Statement of Clinical Significance:
There remains the need to develop implants, surfaces, and materials that can increase the rate of osseointegration. While osteoanabolic agents, like bone morphogenetic protein (BMP), can provide signaling for osteogenesis, the appropriate design of implants can also produce an innate cellular response that may reduce or eliminate the need to use additional agents to stimulate bone formation. Studies show that titanium implant surfaces that mimic the physical properties of osteoclast resorption pits regulate cellular responses of bone marrow stromal cells (MSCs) by altering cell morphology, transcriptomes, and local factor production to increase their differentiation into osteoblasts (OBs) without osteogenic media supplements required for differentiation of MSCs on tissue culture polystyrene (TCPS). The goal of this research was to determine how cells in contact with biomimetic implant surfaces regulate the micro-environment around these surfaces in vitro. Two different approaches were used. First, unidirectional signaling was assessed by treating human MSCs grown on TCPS with conditioned media from MSC cultures grown on Ti6Al4V biomimetic surfaces. In the second set of studies, bidirectional signaling was assessed by co-culturing MSCs grown on mesh inserts that were placed into culture wells in which MSCs were grown on the biomimetic Ti6Al4V substrates. The results show that biomimetic Ti6Al4V surface properties induce MSCs to produce factors within 7 days of culture that stimulate MSCs not in contact with the surface to exhibit an osteoblast phenotype via endogenous BMP2 acting in a paracrine signaling manner.
Keywords: Biomimetic, Titanium, Bone-Implant Interface, Osteoblasts, Spine
INTRODUCTION
Orthopaedic and dental implants are commonly used treatments to restore function and quality of life in patients suffering from pathological bone conditions like osteoporosis or diabetes, or inflammatory joint diseases like osteoarthritis.1–3 Titanium (Ti) and its alloys are often the choice biomaterial for these implants due to their superior biocompatibility, excellent corrosion resistance, and mechanical properties.4–7 Immediately after placement these orthopaedic and dental implants induce a complex biological signaling cascade in order to integrate with native bone tissue.
Our lab and others have shown that the physical properties of an implant surface are important for overall clinical outcomes. Ti implant surfaces possessing biomimetic surface architectures and roughness similar to that of an osteoclast resorption pit, are sensed by bone marrow stromal cells (MSCs) in contact with the proteins adsorbed on the implant surface, causing MSCs to undergo surfaced mediated osteoblastic differentiation and increase production of factors necessary for osteogenesis.7–10 However, this effect is not limited to just metallic implant surfaces, others have shown extensively that changing the morphological physical features and material chemistry are able to control cell fate using calcium phosphate based biomaterials and are osteoinductive in vivo.11–13
Successful osseointegration requires specific local regulation in the peri-implant microenvironment. In patients with compromised bone this process is dysregulated. Clinicians can use recombinant proteins like bone morphogenetic protein 2 (BMP2) and BMP7 in order to augment bone formation. BMPs are a subset of the transforming growth factor beta (TGFβ) superfamily of proteins and are named for their ability to induce bone formation ectopically in vivo,14 indicating the need to appropriately control the signaling as they can also stimulate heterotopic bone formation or inflammation.14–18 Therefore, there is an interest in developing technologies that support osseointegration based on the surface features of the implant.
This research is based on the hypothesis that implant surface properties could be used to augment bone formation peri-implant without the confounding factors of osteoanabolic agents. As a first step, we took advantage of an in vitro model in which human MSCs are cultured on titanium-aluminum-vanadium (Ti6Al4V) substrates with macro/micro/nano-structured surfaces that mimic the physical properties of an osteoclast resorption pit. We assessed whether MSCs grown on these surfaces produce factors that act via paracrine signaling on MSCs cultured on tissue culture polystyrene (TCPS), and if local production of BMPs by surface resident MSCs mediate peri-implant osteogenesis in response to implant surface properties.
MATERIALS AND METHODS
Titanium alloy substrate preparation
To test our hypothesis, we generated surfaces possessing complex surface roughness at the macro/micro/nanoscale (MMN), designed to mimic the surface texture of bone following mineral resorption and tissue processing by osteoclasts. Titanium-aluminum-vanadium substrates were prepared from 15 mm diameter rods of grade 5 alloyed Ti6Al4V machined into 1.6 mm thick disks. Using commercial manufacturing methods (nanoLOCK-, Medtronic Inc, MN), the disks were grit-blasted and acid-etched to produce the MMN surface texture, followed by sterilization via gamma irradiation. These disks were designed to snuggly fit into a 24-well plate.
