Abstract
Objective:
To evaluate the associations of pregestational BMI, gestational weight gain (GWG) and breast-feeding at 1 month postpartum with four patterns of weight change during the first year after delivery: postpartum weight retention (PPWR), postpartum weight gain (PPWG), postpartum weight retention + gain (PPWR + WG) and return to pregestational weight.
Design:
In this secondary analysis of a prospective study, we categorised postpartum weight change into four patterns using pregestational weight and weights at 1, 6 and 12 months postpartum. We evaluated their associations with pregestational BMI, GWG and breast-feeding using multinomial logistic regression. Results are presented as relative risk ratios (RRR) and 95 % CI.
Setting:
Mexico City.
Participants:
Women participating in the Programming Research in Obesity, Growth, Environment and Social Stressors pregnancy cohort.
Results:
Five hundred women were included (53 % of the cohort). Most women returned to their pregestational weight by 1 year postpartum (57 %); 8 % experienced PPWR, 14 % PPWG and 21 % PPWR + WG. Compared with normal weight, pregestational overweight (RRR 2·5, 95 % CI 1·3, 4·8) and obesity (RRR 2·2, 95 % CI 1·0, 4·7) were associated with a higher risk of PPWG. Exclusive breast-feeding, compared with no breast-feeding, was associated with a lower risk of PPWR (RRR 0·3, 95 % CI 0·1, 0·9). Excessive GWG, compared with adequate, was associated with a higher risk of PPWR (RRR 3·3, 95 % CI 1·6, 6·9) and PPWR + WG (RRR 2·4, 95 % CI 1·4, 4·2).
Conclusions:
Targeting women with pregestational overweight or obesity and excessive GWG, as well as promoting breast-feeding, may impact the pattern of weight change after delivery and long-term women’s health.
Keywords: Postpartum weight change, Pregestational BMI, Gestational weight gain, Breast-feeding, Postpartum weight retention
Overweight and obesity have increased at alarming rates worldwide and currently afflict 76 % of adult women in Mexico(1), highlighting the importance of identifying the risk factors for excess weight in this population. Pregnancy and childbearing are risk factors for weight gain and obesity in women(2–5). Weight gain after childbirth may be the result of postpartum weight retention (PPWR) (i.e. retention of gestational weight), postpartum weight gain (PPWG) (i.e. weight gain that occurs entirely in the postpartum period)(6–11) or a combination of weight retention and gain (PPWR + WG) (i.e. retention of gestational weight, followed by postpartum weight gain)(6,11,12). We recently showed that PPWR, PPWG and PPWR + WG were associated with increased adiposity at 6 years postpartum(13). PPWR + WG was the only pattern directly associated with metabolic markers, such as insulin resistance(13). These adverse patterns of weight change are preventable. Therefore, it is essential to understand which characteristics make women more likely to experience them.
Different factors may predispose women to experience weight gain v. retention after delivery. Gestational weight gain (GWG) is a consistent and strong predictor of PPWR(14,15). Pregestational BMI has shown an inverse association with PPWR(14,16), and a positive association with PPWG(9). In some studies, exclusive breast-feeding has been associated with lower PPWR(17), but other studies have not found this association(18,19). To date, most of the published studies have focused on identifying the predictors of PPWR(14–19). Few studies have identified factors associated with PPWG(9), and, to the best of our knowledge, no studies have looked at the predictors of PPWR + WG within the first year after delivery. Furthermore, most of the studies have included primarily white women living in the USA, western Europe or Australia. Little is known about predictors and patterns of weight change in other settings, including Mexico. The objective of the current study was to evaluate the associations of pregestational BMI, GWG and breast-feeding with four mutually exclusive patterns of weight change during the first year postpartum: PPWR, PPWG, PPWR + WG and return to pregestational weight.
Methods
Study population
This was a secondary analysis of 948 mothers participating in the prospective birth cohort Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS). A full description of the cohort is provided elsewhere(20,21). Briefly, PROGRESS is an ongoing prospective cohort study initiated in Mexico City in 2007. Between July 2007 and February 2011, women with a singleton pregnancy who received health insurance and prenatal care through the Mexican Social Security System were invited to participate. They had to be at least 18 years old, <22 weeks pregnant, free of renal or heart diseases, not using steroids or anti-epileptic drugs, have access to a telephone line and plan to live in Mexico City for the next 3 years. Women were excluded if they had a history of infertility, diabetes or psychosis, consumed ≥1 alcoholic drink a day or used drugs or any prescription, herbal or over-the-counter medications regularly.
For the current analysis, we used information collected by in-person interviews during the second and third trimesters of pregnancy, and at 1, 6 and 12 months postpartum. In this analysis, women who delivered a live newborn, free of congenital malformations were included (n 937). Out of the 937 women, 515 had weights available (measured or imputed) to determine their pattern of postpartum weight change, which was the outcome of interest. Out of the 515 women, those who became pregnant again during the first year postpartum were excluded (n 14). We further excluded one woman who had undergone weight loss surgery before pregnancy due to her extreme weight loss during and after pregnancy. The final sample consisted of 500 women, who were comparable in demographic and anthropometric characteristics (e.g. pregestational BMI, GWG), smoking history, lifestyle behaviours (e.g. physical activity and sedentary activities) and breast-feeding practices to the 448 women not included in the analysis. The only exception was gestational age at delivery, which was slightly higher in the analytic sample (38·4 v. 38·1 weeks).
Exposures: predictors of patterns of postpartum weight change
Pregestational BMI
Trained personnel measured women’s height at the first study visit following standardised procedures(22). All participants self-reported their pregestational weight at the initial visit. However, self-reported weight is less reliable in this setting, where few women are regularly weighed. Considering this, we used an estimated pregestational weight obtained from a linear mixed-effects model. The model used weights measured during pregnancy (second and third trimesters) that were available for most women, as well as clinical weight measurements in the 6 months prior to pregnancy through the early pregnancy period that were recovered from Mexican Social Security System clinical records. The model also included days of gestation at the time of weight collection, maternal height, age, socioeconomic status (SES), education, parity and self-reported pregestational weight.
