levels of cytosolic DNA and TREX1 activity in response to radiation, including DNA damage repair factors or cell cycle checkpoints, still need to be elucidated. Nevertheless, the key role of TREX1 in regulation of type-I interferon activation in autoimmunity suggests that it could be a therapeutic target to increase the radiation-induced induction of antitumor immune responses (23). Conversely, IFN-I, as well as type-II interferon, upregulates the immune checkpoint protein, programmed death ligand 1 (PD-L1) (24). Taken together, these findings suggest that IFN induction via the cGAS/STING pathway, which is triggered by radiation-induced DNA damage, may ultimately have dual immune activating and immunosuppressive effects.
The DNA damage caused by radiotherapy also enhances the expression of major histocompatibility complex (MHC) class I molecules in cancer cells. Reits et al. demonstrated that irradiation induced a dose-dependent increase in the levels of intracellular peptides derived from existing proteins, as well as newly increased protein synthesis via mTOR activation (25). Interestingly, in their analysis, the peptides that were uniquely expressed in irradiated cells and presented by MHC class I molecules were derived from proteins involved in DNA repair. These results suggest that the mechanism underlying the radiation-induced expression of MHC class I molecules may be involved in the promotion of DNA repair, as well as the enhancement of existing protein degradation and new protein synthesis.
The induction of the expression of PD-L1 by radiotherapy has been reported in a few studies and mostly attributed to inflammatory cytokines (26,27). We elucidated the mechanism by which the DNA damage signal itself regulates PD-L1 expression. We found that DNA double-strand breaks (DSBs) induced PD-L1 expression in a transcription-dependent manner via DNA damage signaling molecules, including ataxia telangiectasia mutated and ataxia telangiectasia and Rad3-related (ATR), Chk1 and the downstream STAT-IRF1 pathway (28). Furthermore, the depletion of DNA repair proteins, including Ku80 and breast invasive carcinoma 2 (BRCA2), enhanced PD-L1 upregulation induced by DSBs. These results suggest that radiotherapy or DNA damaging chemotherapy may highly induce PD-L1 expression in patients with mutations in these proteins; therefore, PD-L1 inhibition as a consolidation therapy after radiotherapy or chemotherapy may be effective in these cases. In support of our results, the analysis of a mouse model demonstrated that ATR inhibition suppresses PD-L1 expression in cancer cells and enhances the cell-killing effect of T cells (29). Furthermore, a significant delay in tumor growth was observed in combination of ATR inhibitor with radiotherapy (30). Whereas PD-L1 expressed on the surface of cancer cells inhibits activation of T cells by interacting with PD-1, a role that forms the basis for its therapeutic targeting by antibodies (31), a recent report highlights another role of intracellular PD-L1 in stabilizing the mRNA of DNA damage/repair proteins (e.g. NBS1 and BRCA1) (32). This intriguing finding suggests that intracellular PD-L1 may contribute to the therapeutic resistance of cells that survive radiotherapy. Therefore, strategies to inhibit intracellular PD-L1 may enhance the effect of radiotherapy and chemotherapy by inhibiting DNA repair.
In summary, DNA damage, which was previously thought to be the central mechanism underlying the cell-killing effect of radiotherapy, not only induces direct cell death but also modulates the cross-talk between the immune system and the tumor. For its combination with immunotherapy, further research is needed to establish better combination strategies through the elucidation of the radiotherapy-induced DNA damage/repair signals that enhance immune activation. This may also lead to the discovery of new drugs for targets involved in DNA damage/repair, which will enable more effective combination therapies.
Immune responses induced by irradiation in the tumor microenvironment
One of the important effects of radiotherapy on the immune environment is a form of cell death that is called immunogenic cell death (ICD) (33). The release of damage-associated molecular patterns that promote the migration and activation of dendritic cells and antigen presentation to CD8-positive T cells is an important feature of ICD. ICD includes the expression of calreticulin on the cell surface, the release of high-mobility group protein box 1 (HMGB1) and adenosine-5′-triphosphate (ATP) (34). Calreticulin is a chaperone that is present on the surface of the endoplasmic reticulum; moreover, its expression on the cell membrane promotes the phagocytosis of irradiated cells by dendritic cells (35). HMGB1 is a non-histone chromosomal protein that, when released extracellularly, acts as an agonist of the toll-like receptor 4 on the surface of dendritic cells, thus activating them to induce an antigen-specific T-cell response (36,37). ATP activates dendritic cells by binding to their P2X7 receptors and results in IFNγ-producing CD8-positive T cells priming (38). In addition, the tumor-associated antigens released during ICD are taken up by dendritic cells that cross-present them to CD8+ T cells (39,40). Tumor DNA carried by exosomes produced by irradiated cancer cells contributes to dendritic cell activation by stimulating IFN-I production via the cGAS/STING pathway (21). Tumor-derived DNA is transferred to dendritic cells that phagocytize the tumor cell-derived exosomes, promoting the recognition of irradiated tumors by the immune system (41).