Scanning electron microscopy
Surface topography was qualitatively assessed using scanning electron microscopy (SEM; Hitachi SU-70, Tokyo, Japan). Surfaces were secured on SEM imaging mounts by carbon tape and imaged with 56 μA ion current, 5 kV accelerating voltage and 5 mm working distance. Five locations per disk were imaged at each magnification to ensure homogenous MMN surface features, with at least two disks imaged per experimental condition.
Laser confocal microscopy
Surface roughness was quantitatively assessed by laser confocal microscopy (LCM; Zeiss LSM 710). Z-stacks were obtained with a Plan Apochromat 20x/0.8 M27 objective with a 5x optical zoom, using a 405 nm laser in reflection mode at 50% power. Scan parameters were 0.39 μs pixel dwell, and 25 μm pinhole, 705.2 × 705.2 μm image size, and step size of 1μm. Values were obtained using ZEN software (Zeiss) and shown as the mean and standard error.
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) was used to analyze surface chemistry (PHI VersaProbe III Scanning XPS, Physical Electronics Inc., Chanhassen, MN). Copper clips and instrument mount were sonicated in ethanol for 10 min prior to securing samples. Analysis was conducted using a 50 watt, 15kV x-ray gun with a spot size of 200 μm, 20 ms dwelling time and 1eV step size.
Sessile drop contact angle
Surface wettability was determined by sessile drop test using a goniometer (CAM 250, Ramé-Hart). Samples (n=3) were measured in 3 different locations and dried with nitrogen between measurements. 4 μL of ultrapure water was used per drop measurement, and angle measurements were taken every 5 seconds for a total of 20 seconds. Those four measurements were then averaged to produce 1 of the 5 total measurements per disc.
Time course of cellular response
23-year-old human female MSCs donor #8011L (purchased from Texas A&M Institute for Regenerative Medicine, College Station, TX) were cultured in MSC growth medium (GM) comprised of αMEM with 4 μM L-glutamine and 16.5% fetal bovine serum at 37 C in 5% CO2 and 100% humidity and cultured to confluence in T75 flasks (Corning Inc., Oneonta, NY) before plating on the surface. For biological analysis, MMN surfaces were placed in a 24-well plate, and cells were plated at a density of 10,000 cells/cm2 at 0.5 mL per well. MSCs cultured on tissue culture polystyrene (TCPS) served as experimental controls. 24 hours after plating, GM were changed with subsequent media changes every 48 hours after that for seven, fourteen, and twenty-one days. At each designated time point, cells were incubated for 24 hours with fresh GM before harvest. At harvest, conditioned media were subsequently collected and stored at −80°C, and MSCs were rinsed twice with 1x PBS, and placed in 0.5 mL of Triton-X100 and stored at −80°C for biological assays.
Conditioned media treatment of MSCs on TCPS
MMN surfaces were placed in a 24-well plate, and MSCs were plated at a density of 10,000 cells/cm2 at 0.5 mL per well. Human MSCs were cultured on MMN surfaces for 7 days to allow surface mediated differentiation on MMN surfaces. Media were changed every 48 hours. At day 7, separate MSCs were plated on a separate TCPS 24-well plate in two groups. At day 7, media were collected from MMN surfaces and diluted 1:1 in fresh GM and used to treat MSCs cultured on TCPS as a conditioned media (CM) experiment. Media were changed in this fashion daily for 7 days. MSCs receiving fresh GM daily served as experimental controls.
Co-culture during surface mediated differentiation and the role of BMP2
MMN surfaces were placed in a 24-well plate, and MSCs were plated on MMN surfaces and TCPS at a density of 10,000 cells/cm2 at 0.5 mL per well. MSCs were plated on 0.4 μm pore size cell culture inserts at a density of 10,000 cells/cm2 at 0.5 mL per insert. 24 hours after plating, media were changed and the inserts were placed into the 24-well plate and cultured in GM for 7 days. One group of MMN disks received GM supplemented with 15 μg/mL BMP2 blocking antibody (R&D Systems, Inc., Minneapolis, MN). MSCs cultured on inserts and placed into wells without an MSC layer served as an insert control, and MSCs cultured on TCPS on the 24-well plate with MSCs on the cell culture insert served as experimental control. On day 7, the inserts were moved to a separate carrier plate and both inserts and 24-well plates were cultured in fresh GM for 24 hours.