Model performance was assessed with ten-fold cross-validation, which was based on an evaluation of how well the model predictions at the last menstrual period agreed with weights measured at the Mexican Social Security System clinics for a subset of women (n 87). These weights were measured within ±20 d of the last menstrual period. The predictive accuracy assessed by the root mean square error was 3·2 kg. In a post hoc analysis, the model’s predictions were compared with those obtained by a model recently proposed by Thomas et al.(23), achieving similar results (data not shown).
We calculated pregestational BMI as estimated pregestational weight divided by height-squared, and classified women as underweight, normal weight, overweight or obese following the WHO criteria(24). Underweight women were combined with the normal weight category due to the small sample size (n 3).
Gestational weight gain
The study personnel measured women’s weight during the third trimester of pregnancy (mean gestational age 32 (sd 1) weeks, range 29–35 weeks)(22). GWG was calculated as the difference between third-trimester weight and estimated pregestational weight. It was corrected by the length of gestation following the procedure described by Perichart-Perera et al.(25). We classified women as having adequate, insufficient or excessive GWG for their gestational age following the US Institute of Medicine (IOM) guidelines, which have been previously used in a Mexican population(25,26).
Breast-feeding practices
At the first month visit postpartum, women reported whether they were breast-feeding or not and the exclusivity of breast-feeding (i.e. only breast milk or not). They also reported on the introduction of formula, medications and other types of milk, fluids and foods. With this information, we classified breast-feeding as exclusive (i.e. only breast milk), predominant (i.e. breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk) and partial (i.e. breast milk and any food or liquid, including non-human milk)(27).
Outcome: patterns of postpartum weight change
During every visit, the study personnel measured weights with a digital scale following standardised procedures(22). We categorised postpartum weight change into four mutually exclusive patterns using weights at 1, 6 and 12 months postpartum, as well as estimated pregestational weight. We focused on the first year postpartum because weight changes associated with pregnancy are more likely to occur during this time(7), and also to be consistent with published research(28,29). Return to pregestational weight at any postpartum time point was defined as a weight no more than 500 g higher than the estimated pregestational weight. The following definitions were applied for this classification:
Return to pregestational weight: Women who returned to their pregestational weight at 12 months postpartum. Includes women who lost weight compared with the pregestational state.
Postpartum weight retention (PPWR): Women who, on average, lost weight through 12 months postpartum without ever reaching their pregestational weight.
Postpartum weight gain (PPWG): Women who reached their pregestational weight at any point during the first 6 months postpartum and gained weight after that.
Postpartum weight retention and weight gain (PPWR + WG): Women who did not return to their pregestational weight during the first 6 months and, on average, gained weight through 12 months postpartum.
We imputed weight at 12 months for 100 women with missing weight at this time point using a multiple regression model. For this procedure, we used the information from the subsample of women (n 345) with weights available at both 12 and 18 months postpartum. This subsample was statistically comparable in demographic and anthropometric characteristics to the imputed set (n 100) and the overall cohort (see online supplementary material, Supplemental Table S1). Weights at 12 and 18 months were logarithmically transformed to maximise their linear association, as suggested by the Box−Cox family of transformations(30). A basic model included weight at 18 months as the only independent variable and explained 96 % of the variability of weight at 12 months. However, we included additional covariates to improve the model explanatory capacity and to increase precision in the model estimates, namely age, marital status and pregestational BMI. The final model achieved goodness of fit. To predict weight on the original scale, we used the exponential function.
Covariates
At the first study visit, women reported their age, parity (primiparous or multiparous), marital status (single/separated or married/cohabitating) and education (basic: elementary and secondary school; middle: high school; college: at least college). Women were classified into six SES categories (A/B [highest], C+, C, D+, D, E) using a validated questionnaire that included thirteen items on household assets and conditions (i.e. housing quality, services, material goods) and head of household’s education(31). For the current analysis, the six categories were collapsed into three: high (A/B, C+ and C), middle (D+) and low (D, E). Gestational age at delivery was calculated from the child’s birth date and self-reported last menstrual period date. We used the Capurro method, which is based on the newborn’s physical characteristics, as a secondary method to estimate gestational age. In cases where the two methods differed by >3 weeks, we used the Capurro method-derived gestational age(32).
Women provided information on smoking history, physical activity and sedentary activities via a general information questionnaire at the first study visit. Women were categorised as never smokers, smokers around pregnancy and former smokers (i.e. quit at least 1 year before pregnancy). Women reported leisure-time exercise as days per week and time per day invested in activities such as walking, running, swimming and aerobics, among others. Because of the high prevalence of women who did not engage in any leisure-time activity (87 %), we dichotomised this variable as physically active or not (i.e. reported >1 v. 0 min of leisure-time activity per week). Women also reported time spent reading or watching TV during weekdays and weekends. We computed average hours per day spent in these sedentary activities and categorised them as <2 or ≥2 h/d. These cut-offs are consistent with previous research(33). We did not have information on diet, which is a potential confounder on the associations of interest (i.e. GWG and postpartum weight change)(34).
Statistical analysis
We described the distribution of participant’s characteristics by the pattern of postpartum weight change via mean and standard deviation for numeric variables, and percentages for categorical variables. The statistical significance of the associations was evaluated with multinomial logistic regression models.
To evaluate the associations of pregestational BMI, GWG and breast-feeding (all as categorical variables) with patterns of postpartum weight change (dependent variable), we fit multinomial logistic regression models. The reference group was women who returned to their pregestational weight at 12 months postpartum. We first included each predictor individually in separate models to assess their independent effects on patterns of postpartum weight change. All the models were adjusted for relevant confounders selected after a thorough literature review, including maternal age, marital status, education, parity and SES. The models, including GWG as the main predictor, were further adjusted for pregestational BMI, smoking history, physically active and sedentary activities during pregnancy. The models for breast-feeding were additionally adjusted by pregestational BMI, adequacy of GWG, smoking history, gestational age at delivery and pregnancy complications, including pre-eclampsia, gestational hypertension and gestational diabetes. The Hosmer−Lemeshow test was performed to assess the goodness of fit of the models(35). For comparison, we performed the same analysis (i.e. the association of pregestational BMI, GWG and breast-feeding with patterns of postpartum weight change) excluding women with imputed weight at 12 months. The results did not differ substantially; therefore, only the results on the full dataset are presented (see online supplementary material, Supplemental Table S2).