The results of clinical trials of radiotherapy combined with ipilimumab (anti-CTLA-4 antibody) in patients with metastatic non-small cell lung cancer (NSCLC), where ipilimumab has little single-agent activity, identified some of the factors that may determine the success of this combination therapy. Formenti et al. (42) demonstrated that, at the time of radiotherapy completion, IFN-β in the peripheral blood was significantly elevated in patients who exhibited the abscopal effect after radiotherapy to one of the metastases combined with ipilimumab therapy. In contrast, there was no significant increase in IFN-β in patients who showed progressive disease. These data suggest that the ability of radiotherapy to induce IFN-β may contribute to the success or failure of the combination therapy. A detailed analysis revealed the expansion of two CD8-positive T-cell clones that specifically recognized a mutated neoantigen encoded by the KPNA2 gene, which is upregulated by radiation, in a patient with complete response. Together, these findings suggest that induction of IFN-I and exposure of immunogenic mutations may be important mechanisms that contribute to the success of combinations of radiotherapy with immunotherapy.
Although naïve T cells are known to be very radiosensitive, raising a concern that radiotherapy may deplete T cell infiltrating the tumor at the time of treatment, a recent analysis using a long-term image acquisition method in mice showed that most effector T cells that were present in tumors prior to radiotherapy survived radiation administered at clinical doses (43). Furthermore, T cells that survived irradiation retained their activity and their ability to produce IFNγ and kill cancer cells. Several clinical studies analyzed the infiltration of CD8-positive T lymphocytes into tumor tissues using specimens from patients. In most of these studies, specimens were collected about 1 month after the preoperative (chemo)radiotherapy. For example, infiltrating CD8-positive T cells were increased in the surgical specimens after preoperative chemoradiotherapy for NSCLC (44), esophageal cancer (45) and colorectal cancer (46–49). In contrast, the opposite result has also been reported. CD8-positive T-cell infiltration was decreased in oral squamous cell carcinoma (50) and cervical cancer (51) treated with chemoradiotherapy. Because the tumor specimens that were collected after surgery were commonly treated by radiotherapy with concurrent chemotherapy, which affect systemic lymphocytes, and approximately 1 month had elapsed since the completion of preoperative (chemo) radiotherapy, these results may not directly reflect the impact of radiotherapy on the infiltration of CD8-positive T cells. Interestingly, a recent study analyzed specimens that were collected during chemoradiotherapy. Dorta-Estremera et al. analyzed the proportion of CD8-positive T cells among tumor-infiltrating lymphocytes in cervical cancer treated with chemoradiotherapy. They reported that the number of these cells was decreased, whereas the proportion of CD69-positive activated T cells among CD8-positive T cells was increased over time (52), suggesting that chemoradiotherapy has the potential to enhance tumor infiltration by activated T cells, at least during treatment. Thus, although T cells in the irradiated field were reported to be able to survive, because chemotherapy or standard fractionated radiotherapy with a large field conventionally induces systemic lymphopenia (53), and T-cell infiltration changes over time, further clinical studies are required to clarify the optimal timing of the combination of radiotherapy, immunotherapy and chemotherapy to maximize the effect of this approach.
As described above, radiotherapy has the ability to convert the irradiated tumor into an ‘in situ vaccine’. Immunotherapy exerts its effect by promoting the immune response that is inherent to the host. The use of the patients’ own tumor as a source of tumor-specific antigens in radiotherapy can diversify the tumor-specific T-cell response (54,55). This activation of the immune response by radiotherapy creates an environment in which the immunotherapy can function more effectively.