Assessment of the role of BMP2 after surface mediated differentiation
MMN surfaces were placed in a 24-well plate, and MSCs were plated on MMN surfaces and TCPS at a density of 10,000 cells/cm2 at 0.5 mL per well. 24 hours after plating, media were changed and cultured in GM for 7 days with media changes every 48 hours. On day 6, MSCs were plated on 0.4 μm pore size cell culture inserts at a density of 10,000 cells/cm2 at 0.5 mL per insert. On day 7, media were changed and the inserts were placed into the 24-well plate and cultured in GM for 4 days. One group of MMN disks received GM supplemented with 15 μg/mL BMP2 blocking antibody (R&D Systems, Inc.) from days 7 to 11. MSCs cultured on inserts and placed into wells without an MSC layer served as an insert control. On day 11, the inserts were moved to a separate carrier plate and for all groups, both inserts and 24-well plates were cultured in fresh GM for 24 hours.
Assessment of cellular response
Cell layers were lysed by ultrasonication at 40V for 15 seconds/well (VCX 130; Vibra-Cell, Newtown, CT). The QuantiFluor* dsDNA system (Promega, Madison, WI) was used to determine total DNA content by fluorescence. Alkaline phosphatase activity was measured in the cell layer lysates by the release of para-nitrophenol from para-nitrophenyl phosphate (pH 10.2) and normalized to time and protein content measured by bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA).
Enzyme-linked immunosorbent assays were used to determine the levels of osteogenic factors in the conditioned media. Osteocalcin (OCN; Thermo Fisher), osteoprotegerin (OPG; R&D Systems, Inc.), osteopontin (OPN; R&D Systems, Inc.), vascular endothelial growth factor A (VEGF-A; R&D Systems, Inc.), BMP2 (R&D Systems, Inc.), bone morphogenetic protein 4 (BMP4; R&D Systems, Inc.), interleukin 6 (IL6; R&D Systems, Inc.), and interleukin 10 (IL10; R&D Systems, Inc.) were quantified according to the manufacturer’s protocol.
Statistical analysis
Data are means ± standard error mean of six independent cultures/variable. All experiments were repeated to ensure the validity of observations, with results from individual experiments shown. Statistical analysis among groups was performed by one-way analysis of variance (ANOVA) and multiple comparisons between the groups were conducted with a two-tailed Tukey correction. A p-value of less than 0.05 was considered statistically significant.
RESULTS
MMN implant surfaces exhibit an osteoclast resorption pit biomimetic topography.
Surface macro roughness and micro roughness were seen by SEM at lower magnifications (Fig. 1A, B). Higher magnification imaging at the submicron and nanoscale showed homogenous nanostructures and submicron ridges across the MMN surfaces (Fig. 1C, D). Both the average areal and linear microroughness were increased at ~2.5 μm (Fig. 1E). XPS analysis showed that oxygen was the most present element on the implant surface, followed by carbon and titanium (Fig. 1F). MMN surfaces were slightly hydrophobic with an average contact angle of 95 degrees (Fig. 1G).
Fig 1. Characterization of MMN Ti6Al4V Implant Surfaces.