In a sensitivity analysis, we used a modified definition of patterns of postpartum weight change considering a 0 g instead of the 500 g margin as a threshold to return to pregestational weight. Additionally, to compare our proposed classification of patterns of weight change with an outcome commonly reported in the literature(9,29,36), we evaluated the association of pregestational BMI, GWG and breast-feeding at 1 month with the odds of substantial postpartum weight retention using logistic regression models. Women were classified as having substantial postpartum weight retention if the difference between their weight at 12 months and their pregestational weight was ≥4·5 kg.
We performed all the analyses in STATA 15 (StataCorp LP). A P-value <0·05 was considered statistically significant.
Results
Women were 27 years on average, had basic education (42 %), were married or cohabitating (82 %), had low SES (53 %) and were multiparous (64 %) (Table 1). Pregestational overweight affected 41 %, and obesity 18 %, of the women. Mean gestational weight gain was 7·4 (sd 3·1) kg, and adequate gestational weight gain (47 %) was more common than insufficient (26 %) and excessive (27 %) gain. The majority (53 %) practiced partial breast-feeding at 1 month postpartum, and 28 % practiced exclusive breast-feeding.
Table 1.
Participants’ characteristics according to patterns of postpartum weight change in 500 women participating in the Programming Research in Obesity, Growth, Environment and Social Stressors cohort*
| Characteristics | All women, n 500 (%) | Patterns of postpartum weight change | P-value† | |||
|---|---|---|---|---|---|---|
| Return to pregestational weight | PPWR | PPWG | PPWR + WG | |||
| n 286 (57·2 %) | n 40 (8 %) | n 70 (14 %) | n 104 (20·8 %) | |||
| % | % | % | % | |||
| Pre-pregnancy | ||||||
| Maternal age (years) | 0·582 | |||||
| Mean | 27·2 | 27·3 | 26·4 | 27·7 | 26·8 | |
| sd | 5·5 | 5·4 | 4·3 | 6·3 | 5·4 | |
| Education (%) | ||||||
| Basic | 42·2 | 40·9 | 57·5 | 40·0 | 41·4 | 0·518 |
| Middle | 35·2 | 36·4 | 30·0 | 34·3 | 34·6 | |
| College | 22·6 | 22·7 | 12·5 | 25·7 | 24·0 | |
| Marital status (%) | ||||||
| Married/cohabitating | 82·0 | 85·7 | 85·0 | 74·3 | 76·0 | 0·047 |
| Without partner | 18·0 | 14·3 | 15·0 | 25·7 | 24·0 | |
| SES (%) | ||||||
| Low | 52·6 | 51·8 | 55·0 | 44·3 | 59·6 | 0·181 |
| Medium | 22·4 | 21·0 | 22·5 | 24·3 | 25·0 | |
| High | 25·0 | 27·3 | 22·5 | 31·4 | 15·4 | |
| Parity (%) | ||||||
| Multiparous | 64·0 | 69·6 | 72·5 | 55·7 | 51·0 | 0·002 |
| Nulliparous | 36·0 | 30·4 | 27·5 | 44·3 | 49·0 | |
| Pregestational BMI (%) | ||||||
| Normal | 41·0 | 43·4 | 55·0 | 24·3 | 40·4 | 0·033 |
| Weight | ||||||
| Overweight | 40·6 | 37·8 | 35·0 | 52·9 | 42·3 | |
| Obese | 18·4 | 18·9 | 10·0 | 22·9 | 17·3 | |
| Pregnancy | ||||||
| Gestational age at delivery (weeks) | 0·203 | |||||
| Mean | 38·4 | 38·3 | 38·4 | 38·2 | 38·7 | |
| sd | 1·6 | 1·6 | 1·7 | 1·5 | 1·7 | |
| Smoking history (%) | ||||||
| Smokers around pregnancy | 24·4 | 23·4 | 35·0 | 24·3 | 23·1 | 0·104 |
| Former smokers | 8·8 | 8·7 | 5·0 | 2·9 | 14·4 | |
| Never smokers | 66·8 | 67·8 | 60·0 | 72·9 | 62·5 | |
| Physically active (%) | ||||||
| No | 87·2 | 85·3 | 95·0 | 88·6 | 88·5 | 0·272 |
| Yes | 12·8 | 14·7 | 5·0 | 11·4 | 11·5 | |
| Time in sedentary activities‡ (%) | ||||||
| ≥2h/d | 55·8 | 51·8 | 65·0 | 57·1 | 62·5 | 0·153 |
| <2h/d | 44·2 | 48·2 | 35·0 | 42·9 | 37·5 | |
| GWG§ | <0·001 | |||||
| Mean | 7·4 | 6·7 | 10·5 | 6·4 | 8·7 | |
| sd | 3·1 | 3·0 | 2·7 | 2·5 | 2·5 | |
| Adequacy of GWG (%)§ | ||||||
| Insufficient | 26·1 | 32·8 | 0·0 | 32·8 | 13·0 | <0·001 |
| Adequate | 46·8 | 48·4 | 43·2 | 45·3 | 44·6 | |
| Excessive | 27·2 | 18·8 | 56·8 | 21·9 | 42·4 | |
| Postpartum | ||||||
| Type of breast-feeding at 1 month (%)§ | ||||||
| No breast-feeding | 13·3 | 11·2 | 22·5 | 9·2 | 17·3 | 0·645 |
| Partial | 53·3 | 54·2 | 45·0 | 55·4 | 52·9 | |
| Predominant | 5·5 | 5·6 | 7·5 | 6·2 | 3·9 | |
| Exclusive | 28·0 | 28·9 | 25·0 | 29·2 | 26·0 | |
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; SES, socioeconomic status; GWG, gestational weight gain.