Clinical application of the combined therapy
Published evidence
Based on the accumulation of the abovementioned preclinical data (Fig. 1), the combination of radiotherapy with immunotherapy has expanded rapidly since the advent of ICIs. In the clinical studies performed to date, this combination therapy was broadly divided into strategies aimed at the cure of localized tumors and at eliciting systemic effects in metastatic cancers. An excellent example of the former is the so-called PACIFIC trial, which is a phase III clinical trial of durvalumab (an anti-PD-L1 antibody) as consolidation therapy after radical chemoradiotherapy for stage III NSCLC. This clinical study showed a significant prolongation of overall survival (OS) and progression-free survival (PFS) without a significant increase in adverse events in the treatment of stage III NSCLC in the arm given durvalumab (56,57). The PACIFIC trial does not allow the evaluation of the contribution of radiotherapy since all patients received standard-of-care chemoradiation. It is intriguing to consider if the elucidation of the optimal radiotherapy dose and fractionation to be used in this setting could further improve patients’ outcome.
Figure 1.
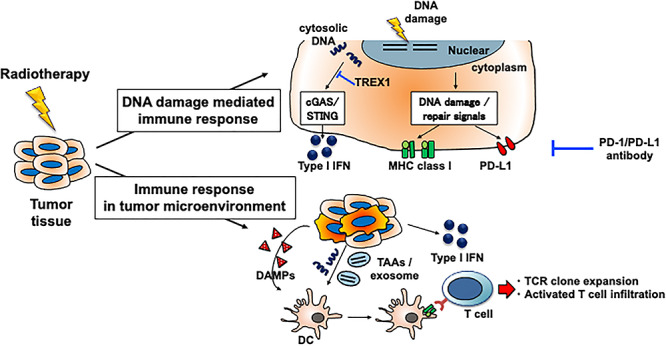
Immune responses induced by radiotherapy include those caused by DNA damage and those that occur in the tumor microenvironment. The immune response to radiotherapy creates an environment in which immune checkpoint inhibitor can more effectively eliminate tumors. Immune response to DNA damage includes programmed death ligand 1 (PD-L1) upregulation, which contributes to immunosuppression, but can be a target of the PD-1/PD-L1 blockade. DAMP, damage-associated molecular pattern; DC, dendritic cell; IFN, interferon; MHC, major histocompatibility complex; TAA, tumor-associated antigen.
In metastatic cancer, a randomized phase III trial was performed in patients with metastatic castration-resistant prostate cancer. Patients with at least one bone metastasis that had progressed after docetaxel treatment were randomly divided into two groups, i.e. bone-directed palliative radiotherapy followed by either ipilimumab or placebo therapy. A significantly longer median PFS and higher OS rate at 2–5 years were observed in the ipilimumab group (58,59). It should be noted that the results of this trial were calculated by an exploratory analysis using piecewise hazard model because the OS curves were crossed at 7–8 months and the proportional hazard ratio assumption was not met, but it is pointed out to be inappropriate for this study (60). Therefore, an alternative method of re-evaluation by mean survival time (MST) was suggested, which showed that the MST up to 52 months was significantly longer in the ipilimumab group compared with the placebo group (60).
The results of aforementioned phase II clinical trial for patients with metastatic NSCLC showed that hypofractionated radiotherapy (6 Gy × 5 or 9 Gy × 3) to a single metastasis with initiation of ipilimumab on the first day of radiation resulted in intent-to-treat overall response rate (ORR) of non-irradiated metastases in 18% of patients, and clinical benefit in 31% of patients, without an increase in side effects due to the combination (42). Another phase II randomized trial in patients with metastatic NSCLC compared SBRT to a metastasis before the initiation of pembrolizumab (an anti-PD-1 antibody) with pembrolizumab monotherapy. The results revealed that OS, PFS and ORR of non-irradiated lesion showed improved trends in the SBRT combination group without an increase in treatment-related adverse events (61). A pooled analysis of this study and another phase I/II study of SBRT in combination with pembrolizumab demonstrates the significant superiority of the SBRT combination group in non-irradiated tumor response and control rates, OS and PFS (62). Furthermore, in a prospective study of patients with metastatic melanoma treated with radiotherapy to one or two disease sites with concurrent ipilimumab, half of all patients had a clinical benefit during the observation period, including durable CR in 3 of 22 patients (63). In another phase I trial, hypofractionated radiotherapy against a metastasis combined with pembrolizumab resulted in PR in 2 of 12 patients with NSCLC or melanoma who were previously treated with anti-PD-1 antibodies and CR in 1 of 12 patients with other tumors not previously treated with anti-PD-1 antibodies. Furthermore, importantly, there were no grade 3 or higher adverse events (64). The results of these clinical trials suggest the potential of combined therapy for both locally advanced and systemically metastatic cancer.