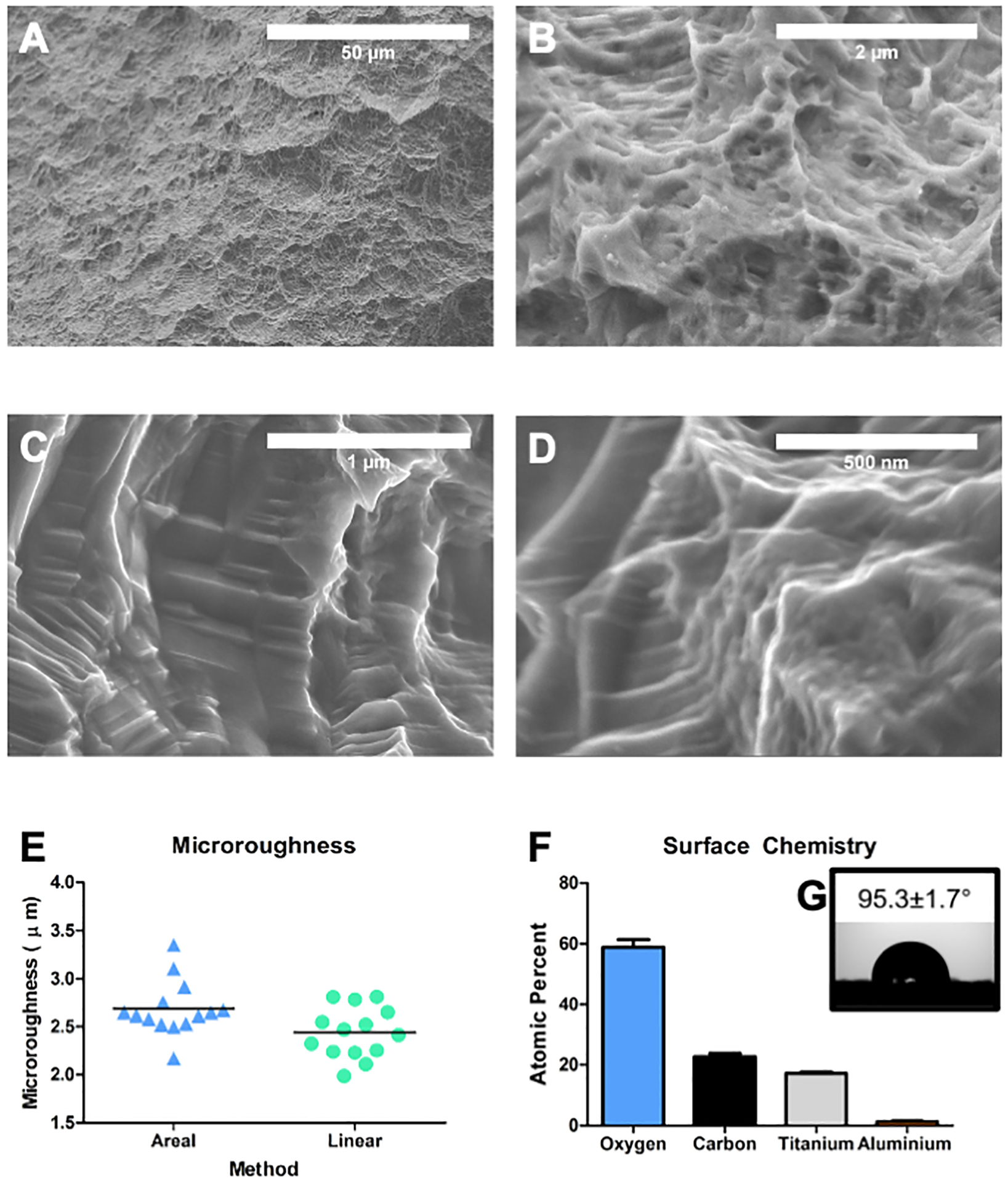
Machined titanium-aluminum-vanadium implant surfaces (MMN) were grit blasted and acid-etched and surface topography was qualitatively examined by SEM. Scanning electron micrographs show implant surfaces possessing complex topographies at the (A) macro, (B) micro, and (C, D) nanoscales. (E) Optical profilometry was used to quantify areal and linear surface microroughness. (F) Surface chemistry was evaluated by XPS and oxygen was the high atomic percentage on the implant surfaces followed by carbon and titanium, trace amount of aluminum were detected. (G) Sessile drop contact angle show a slightly hydrophobic surface.
MSCs on MMN substrates exhibit an osteoblast phenotype and produce osteogenic factors within 7 days whereas MSCs on TCPS require 21 days of culture in growth media.