Education: basic (elementary and secondary school), middle (high school) and college (at least college). SES was obtained with a validated scale and categorised as high (A/B, C+ and C), middle (D+) and low (D, E). Pregestational BMI: normal weight (<25 kg/m2), overweight (≥25 to <30 kg/m2) and obese (≥30 kg/m2). GWG: insufficient (below the expected weight for gestational age), adequate (between the expected minimum and maximum weights for gestational age) and excessive (above the expected weight for gestational age). Physically active: no (did not engage in leisure-time activity), or yes (engaged in any leisure-time activity). Type of breast-feeding at 1 month: exclusive (only breast milk), predominant (breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk), partial (breast milk and any food or liquid, including non-human milk) and no breast-feeding.
P-value for the comparison between patterns of postpartum weight change using multinomial logistic regression models. A P-value <0·05 was considered statistically significant.
Time in sedentary activities includes reading and watching TV.
GWG and adequacy of GWG: n 449 (n 256 for return to pregestational weight, n 37 for PPWR, n 64 for PPWG and n 92 for PPWR + WG); type of breast-feeding at 1 month: n 458 (n 249 for return to pregestational weight, n 40 for PPWR, n 65 for PPWG and n 104 for PPWR + WG).
Most women returned to their pregestational weight by 12 months postpartum (57 %), while 8 % had PPWR, 14 % PPWG and 21 % PPWR + WG. Marital status, parity, pregestational BMI and GWG differed between patterns of postpartum weight change (Table 1). For example, the pattern of PPWR had a high proportion of women who were married or cohabitating (85 %), multiparous (72·5 %), normal weight prior to pregnancy (55 %) and who had excessive GWG (56·8 %). Most women who experienced PPWG had overweight or obesity before pregnancy (75·7 %), and a high proportion had adequate (45·3 %) or insufficient (32·8 %) GWG. Almost half of women with PPWR + WG were primiparous (49 %), 59·6 % had overweight or obesity before pregnancy, and 42·4 % had excessive GWG.
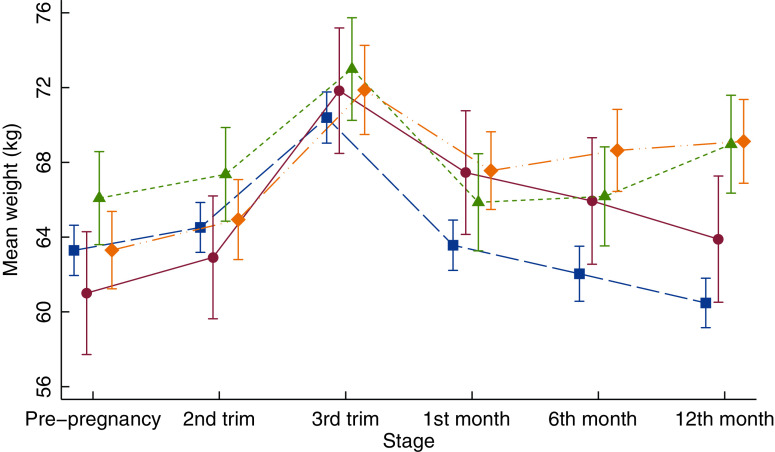
Mean weight change at 12 months postpartum for all women was 0·2 (sd 4·6) kg, with a range of –14·7 to 18·2 kg. Women who returned to their pregestational weight lost on average 2·8 (sd 2·7) kg by 12 months postpartum. Those with PPWR and PPWG gained 2·9 (sd 2·1) and 2·9 (sd 1·8) kg, respectively. Women classified as PPWR + WG experienced the highest weight gain from pregestational to 12 months postpartum with a mean of 5·8 (sd 3·6) kg. As shown in Fig. 1, weight trajectories from before pregnancy to 12 months postpartum also differed between the four patterns of postpartum weight change.
Fig. 1.
Weight changes from pre-pregnancy to 12 months postpartum by patterns of weight change. The graph displays the mean weight in kg and 95 % CI at each time point within each category.  , return to pregestational weight;
, return to pregestational weight;  , retention;
, retention;  , gain;
, gain;  , retention/gain
, retention/gain
Tables 2−4 present the association between each predictor and patterns of postpartum weight change. Results are presented as relative risk ratios (RRR) and 95 % CI. In the age-adjusted model (Table 2), pregestational overweight and obesity, compared with normal weight, were each associated with a higher risk of PPWG (overweight: RRR 2·5, 95 % CI 1·3, 4·8; obesity: RRR 2·2, 95 % CI 1·0, 4·7). They were not associated with PPWR or PPWR + WG. These results persisted after adjustment for parity and sociodemographic covariates (model 2).
Table 2.
Association between pregestational BMI and patterns of postpartum weight change
| Reference group: return to pregestational weight | PPWR | PPWG | PPWR + WG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pregestational BMI* | n | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value |
| Model 1† | ||||||||||
| Normal weight | 205 | Reference | Reference | Reference | ||||||
| Overweight | 203 | 0·77 | 0·37, 1·61 | 0·491 | 2·53 | 1·33, 4·81 | 0·005 | 1·26 | 0·76, 2·09 | 0·370 |
| Obese | 92 | 0·44 | 0·14, 1·37 | 0·158 | 2·19 | 1·02, 4·71 | 0·045 | 1·04 | 0·54, 1·99 | 0·911 |
| Model 2‡ | ||||||||||
| Normal weight | 205 | Reference | Reference | Reference | ||||||
| Overweight | 203 | 0·81 | 0·39, 1·70 | 0·583 | 2·49 | 1·30, 4·77 | 0·006 | 1·27 | 0·75, 2·13 | 0·373 |
| Obese | 92 | 0·42 | 0·13, 1·29 | 0·129 | 2·19 | 1·00, 4·79 | 0·049 | 1·03 | 0·53, 2·00 | 0·940 |
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
Pregestational BMI: normal weight (<25 kg/m2), overweight (≥25 to <30 kg/m2) and obese (≥30 kg/m2).
Model 1: adjusted for age.
Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous) and socioeconomic status (high, middle and low).
Table 4.