Several retrospective analyses also support the potential benefit of ICIs combined with radiotherapy. For example, the secondary analysis of a phase I clinical trial of pembrolizumab monotherapy for patients with locally advanced or metastatic NSCLC, the KEYNOTE-001 trial, revealed that patients who previously received any radiotherapy had a significantly longer OS and PFS compared with patients who did not receive radiotherapy (65). Similarly, in another retrospective analysis of nivolumab (an anti-PD-1 antibody) for patients with advanced NSCLC, a group of patients who had a history of radiotherapy, regardless of its radical or palliative nature, had a significantly better OS and PFS than did those without a history of radiotherapy (66). Significantly, the incidence of treatment-related pulmonary toxicity was greater in the radiation-treated group, but the incidence of grade 3 or higher pneumonitis was not significantly different between the groups in these analyses. Moreover, among patients with metastatic lung tumors treated with anti-PD-1/PD-L1 antibodies, a trend toward longer OS was reported in patients with prior thoracic radiotherapy than in those without radiotherapy, despite including fewer targetable mutations (67). Another retrospective analysis reported that palliative radiotherapy for advanced metastatic melanoma patients with progression after ipilimumab treatment resulted in an abscopal effect in 11 of 21 patients and significantly longer OS in those who showed the abscopal effect than in those who did not (68). Overall, these reports have stimulated a several clinical trials of radiotherapy combined with immunotherapy that are currently under way.
Ongoing clinical trials
A search of ClinicalTrials.gov for phase III clinical trials aimed at investigating the efficacy of the combination of radiotherapy and ICI therapy identified 25 combined clinical trials (Table 1). Most of these trials included radiotherapy in both arms, examining the additional effect of ICI on radiotherapy. The breakdown of target organs in these clinical studies was as follows: nine trials in the head and neck region, six trials in the lung, three trials in the esophagus, two trials in the cervix and one trial each in the brain, liver, lower gastrointestinal tract, skin and lymphoma. Many clinical trials seem to be under way that target sites, such as head and neck cancer and lung cancer, which are treated by high-precision radiotherapy modalities, such as IMRT. In fact, the modality of radiotherapy in these trials was as follows: seven trials used IMRT and four trials used SBRT, which means that the so-called high-precision radiotherapy is often used in combination trials. Interestingly, with regard to the timing of the combination, most of these ongoing trials use concurrent combinations of radiotherapy with ICIs, in contrast to the PACIFIC trial, which is the sequential combination of chemoradiotherapy with conventional fractionation and anti-PD-L1 antibody therapy. The results of these studies will clarify the effectiveness of the combination strategy, which has been supported by many preclinical studies.
Table 1.
Active (i.e. ‘Recruiting’ and ‘Active, not recruiting’ in ClinicalTrials.gov) phase III clinical trials of immune checkpoint inhibitors combined with (chemo)radiotherapy
| Primary lesion | Study title | Radiotherapy | Immune checkpoint inhibitor | Design | NCT number |
|---|---|---|---|---|---|
| Anti-PD-1/PD-L1 antibody | |||||
| Head and neck | Study of Pembrolizumab Given Prior to Surgery and in Combination With Radiotherapy Given Post-surgery for Advanced Head and Neck Squamous Cell Carcinoma (MK-3475-689) | EBRT (60 Gy/30 fr. or 66 Gy/33 fr. or 70 Gy/35 fr.) | Pembrolizumab | Experimental: Pembrolizumab > surgery > Pembrolizumab + CRT Comparator: Surgery > CRT | NCT03765918 |