At 7 and 14 days, total DNA content of MSCs on MMN surfaces was decreased significantly compared to TCPS. However, at day 21 DNA content was similar between the two groups (Fig. 2A). Both OCN and OPN were produced in a greater quantity by MSCs cultured on MMN surfaces at all points than on TCPS. OCN was maximally produced at 21 days on MMN surfaces compared to other time points, and OPN was greatest at day 7 (Fig. 2B, C). OPG and BMP2 were upregulated on MMN surfaces at day 7, however, at day 14 only BMP2 was upregulated. At day 21, MSCs on both TCPS and MMN substrates produced similar concentrations of BMP2 (Fig. 2D, E).
Fig 2. Surface Mediated Differentiation of MSCs into OBs over 21 Days.
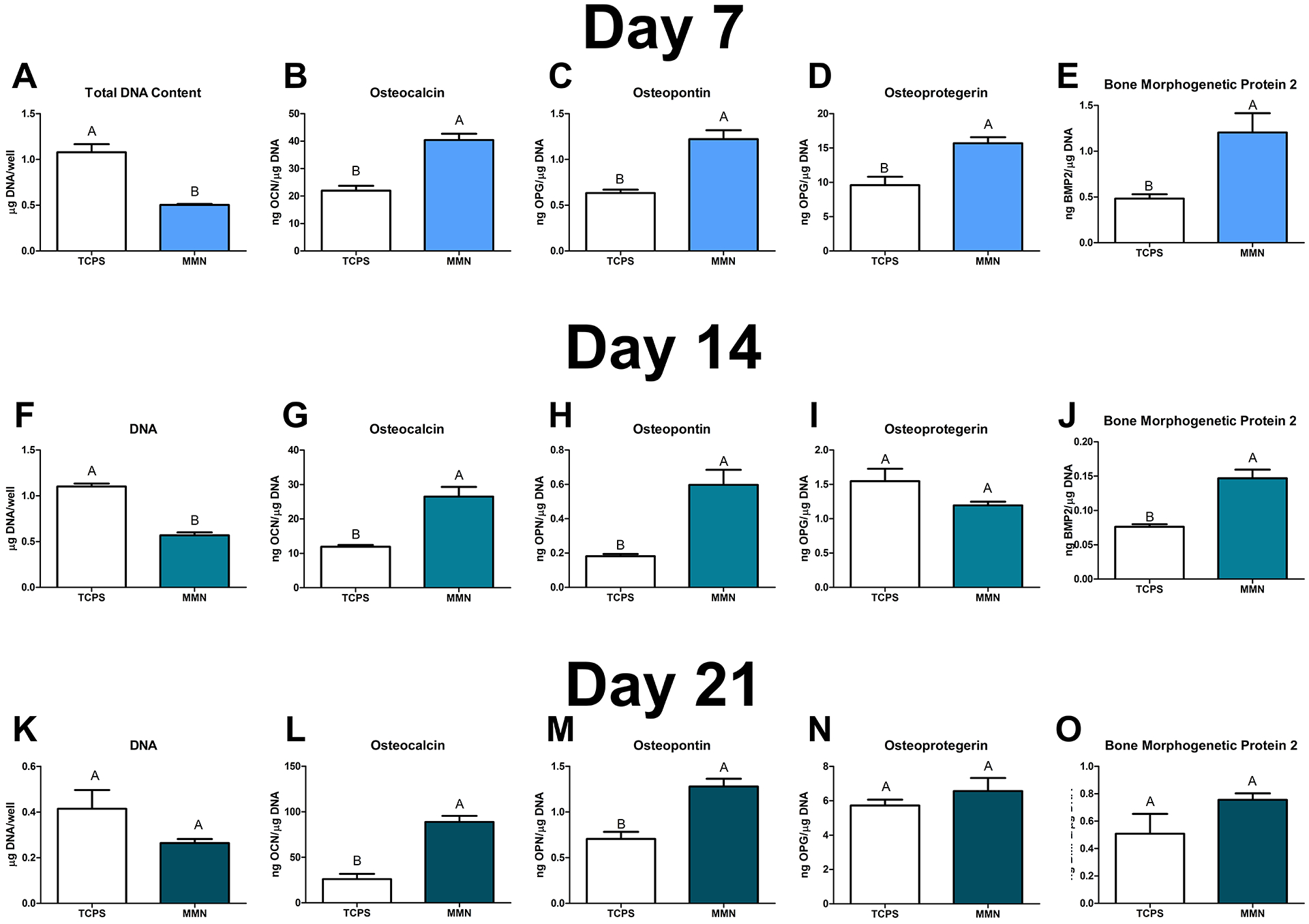
MSCs were cultured on MMN surfaces for 21 days in growth media (GM). (A, F, K) DNA content was determined in the cell layer lysate and was decreased compared to TCPS at 7 and 14 days. (B, G, L) Osteocalcin, (C, H, M) osteopontin, (D, I, N) osteoprotegerin, and (E, J, O) bone morphogenetic protein 2 were determined in the conditioned media. The data are means ± SEM & cultures/variable via one-way ANOVA and Tukey post-test. Groups not sharing letters are considered statistically significant at a p-value < 0.05 and n = 6.