Association between breast-feeding and patterns of postpartum weight change
| Reference group: return to pregestational weight | PPWR | PPWG | PPWR + WG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of breast-feeding at 1 month* | n | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value |
| Model 1† | ||||||||||
| No breast-feeding | 61 | Reference | Reference | Reference | ||||||
| Partial | 244 | 0·43 | 0·17, 1·05 | 0·063 | 1·24 | 0·47, 3·22 | 0·665 | 0·64 | 0·33, 1·26 | 0·197 |
| Predominant | 25 | 0·65 | 0·15, 2·81 | 0·567 | 1·34 | 0·33, 5·56 | 0·683 | 0·44 | 0·12, 1·55 | 0·200 |
| Exclusive | 128 | 0·44 | 0·16, 1·19 | 0·104 | 1·23 | 0·45, 3·40 | 0·690 | 0·59 | 0·28, 1·23 | 0·156 |
| Model 2‡ | ||||||||||
| No breast-feeding | 55 | Reference | Reference | Reference | ||||||
| Partial | 222 | 0·38 | 0·13, 1·09 | 0·071 | 1·23 | 0·44, 3·38 | 0·693 | 0·61 | 0·28, 1·33 | 0·214 |
| Predominant | 22 | 0·76 | 0·12, 4·61 | 0·761 | 2·18 | 0·47, 10·02 | 0·316 | 0·51 | 0·12, 2·19 | 0·367 |
| Exclusive | 114 | 0·27 | 0·08, 0·91 | 0·035 | 1·57 | 0·52, 4·72 | 0·419 | 0·69 | 0·29, 1·69 | 0·421 |
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
Type of breast-feeding at 1 month: exclusive (only breast milk), predominant (breast milk and certain liquids such as water and water-based drinks, but excluding non-human milk), partial (breast milk and any food or liquid, including non-human milk) and no breast-feeding.
Model 1: adjusted for age.
Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous), socioeconomic status (high, middle and low), pregestational BMI (normal weight, overweight and obese), smoking history (never smokers, smokers around pregnancy and former smokers), adequacy of GWG (insufficient, adequate and excessive), gestational age at delivery and pregnancy complications (yes and no).
Excessive GWG, in comparison with adequate GWG, was associated with a higher risk of PPWR (RRR 3·3, 95 % CI 1·6, 6·9) and PPWR + WG (RRR 2·4, 95 % CI 1·4, 4·2). On the other hand, insufficient GWG was associated with a lower risk of PPWR + WG (RRR 0·4, 95 % CI 0·2, 0·9) (Table 3, model 1). These associations became somewhat stronger after adjustment for pregestational BMI, sociodemographic characteristics and lifestyle behaviours during pregnancy (model 2). GWG was not associated with PPWG in any of the models.
Table 3.
Association between gestational weight gain and patterns of postpartum weight change
| Reference group: return to pregestational weight | PPWR | PPWG | PPWR + WG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adequacy of GWG* | n | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value | RRR | 95 % CI | P-value |
| Model 1† | ||||||||||
| Insufficient‡ | 117 | – | – | – | 1·08 | 0·58, 2·02 | 0·814 | 0·43 | 0·21, 0·87 | 0·018 |
| Adequate | 210 | Reference | Reference | Reference | ||||||
| Excessive | 122 | 3·32 | 1·59, 6·92 | 0·001 | 1·29 | 0·62, 2·65 | 0·496 | 2·43 | 1·40, 4·23 | 0·002 |
| Model 2§ | ||||||||||
| Insufficient‡ | 117 | – | – | – | 1·30 | 0·67, 2·53 | 0·439 | 0·42 | 0·20, 0·88 | 0·021 |
| Adequate | 210 | Reference | Reference | Reference | ||||||
| Excessive | 122 | 6·16 | 2·62, 14·50 | <0·001 | 0·97 | 0·45, 2·10 | 0·937 | 3·04 | 1·62, 5·69 | 0·001 |
PPWR, postpartum weight retention; PPWG, postpartum weight gain; PPWR + WG, postpartum weight retention + weight gain; GWG, gestational weight gain; RRR, relative risk ratio.
Results from multinomial logistic regression models.
GWG: insufficient (below the expected weight for gestational age), adequate (between the expected minimum and maximum weights for gestational age) and excessive (above the expected weight for gestational age).
Model 1: adjusted for age.
n 0 for insufficient GWG and postpartum weight retention.
Model 2: adjusted for age, marital status (single/separated and married/cohabitating), education (basic: elementary and secondary school; middle: high school; college: at least college), parity (primiparous and multiparous), socioeconomic status (high, middle and low), pregestational BMI (normal weight, overweight and obese), smoking history (never smokers, smokers around pregnancy and former smokers), physically active (yes and no) and sedentary activities in pregnancy (<2 and ≥2 h/d).
In the age-adjusted model, partial and exclusive breast-feeding at 1 month, compared with no breast-feeding, appeared to be protective for PPWR but not for PPWG or PPWR + WG (Table 4, model 1). In both cases, the results included the null value. After full adjustment of covariates (model 2), the association between exclusive breast-feeding and lower risk of PPWR became stronger, and the CI excluded the null (RRR 0·3, 95 % CI 0·1, 0·9).
In a sensitivity analysis using a modified definition of the outcome, we observed similar associations between pregestational BMI, GWG and patterns of postpartum weight change (see online supplementary material, Supplemental Table S3). In this supplementary analysis, exclusive breast-feeding at 1 month, compared with no breast-feeding, was not associated with a lower risk of PPWR (RRR 0·5, 95 % CI 0·2, 1·4). We also wanted to assess how our proposed classification of patterns of postpartum weight change compared with the widely used definition of substantial postpartum weight retention (i.e. retaining ≥4·5 kg at 12 months postpartum). In our sample, 17 % of women experienced substantial postpartum weight retention (see online supplementary material, Supplemental Table S4). Of these women, 13, 17 and 70 % would be classified as PPWR, PPWG and PPWR + WG, respectively, according to our classification. In age-adjusted and fully adjusted models, pregestational BMI and breast-feeding at 1 month were not associated with the odds of retaining ≥4·5 kg. Insufficient GWG, compared with adequate GWG, was associated with lower odds of substantial postpartum weight retention (OR 0·4, 95 % CI 0·2, 0·9). In contrast, excessive GWG was associated with higher odds of PPWR (OR 3·3, 95 % CI 1·8, 6·0).