| Study of Pembrolizumab (MK-3475) or Placebo With Chemoradiation in Participants With Locally Advanced Head and Neck Squamous Cell Carcinoma (MK-3475-412/KEYNOTE-412) | EBRT (70 Gy/35 fr.) | Pembrolizumab | Experimental: Pembrolizumab > CRT + Pembrolizumab > Pembrolizumab Comparator: Placebo > CRT + Placebo > Placebo | NCT03040999 | |
| Testing Immunotherapy Versus Observation in Patients With HPV Throat Cancer | IMRT (70 Gy/35 fr.) | Nivolumab | Experimental (Arm A): CRT > Nivolumab Comparator (Arm B): CRT Experimental (Arm C): Nivolumab |
NCT03811015 | |
| Sintilimab (PD-1 Antibody) and Chemoradiotherapy in Locoregionally advanced Nasopharyngeal Carcinoma | IMRT (70 Gy in 6–7 weeks) | Sintilimab | Experimental: chemotherapy + Sintilimab > CRT + Sintilimab Comparator: Chemotherapy > CRT | NCT03700476 | |
| Programmed Death-1 (PD-1) Antibody Combined With IMRT in Recurrent Nasopharyngeal Carcinoma Patients | IMRT (60–66 Gy/30–33 fr.) | Toripalimab | Experimental: RT + toripalimab Comparator: RT alone | NCT03907826 | |
| Toripalimab Plus Concurrent Chemo-radiotherapy for Unresectable Locally Recurrent Nasopharyngeal Carcinoma | IMRT: (60–64 Gy/27 fr.) | Toripalimab | Experimental: CRT + toripalimab Comparator: CRT | NCT04453813 | |
| Radiation Therapy With Durvalumab or Cetuximab in Treating Patients With Locoregionally Advanced Head and Neck Cancer Who Cannot Take Cisplatin | IMRT | Durvalumab | Experimental: IMRT + Durvalumab Comparator: IMRT + cetuximab | NCT03258554 | |
| Concurrent and Adjuvant PD1 Treatment Combined With Chemo-radiotherapy for High-risk Nasopharyngeal Carcinoma | IMRT | Camrelizumab | Experimental: Camrelizumab + CRT Comparator: CRT | NCT04453826 | |
| Lung | PD-1 Inhibitors and Chemotherapy With Concurrent Irradiation at Varied Tumour Sites in Advanced Non-small Cell Lung Cancer | EBRT (at least 18 Gy/3 fr.) | Pembrolizumab | Experimental: Pembrolizumab + CRT Comparator: Pembrolizumab + chemotherapy | NCT03774732 |
| Efficacy and Safety Study of Stereotactic Body Radiotherapy (SBRT) With or Without Pembrolizumab (MK-3475) in Adults With Medically Inoperable Stage I or IIA Non-Small Cell Lung Cancer (NSCLC) (MK-3475-867/KEYNOTE-867) | SBRT (45–54 Gy/3–5 fr.) | Pembrolizumab | Experimental: SBRT + Pembrolizumab Comparator: SBRT + placebo | NCT03924869 | |
| Study of Pembrolizumab With Concurrent Chemoradiation Therapy Followed by Pembrolizumab With or Without Olaparib in Stage III Non-Small Cell Lung Cancer (NSCLC) (MK-7339-012/KEYLYNK-012) | EBRT (60 Gy/30 fr.) | Pembrolizumab | Experimental: Pembrolizumab + concurrent CRT > Pembrolizumab + placebo Experimental: Pembrolizumab + concurrent CRT > Pembrolizumab + olaparib Comparator: CRT > durvalumab | NCT04380636 | |
| Immunotherapy With or Without SBRT in Patients With Stage IV Non-small Cell Lung Cancer | SBRT | Pembrolizumab | Experimental: SBRT + Pembrolizumab Comparator: Pembrolizumab alone | NCT03867175 | |
| Durvalumab vs. Placebo Following Stereotactic Body Radiation Therapy in Early Stage Unresected Non-small Cell Lung Cancer Patients (PACIFIC-4) | SBRT | Durvalumab | Experimental: SBRT > Durvalumab Comparator: SBRT > Placebo | NCT03833154 | |
| Esophagus | Study of Pembrolizumab (MK-3475) Versus Placebo in Participants With Esophageal Carcinoma Who Are Receiving Chemotherapy and Radiation Therapy (MK-3475-975/KEYNOTE-975) | EBRT (50 Gy/25 fr. or 60 Gy/30 fr.) | Pembrolizumab | Experimental: Pembrolizumab > Pembrolizumab + CRT Comparator: Placebo > Placebo + CRT | NCT04210115 |