Conditioned media produced by MSCs grown on MMN substrates for 7 days induced MSC differentiation in cultures grown on TCPS.
Total DNA content after 7 days of CM:GM treatment of MSCs on TCPS was decreased compared to growth media control (Fig. 3A). Optical microscopic visualization of the MSCs did not show any distinct visual differences in morphology between control and MMN-CM outside of confluency. Analysis of OCN (Fig. 3B), OPN (Fig. 3C), BMP2 (Fig. 3D), and OPG (Fig. 3E) showed increased production by MSCs exposed to CM from MSCs cultured on MMN surfaces.
Fig 3. Conditioned Media Collected from MSCs Cultured on MMN Surfaces can Influence MSCs Treated with MMN Media Cultured on TCPS to an Osteoblast Phenotype.
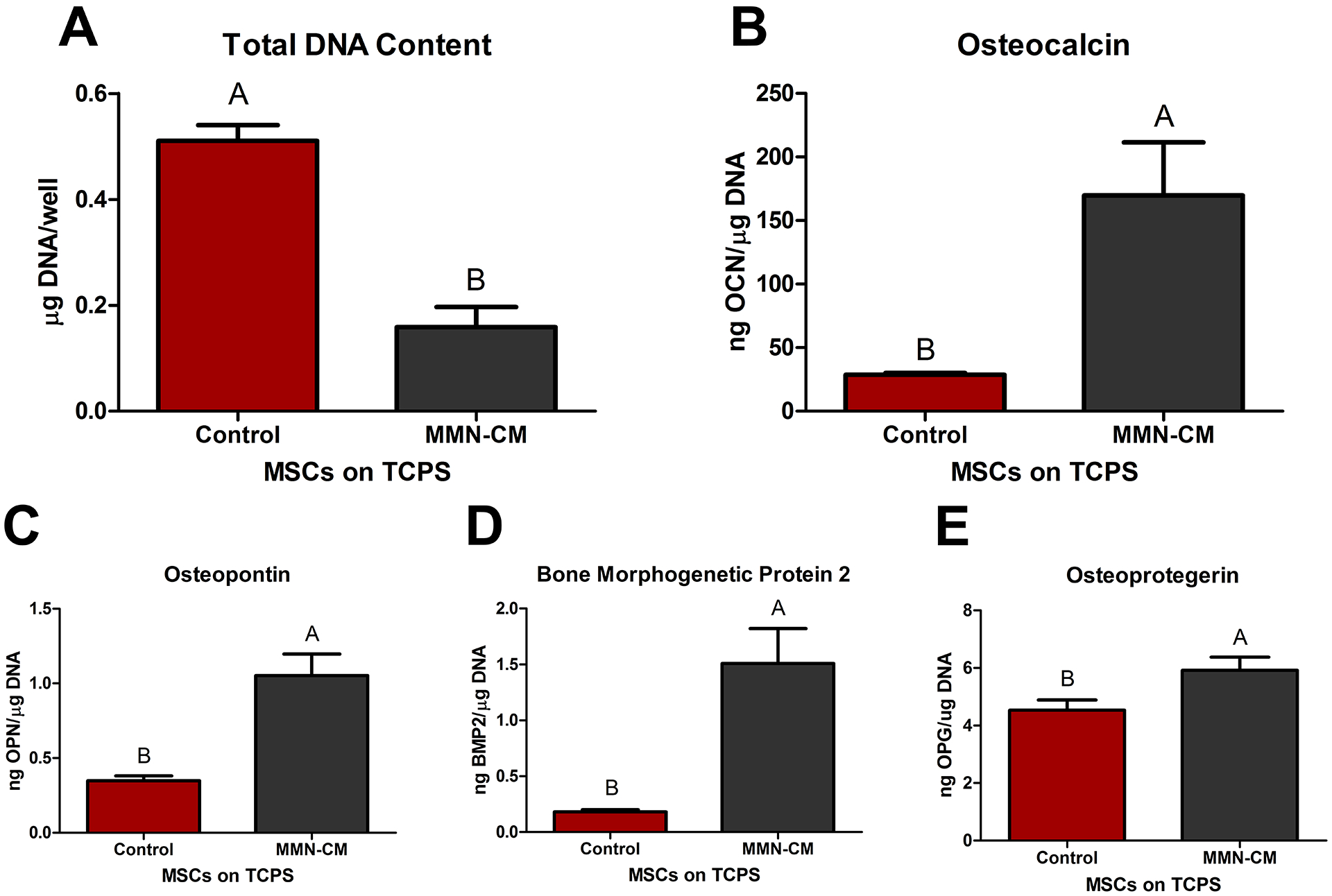
MSCs were cultured on MMN surfaces for 7 days. After 1 week, media was collected daily to create conditioned media (MMN-CM). MMN-CM was mixed with fresh GM and used to treat MSCs cultured on tissue culture polystyrene (TCPS) for 7 days. (A) DNA content was determined in the cell layer lysate. (B) OCN, (C) OPN, (D) BMP2, and (E) OPG were determined after a 24 hour incubation with fresh GM. The data are means ± SEM & cultures/variable via one-way ANOVA and Tukey post-test. Groups not sharing letters are considered statistically significant at a p-value < 0.05 and n = 6.