Discussion
Weight gain after delivery may be the result of retaining gestational weight, gaining weight postpartum or both. These patterns of weight change are associated with potentially modifiable factors. In the current study, pregestational overweight and obesity were associated with a higher risk of PPWG during the first year postpartum, whereas excessive GWG was associated with a higher risk of PPWR and PPWR + WG. Exclusive breast-feeding at 1 month postpartum decreased the risk of PPWR.
In our study, 21 % of women experienced PPWR + WG the first year after delivery, while 14 % experienced PPWG and 8 % PPWR. To date, most of the studies have lumped all women who do not return to their pregestational weight as having PPWR. None of these studies have differentiated between patterns of postpartum weight change(29,37–41). Some studies have shown that PPWG occurs in the late postpartum period (≥12 or ≥18 months)(7,9,10,33). We build on these results by showing that a considerable proportion of women gain weight, either alone or combined with retention, within the first 12 months after delivery. To the best of our knowledge, only one study has characterised different patterns of weight change the first year after delivery, using repeated weight assessments(6). In the current study of 305 Canadian women, 15 % weighted more at 3 months postpartum, compared with their pregestational weight, and gained weight from 3 to 12 months. Sixty-six per cent also weighted more at 3 months but lost weight from 3 to 12 months. However, on average, they still weighted more by 12 months, compared with their pregestational weight. Another 11 % had lost weight by 3 months, compared with their pregestational weight, and regained weight from 3 to 12 months(6). The three groups described in the current study are comparable with our patterns of PPWR + WG, PPWR and PPWG, respectively.
Women with pregestational overweight and obesity, compared with normal weight, had a higher risk of PPWG. Similar to our cohort, in the study of Kew et al.(6), women who regained weight, after having reached their pregestational weight, had the highest BMI before pregnancy. Lipsky et al.(9), in a study of 413 American women, found that women with overweight and obesity, compared with normal weight, had higher odds of gaining ≥2·25 kg from 1 to 2 years postpartum (overweight: OR 2·4, 95 % CI 1·3, 4·4; obesity: OR 3·0, 95 % CI 1·6, 5·6). Gunderson et al.(42) also showed that women with overweight and obesity were more likely to gain ≥2 kg from 6 weeks to a median of 2 years postpartum. The reason why women with pregestational overweight or obesity are more likely to gain weight postpartum is unclear. It is possible that behavioural factors associated with weight gain, such as lower physical activity, longer time in sedentary activities and unhealthy dietary patterns, are more prevalent among these groups of women(33,43).
We found that excessive GWG was associated with an increased risk of PPWR and PPWR + WG, while insufficient GWG was associated with a lower risk of PPWR + WG. Higher GWG has been consistently associated with PPWR(14–16,29,38,44,45), although none of these studies have made the distinction between retention alone v. retention and gain. In a recent meta-analysis, GWG above the IOM recommendations, compared with ‘within recommendations’, was associated with a higher weight retention (3·2 kg, 95 % CI 2·8, 3·6 kg). In contrast, GWG below the IOM recommendations was associated with a lower weight retention (−2·1 kg, 95 % CI −2·4, −1·9 kg)(14). Few studies have evaluated the association between GWG and patterns of weight change other from PPWR(11,12). An analysis of postpartum weight trajectories in Norwegian women found that excessive GWG was associated with two weight trajectories characterised by high initial weight retention (6 months) followed by either weight loss or sustained weight gain through 3 years postpartum. Insufficient GWG was associated with a lower risk of these trajectories(12). A recent study showed that excess GWG represents mostly gains in maternal fat(46). This additional fat mass may persist beyond delivery and explain the increased risk of PPWR or PPWR + WG observed in our study. Our results of lower risk of PPWR + WG among women with insufficient GWG must be interpreted with caution. Insufficient GWG, although it may provide some benefits in terms of postpartum weight, has been associated with adverse neonatal outcomes, including a higher risk of small-for-gestational-age and preterm birth(47).
In the current study, exclusive breast-feeding at 1 month was associated with a decreased risk of PPWR. Martin et al.(40), in a study of Australian women, did not find any association between the type of breast-feeding at 3 months and weight retention at 12 months. Consistent with our findings, López-Olmedo et al.(48), in a group of Mexican women, found that those who breastfed exclusively until the third month postpartum had greater weight loss in comparison with those non-breast-feeding. One recent meta-analysis did not find an association between breast-feeding and postpartum weight change(18). Methodological differences between studies, including different exposure times or definitions of breast-feeding, and lack of adjustment by relevant covariates might explain the inconsistency in findings.
Our study has several implications. To modify the impact of pregnancy and childbearing on women’s weight and health status, we need to improve our understanding of the course of weight change after delivery and the characteristics associated with the different patterns of weight change. In the current study, we proposed a different approach to characterise postpartum weight trajectories in women. Although some authors have suggested using the term PPWR within a limited period following delivery, for example, 12−18 months(7,9), we showed that women also gain weight during this time. We found that PPWR + WG and PPWG were more common than PPWR among women who did not reach their pregestational weight during the first year after delivery. In a previous study, we found that women with PPWR, PPWG and PPWR + WG, compared with those who returned to their pregestational weight by 1 year postpartum, had increased adiposity 6 years after delivery. We also found that PPWR + WG was associated with metabolic alterations such as insulin resistance(13). In the current study, we extend these results by identifying the contribution of specific modifiable factors to the risk of each of the patterns of weight change.
Our findings should be evaluated within the strengths and limitations of the study. Generalisability may be limited because our population consisted primarily of women of low SES living in Mexico City. Only 53 % of the original cohort had information available for this analysis, which may have increased the possibility of selection bias. This type of bias is unlikely because analysed and non-analysed women were identical in all characteristics, including the exposures of interest (except for gestational age at delivery). The potential for confounding was minimised by adjusting each of the associations studied for relevant covariates. However, we did not have information on diet, which likely plays a role in the variability of postpartum weight change(34,49,50). In specific, diet during pregnancy has been associated with both GWG and PPWR(34); therefore, it is a potential confounder of this association. We cannot completely rule out the possibility of residual confounding for the lack of adjustment by diet. However, other studies have shown that the association between GWG and postpartum weight change is strong and persists after adjustment by dietary patterns and energy intake(8,29). Finally, the results on the association between breast-feeding and patterns of postpartum weight change must be interpreted with caution because some categories had very small sample sizes (i.e. predominant breast-feeding, n 22). We recognise that we may have been underpowered to detect any association between these categories and the outcome.