| Study of Camrelizumab (SHR-1210) in Combination With Concurrent Chemoradiotherapy in Locally Advanced Esophageal Cancer | NS | Camrelizumab | Experimental: Camrelizumab + Paclitaxel + Cisplatin + radiotherapy Comparator: Placebo + Paclitaxel + Cisplatin + radiotherapy | NCT04426955 | |
| Liver | Combination of Sintilimab and Stereotactic Body Radiotherapy in Hepatocellular Carcinoma (ISBRT01) | SBRT (30–54 Gy/3–6 fr.) | Sintilimab | Experimental: SBRT > Sintilimab Comparator: SBRT | NCT04167293 |
| Uterine cervix | Study of Durvalumab With Chemoradiotherapy for Women With Locally Advanced Cervical Cancer (CALLA) | EBRT + brachytherapy | Durvalumab | Experimental: durvalumab + CRT > durvalumab Comparator: placebo + CRT | NCT03830866 |
| Study of Chemoradiotherapy With or Without Pembrolizumab (MK-3475) For The Treatment of Locally Advanced Cervical Cancer (MK-3475-A18/KEYNOTE-A18/ENGOT-cx11) | EBRT (45–50 Gy/23–28 fr.) + brachytherapy (25–30 Gy/4–6 fr.) | Pembrolizumab | Experimental: Pembrolizumab + CRT Experimental: Placebo + CRT | NCT04221945 | |
| Intestine | PD1 Antibody Sintilimab ± Chemoradiotherapy for Locally Advanced Rectal Cancer | EBRT (50 Gy/25 fr.) | Sintilimab | Experimental: Sintilimab > surgery or watch and wait > Sintilimab ± chemotherapy Experimental cohort B (arm-1): Sintilimab + CRT > surgery or watch and wait > chemotherapy Comparator cohort B (arm-2): CRT > surgery or watch and wait > chemotherapy | NCT04304209 |
| Skin | Pembrolizumab Versus Placebo Following Surgery and Radiation in Participants With Locally Advanced Cutaneous Squamous Cell Carcinoma (MK-3475-630/KEYNOTE-630) | NS | Pembrolizumab | Experimental: surgery > radiotherapy > Pembrolizumab Comparator: surgery > radiotherapy | NCT03833167 |
| Lymphoma | A Multicenter, Phase 3, Randomized Trial of Sequencial Chemoradiotherapy With or Without Toripalimab (PD-1 Antibody) in Newly Diagnosed Early-Stage Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (ENKTL) | IMRT (54–56 Gy in 25–26 weeks) | Toripalimab | Experimental: toripalimab + chemotherapy > toripalimab + radiotherapy > toripalimab Comparator: chemotherapy > radiotherapy | NCT04365036 |
| Anti-PD-1/PD-L1 antibody + anti-CTLA-4 antibody | |||||
| Brain | Testing the Use of the Immunotherapy Drugs Ipilimumab and Nivolumab Plus Radiation Therapy Compared to the Usual Treatment (Temozolomide and Radiation Therapy) for Newly Diagnosed MGMT Unmethylated Glioblastoma | NS | Nivolumab and Ipilimumab | Experimental: RT + Nivolumab + Ipilimumab Comparator: RT + Temozolomide > Temozolomide |
NCT04396860 |
| Head and neck | Study of Nivolumab Alone or in Combination With Ipilimumab as Immunotherapy vs. Standard Follow-up in Surgical Resectable HNSCC After Adjuvant Therapy | EBRT (56–66 Gy) | Nivolumab and Ipilimumab | Experimental: Nivolumab > surgery > RT or CRT > Nivolumab (arm Ia) or Nivolumab + Ipilimumab (arm Ib) Comparator: Surgery > RT or CRT |
NCT03700905 |
| Lung | Phase III Trial of (Local Consolidation Therapy; LCT) After Nivolumab and Ipilimumab | NS | Nivolumab and Ipilimumab | Experimental: Arm A (ipilimumab, nivolumab): (INDUCTION) Nivolumab + Ipilimumab > Nivolumab + Ipilimumab Experimental: Arm B (ipilimumab, nivolumab, LCT): (INDUCTION) Nivolumab + Ipilimumab > LCT (surgery and/or RT) > Nivolumab + Ipilimumab |
NCT03391869 |
| Esophagus | Nivolumab and Ipilimumab in Treating Patients With Esophageal and Gastroesophageal Junction Adenocarcinoma Undergoing Surgery | NS | Nivolumab and Ipilimumab | Experimental: Arm A: CRT Experimental: Arm B: CRT + Nivolumab Experimental: Arm C: Nivolumab Experimental: Arm D: Nivolumab + Ipilimumab |
NCT03604991 |
EBRT, externalbeam radiotherapy; fr., fractions; CRT, chemoradiotherapy; IMRT, intensity-modulated radiotherapy; RT, radiotherapy; SBRT, stereotactic body radiotherapy; LCT, local consolidation therapy.