Some strengths must be acknowledged. We categorised breast-feeding practices according to the WHO(27) and adjusted by pregestational BMI, GWG and parity, which are important confounders of the association between breast-feeding and postpartum weight change(19). Studying breast-feeding at 1 month postpartum may be seen as a limitation but, by doing this, we were confident that the exposure (i.e. breast-feeding) preceded the outcome of interest (i.e. patterns of weight change). The WHO recommends practicing exclusive breast-feeding for at least 6 months postpartum(51). Therefore, our results on the association between breast-feeding and PPWR must be interpreted with caution and should be replicated in future studies. A strength of the current study is that most weights used for the analysis were objectively measured by trained personnel. One exception was weight at 12 months that was imputed for a subset of women. The results excluding this subset of women were comparable with those of the primary analysis that included women with imputed and non-imputed weights. Self-reported pregestational weight is highly subject to error in this population, and misreporting can be substantial(23). In the absence of measured pregestational weight, using an estimated pregestational weight rigorously validated, instead of self-reported, might be a strength of this analysis.
Conclusions
We found that while most women return to their pregestational weight 1 year after delivery, an important subset experiences retention of gestational weight, weight gain or both, which may increase their long-term risk of obesity and metabolic diseases. Although our results need to be replicated in a different population, they suggest that these adverse patterns of weight change may be prevented by targeting women with pregestational overweight or obesity and excessive GWG, and by promoting exclusive breast-feeding. Future studies should test whether targeting these high-risk women and promoting breast-feeding has an impact on postpartum weight change and long-term women’s health.
Acknowledgements
Acknowledgements: We thank the Centro Médico ABC and the National Institute of Perinatology, México, for their support with the current research. Financial support: The current work was supported by the National Institute of Environmental Health Sciences, grants nos. R01 ES013744, P30 ES023515, R01 ES021357, R24 ES028522, and partially funded by the National Institute of Public Health/Ministry of Health of Mexico. The funding sources had no role in the design of the study, in the analysis, interpretation of the data or the writing of the manuscript. Conflict of interest: The authors declare that they have no competing interests. Authorship: D.C.S.C. conceptualised and designed the study, carried out the statistical analysis, interpretation of the data and drafted the manuscript M.M.T.R., B.T.V. and R.L.R. participated in the conceptualisation and design of the study, interpretation of the data and critically reviewed the manuscript. E.O. helped in the interpretation of the data and critically reviewed the manuscript. R.O.W., A.C., M.L.P.Z., A.A.B., A.C.J., M.A.O. and I.R.S. critically reviewed the manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Committees on Ethics, Biosafety and Research at the Mexican National Institute of Public Health, as well as the Institutional Review Boards of the participating institutions. Written informed consent was obtained from all subjects/patients.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020002803.
click here to view supplementary material
References
- 1. Shamah-Levy T, Cuevas-Nasu L, Dommarco-Rivera J et al. (2016) Encuesta Nacional de Salud y Nutrición de Medio Camino. Cuernavaca, Morelos, México: Instituto Nacional de Salud Pública; available at https://www.gob.mx/cms/uploads/attachment/file/209093/ENSANUT.pdf (accessed December 2018). [Google Scholar]
- 2. Cohen AK, Chaffee BW, Rehkopf DH et al. (2014) Excessive gestational weight gain over multiple pregnancies and the prevalence of obesity at age 40. Int J Obes 38, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hutchins F, Abrams B, Brooks M et al. (2020) The effect of gestational weight gain across reproductive history on maternal body mass index in midlife: the Study of Women’s Health Across the Nation. J Womens Health 29, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis EM, Babineau DC, Wang X et al. (2014) Short inter-pregnancy intervals, parity, excessive pregnancy weight gain and risk of maternal obesity. Matern Child Health J 18, 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazariegos M, Ortiz-Panozo E, Gonzalez de Cosio T et al. (2020) Parity, lactation, and long-term weight change in Mexican women. Matern Child Nutr 16, e12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kew S, Ye C, Hanley AJ et al. (2014) Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 37, 1998–2006. [DOI] [PubMed] [Google Scholar]
- 7. Schmitt NM, Nicholson WK & Schmitt J (2007) The association of pregnancy and the development of obesity − results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes 31, 1642–1651. [DOI] [PubMed] [Google Scholar]
- 8. Kirkegaard H, Stovring H, Rasmussen K et al. (2014) How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr 99, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipsky LM, Strawderman MS & Olson CM (2012) Maternal weight change between 1 and 2 years postpartum: the importance of 1 year weight retention. Obesity 20, 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onyango AW, Nommsen-Rivers L, Siyam A et al. (2011) Post-partum weight change patterns in the WHO Multicentre Growth Reference Study. Matern Child Nutr 7, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soria-Contreras DC, Rifas-Shiman SL, Aris IM et al. Weight trajectories after delivery are associated with adiposity and cardiometabolic markers at 3 years postpartum among women in project viva. J Nutr 150, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abebe DS, Von Soest T, Von Holle A et al. (2015) Developmental trajectories of postpartum weight 3 years after birth: Norwegian Mother And Child Cohort Study. Matern Child Health J 19, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soria-Contreras DC, Trejo-Valdivia B, Cantoral A et al. (2020) Patterns of weight change one year after delivery are associated with cardiometabolic risk factors at six years postpartum in Mexican women. Nutrients 12, E170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rong K, Yu K, Han X et al. (2015) Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 18, 2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mannan M, Doi S & Mamun AA (2013) Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev 71, 343–352. [DOI] [PubMed] [Google Scholar]
- 16. Rode L, Kjærgaard H, Ottesen B et al. (2012) Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Matern Child Health J 16, 406–413. [DOI] [PubMed] [Google Scholar]
- 17. Brandhagen M, Lissner L, Brantsaeter AL et al. (2014) Breast-feeding in relation to weight retention up to 36 months postpartum in the Norwegian Mother and Child Cohort Study: modification by socio-economic status? Public Health Nutr 17, 1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chowdhury R, Sinha B, Sankar MJ et al. (2015) Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr 104, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neville CE, McKinley MC, Holmes VA et al. (2014) The relationship between breastfeeding and postpartum weight change – a systematic review and critical evaluation. Int J Obes 38, 577–590. [DOI] [PubMed] [Google Scholar]
- 20. Braun JM, Wright RJ, Just AC et al. (2014) Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamayo Y, Ortiz M, Téllez-Rojo MM et al. (2016) Longitudinal associations of age and prenatal lead exposure on cortisol secretion of 12–24 month-old infants from Mexico City. Environ Health 15, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Health and Nutrition Examination Survey (2007) Anthropometry Procedures Manual; available at https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed June 2019).