Perspectives
High-precision radiotherapy has made it possible to increase the dose size of radiotherapy relatively safely, and the role of radiotherapy as an option for cancer treatment is expanding at both early and advanced stages of the disease. On considering the combination with immunotherapy, which is aimed at the local and systemic disease control, a radiotherapy protocol that maximizes the immune response must be established. Specifically, it will be useful to elucidate how DNA damage signaling affects the immune response after radiotherapy. Furthermore, the elucidation of the optimal radiotherapy method premised on the combined therapy, such as the irradiation dose, the number of fractions and the timing of the combined use of ICIs, is necessary. The survival term was significantly longer in mice that started the anti-PD-L1 antibody during fractional radiotherapy compared with those that received sequential administration after the completion of radiotherapy (26). In another mouse study, the timing of 20 Gy × 1 fraction and anti-CTLA4 antibodies reported that the best tumor control and survival advantage was observed in the group that started the anti-CTLA4 antibodies before radiotherapy, compared with the group that started it after radiotherapy (69). In clinical practice, a retrospective analysis of the timing of palliative radiotherapy and ipilimumab for metastatic melanoma reported that the median OS was 9 months in patients who received radiotherapy during induction of ipilimumab and 39 months in patients who received radiotherapy while continuing ipilimumab (70). These results suggest that ICIs should be initiated prior to or concurrently with radiotherapy, rather than after radiotherapy, although accurate evaluation requires comparison by prospective clinical trials due to differences in dosages and ICI used in these studies. Regarding the irradiation field, when it includes the draining lymph nodes, it can suppress the radiotherapy-induced immune response, as shown in preclinical studies (71). Therefore, the radiotherapy method used in the combination treatment with immunotherapy may differ from the conventional radiotherapeutic treatment strategies. Taken together, these results imply that ICI may be preferable prior to or concurrent with radiotherapy in a single 10 Gy fractionated dose that avoids draining lymph nodes. However, despite a wealth of preclinical evidence, clinical evidence that radiotherapy enhances immunotherapy responses is limited, calling for clinical studies that include an in-depth immunomonitoring to elucidate the mechanisms of success or failure in patients.
Conclusion
The role of radiotherapy in the age of ICIs and precision medicine is evolving, and in the future, radiotherapy may be used not only as a local treatment but also as a systemic one in combination with immunotherapy. Whereas the results of recent clinical studies are promising, evidence that radiotherapy can reliably improve responses to immunotherapy is still lacking. Thus, more studies are needed to understand the immunogenic effects of radiation in preclinical models and in patients.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Contributor Information
Hiro Sato, Department of Radiation Oncology, Graduate School of Medicine, Gunma University, Maebashi, Gunma, Japan; Gunma University Heavy Ion Medical Center, Maebashi, Gunma, Japan.
Sandra Demaria, Department of Radiation Oncology, Weill Cornell Medical College, New York, NY, USA.
Tatsuya Ohno, Department of Radiation Oncology, Graduate School of Medicine, Gunma University, Maebashi, Gunma, Japan.
Funding
The work was supported by the JSPS KAKENHI Grants provided to H.S. [grant number JP 19K08195].
Conflict of interest statement
The authors declare no potential conflicts of interest related to this manuscript, but Sandra Demaria has received compensation for consultant/advisory services from Lytix Biopharma, Mersana Therapeutics, EMD Serono and Ono Pharmaceutical and research support from Lytix Biopharma and Nanobiotix.
References
- 1. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- 2. O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67. [DOI] [PubMed] [Google Scholar]
- 3. Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demaria S, Formenti SC. The abscopal effect 67 years later: from a side story to center stage. Br J Radiol. 2020;93:20200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MOLE RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–41. [DOI] [PubMed] [Google Scholar]
- 6. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- 7. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1–12. [DOI] [PubMed] [Google Scholar]
- 10. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamazaki T, Kirchmair A, Sato A, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–71. [DOI] [PubMed] [Google Scholar]
- 15. Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- 18. Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ablasser A, Hemmerling I, Schmid-Burgk JL, Behrendt R, Roers A, Hornung V. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J Immunol. 2014;192:5993–7. [DOI] [PubMed] [Google Scholar]
- 21. Diamond JM, Vanpouille-Box C, Spada S, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. 2018;6:910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luke JJ, Onderdonk BE, Bhave SR, et al. Improved survival associated with local tumor response following multisite radiotherapy and pembrolizumab: secondary analysis of a phase I trial. Clin Cancer Res. 2020;26:6437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamazaki T, Galluzzi L. TREX1 cuts down on cancer immunogenicity. Trends Cell Biol. 2017;27:543–5. [DOI] [PubMed] [Google Scholar]
- 24. Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. [DOI] [PubMed] [Google Scholar]
- 27. Azad A, Yin Lim S, D'Costa Z, et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med. 2017;9:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun LL, Yang RY, Li CW, et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. 2018;8:1307–16. [PMC free article] [PubMed] [Google Scholar]
- 30. Vendetti FP, Karukonda P, Clump DA, et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest. 2018;128:3926–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tu X, Qin B, Zhang Y, et al. PD-L1 (B7-H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol Cell 2019;74:1215–26.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Ruiz ME, Rodriguez I, Leaman O, et al. Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol Ther. 2019;196:195–203. [DOI] [PubMed] [Google Scholar]
- 35. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. [DOI] [PubMed] [Google Scholar]
- 36. Messmer D, Yang H, Telusma G, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–13. [DOI] [PubMed] [Google Scholar]
- 37. Yamazaki T, Hannani D, Poirier-Colame V, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkins AC, Patin EC, Harrington KJ, Melcher AA. The immunological consequences of radiation-induced DNA damage. J Pathol. 2019;247:606–14. [DOI] [PubMed] [Google Scholar]
- 41. Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic Tumors. Immunity. 2014;41:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arina A, Beckett M, Fernandez C, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun. 2019;10:3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoneda K, Kuwata T, Kanayama M, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelly RJ, Zaidi AH, Smith MA, et al. The dynamic and transient immune microenvironment in locally advanced esophageal adenocarcinoma post chemoradiation. Ann Surg. 2018;268:992–9. [DOI] [PubMed] [Google Scholar]
- 46. Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S414–21. [DOI] [PubMed] [Google Scholar]
- 47. Lim SH, Chua W, Cheng C, et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34:6505–13. [PubMed] [Google Scholar]
- 48. Lim YJ, Koh J, Kim S, et al. Chemoradiation-induced alteration of programmed death-ligand 1 and CD8. Int J Radiat Oncol Biol Phys. 2017;99:1216–24. [DOI] [PubMed] [Google Scholar]
- 49. Matsutani S, Shibutani M, Maeda K, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tabachnyk M, Distel LV, Büttner M, et al. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;48:594–601. [DOI] [PubMed] [Google Scholar]
- 51. Tsuchiya T, Someya M, Takada Y, et al. Association between radiotherapy-induced alteration of programmed death ligand 1 and survival in patients with uterine cervical cancer undergoing preoperative radiotherapy. Strahlenther Onkol. 2020;196:725–35. [DOI] [PubMed] [Google Scholar]
- 52. Dorta-Estremera S, Colbert LE, Nookala SS, et al. Kinetics of intratumoral immune cell activation during chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys. 2018;102:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol. 2018;3:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rudqvist NP, Pilones KA, Lhuillier C, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res. 2018;6:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 57. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 58. Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fizazi K, Drake CG, Beer TM, et al. Final analysis of the Ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ludmir EB, McCaw ZR, Re WLJ, et al. Re: Karim Fizazi, Charles G. Drake, Tomasz M. Beer, et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2021;79:e10–e11. doi: 10.1016/j.eururo.2020.07.032. Interpreting the Effect of Ipilimumab Following Radiotherapy for Patients with Postdocetaxel Metastatic Castration-resistant Prostate Cancer. [DOI] [PubMed] [Google Scholar]
- 61. Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;11:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2020;S2213–2600(20)30391–X. doi: 10.1016/S2213-2600(20)30391-X. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 63. Hiniker SM, Reddy SA, Maecker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maity A, Mick R, Huang AC, et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br J Cancer. 2018;119:1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamaguchi O, Kaira K, Hashimoto K, et al. Radiotherapy is an independent prognostic marker of favorable prognosis in non-small cell lung cancer patients after treatment with the immune checkpoint inhibitor, nivolumab. Thorac Cancer. 2019;10:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hwang WL, Niemierko A, Hwang KL, et al. Clinical outcomes in patients with metastatic lung cancer treated with PD-1/PD-L1 inhibitors and thoracic radiotherapy. JAMA Oncol. 2018;4:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11:e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24:5058–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