- 23. Thomas DM, Oken E, Rifas-Shiman SL et al. (2019) Do women know their prepregnancy weight? Obesity 27, 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organ Tech Rep Ser (2000) Obesity: preventing and managing the global epidemic. Report of a WHO Consultation; available at http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf (accessed December 2018). [PubMed]
- 25. Perichart-Perera O, Muñoz-Manrique C, Reyes-López A et al. (2017) Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS One 12, e0180874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasmussen KM & Yaktine AL (2009) Weight Gain During Pregnancy: Reexamining the Guidelines, vol. 184. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 27. World Health Organization (2008) Indicators for Assessing Infant and Young Child Feeding Practices Part 1: Definitions. Geneva, Switzerland; available at https://apps.who.int/iris/bitstream/handle/10665/43895/9789241596664_eng.pdf?sequence=1 (accessed November 2018). [Google Scholar]
- 28. Provenzano AM, Rifas-Shiman SL, Herring SJ et al. (2015) Associations of maternal material hardships during childhood and adulthood with prepregnancy weight, gestational weight gain, and postpartum weight retention. J Womens Health 24, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siega-Riz AM, Herring AH, Carrier K et al. (2010) Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity 18, 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Box GEP & Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B 26, 211–252. [Google Scholar]
- 31. Villegas-Carrasco A (2002) The AMAI system of classifying households by socio-economic level. The experience of Mexico and its comparison with Brazil and Argentina. Proceedings of the Latin American Conference, Sao Paulo, Brazil.
- 32. Sanders AP, Svensson K, Gennings C et al. (2018) Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ Int 120, 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkegaard H, Stovring H, Rasmussen KM et al. (2015) Maternal weight change from prepregnancy to 7 years postpartum−the influence of behavioral factors. Obesity 23, 870–878. [DOI] [PubMed] [Google Scholar]
- 34. Knudsen VK, Heitmann BL, Halldorsson TI et al. (2013) Maternal dietary glycaemic load during pregnancy and gestational weight gain, birth weight and postpartum weight retention: a study within the Danish National Birth Cohort. Br J Nutr 109, 1471–1478. [DOI] [PubMed] [Google Scholar]
- 35. Fagerland M & Hosmer D (2012) A generalized Hosmer-Lemeshow goodness-of-fit test for multinomial logistic regression models. Stata J 12, 447–453. [Google Scholar]
- 36. Olson C, Strawderman MS, Hinton PS et al. (2003) Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord 27, 117–127. [DOI] [PubMed] [Google Scholar]
- 37. Li A, Teo KK, Morrison KM et al. (2017) A genetic link between prepregnancy body mass index, postpartum weight retention, and offspring weight in early childhood. Obesity 25, 236–243. [DOI] [PubMed] [Google Scholar]
- 38. Oken E, Kleinman KP, Belfort MB et al. (2009) Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol 170, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Endres LK, Straub H, McKinney C et al. (2015) Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 125, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin JE, Hure AJ, Macdonald-Wicks L et al. (2014) Predictors of post-partum weight retention in a prospective longitudinal study. Matern Child Nutr 10, 496–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Althuizen E, Van Poppel MN, De Vries JH et al. (2011) Postpartum behaviour as predictor of weight change from before pregnancy to one year postpartum. BMC Public Health 11, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gunderson EP, Abrams B & Selvin S (2001) Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord 25, 853–862. [DOI] [PubMed] [Google Scholar]
- 43. Ostbye T, Peterson BL, Krause KM et al. (2012) Predictors of postpartum weight change among overweight and obese women: results from the Active Mothers Postpartum Study. J Womens Health 21, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nehring I, Schmoll S, Beyerlein A et al. (2011) Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr 94, 1225–1231. [DOI] [PubMed] [Google Scholar]
- 45. Kac G, Benicio M, Velasquez-Melendez G et al. (2004) Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of Brazilian women. J Nutr 134, 661–666. [DOI] [PubMed] [Google Scholar]
- 46. Berggren EK, Groh-Wargo S, Presley L et al. (2016) Maternal fat, but not lean, mass is increased among overweight/obese women with excess gestational weight gain. Am J Obstet Gynecol 214, 745.e1–745.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldstein RF, Abell SK, Ranasinha S et al. (2017) Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317, 2207–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. López-Olmedo N, Hernández-Cordero S, Neufeld LM et al. (2016) The associations of maternal weight change with breastfeeding, diet and physical activity during the postpartum period. Matern Child Health J 20, 270–280. [DOI] [PubMed] [Google Scholar]
- 49. Oken E, Taveras EM, Popoola FA et al. (2007) Television, walking, and diet: associations with postpartum weight retention. Am J Prev Med 32, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boghossian NS, Yeung EH, Lipsky LM et al. (2013) Dietary patterns in association with postpartum weight retention. Am J Clin Nutr 97, 1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization (2001) Global Strategy for Infant and Young Child Feeding: 54th World Health Assembly; available at https://apps.who.int/gb/archive/pdf_files/WHA54/ea54id4.pdf?ua=1 (accessed April 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020002803.
click here to view supplementary material