Reciprocal paracrine signaling in co-cultures of MSCs on MMN surfaces and MSCs on TCPS results in osteoblast differentiation via BMP2.
MSCs cultured on MMN surfaces had decreased DNA compared to cells grown on TCPS (Fig. 4A). Treatment with the BMP2 blocking antibody (abBMP2) increased DNA content compared to cells grown on MMN surfaces. Analysis of early differentiation marker alkaline phosphatase activity (Fig. 4B) showed that cells grown on MMN surfaces had decreased alkaline phosphatase activity compared to MSCs on TCPS, whether or not anti-BMP2 antibody was used. OCN and OPN were increased on MMN surfaces but treatment with abBMP2 reduced production to levels similar to that of cells on TCPS (Fig. 4C, D).
Fig 4. MSCs Cultured on MMN Surfaces for 7 Days Release Growth Factors that Direct MSCs Distal to Differentiate into Osteoblasts.
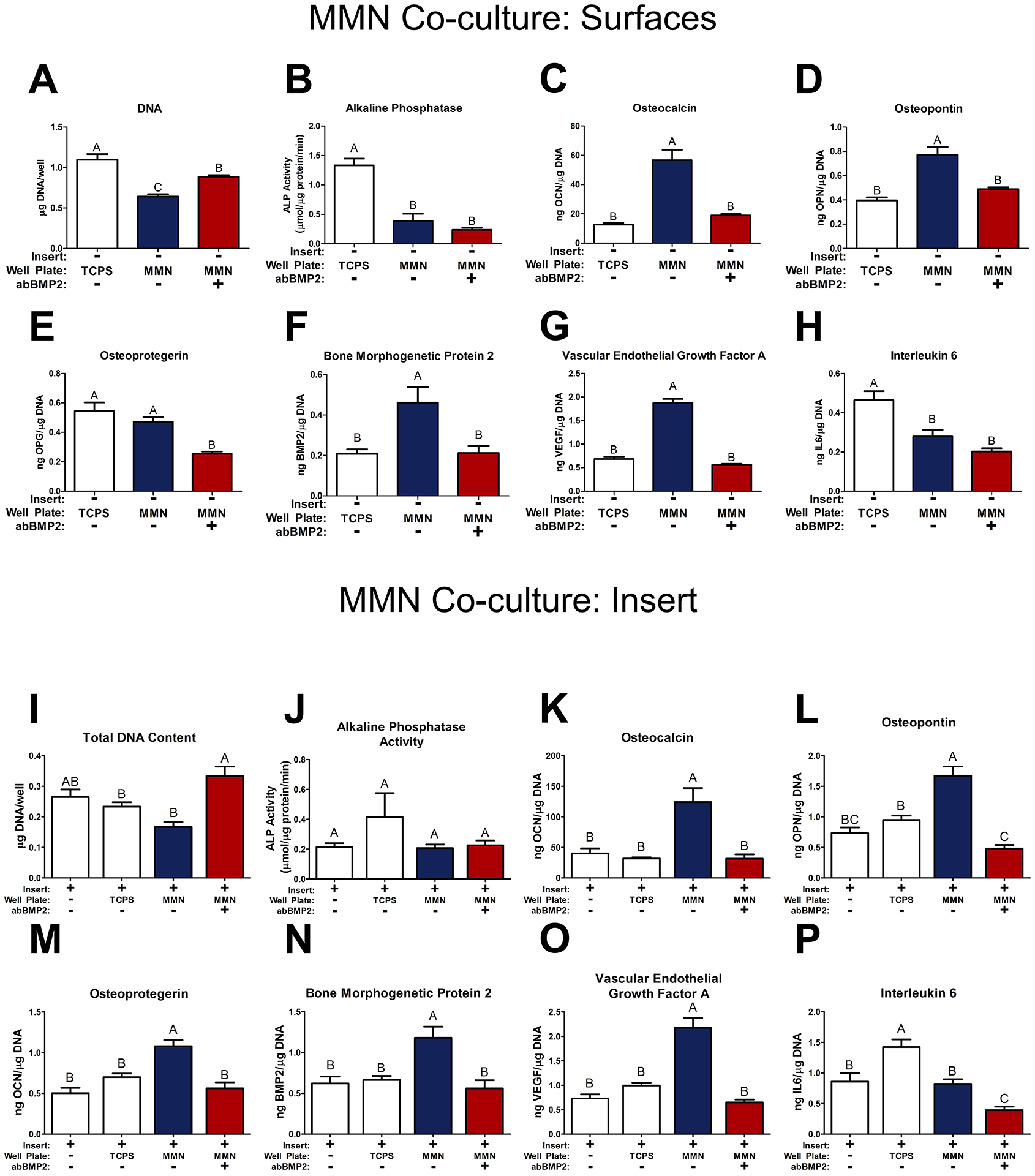
In a co-culture model, MSCs were cultured on TCPS, MMN surfaces, and distal mesh inserts for 7 days. 24 hours prior to harvest, the mesh insert and baseplate were cultured separately to evaluate insert and surface cultures individually. One MMN surface group received a blocking antibody for BMP2 (abBMP2) for the duration of the experiment. (A, I) DNA content and (B, J) alkaline phosphatase activity (ALP) was determined in the cell layer lysate on surfaces and insert respectively. Osteoblast differentiation markers (C, K) osteocalcin, (D, L) osteopontin, as well as paracrine signaling factors (E, M) osteoprotegerin, (F, N) bone morphogenetic protein 2, (G, O) vascular endothelial growth factor A, and (H, P) interleukin 6 were determined in the collected media from the surfaces and inserts respectively. The data are means ± SEM & cultures/variable via one-way ANOVA and Tukey post-test. Groups not sharing letters are considered statistically significant at a p-value < 0.05 and n = 6.
In contrast to results shown in Figure 2, there was no difference in OPG produced by cells cultured on MMN and TCPS surfaces in the reciprocal model. Treatment with abBMP2 decreased OPG production (Fig. 4E). BMP2 was increased on MMN surfaces and production was blocked by abBMP2 treatment (Fig. 4F). VEGF-A, a robust vasculogenic factor, was increased on MMN surfaces and blocked by inhibition of differentiation using abBMP2 (Fig. 4G). IL6, a proinflammatory marker of immune regulation, was decreased on all Ti6Al4V MMN surfaces regardless of abBMP2 treatment (Fig. 4H).
MSCs cultured on cell culture inserts above each surface group were assessed for the same cellular response markers. Total DNA content was the lowest on MSCs cultured on inserts placed in MMN surface wells, but was not different from insert control and TCPS co-culture groups. Treatment with BMP2 blocking antibody increased total DNA content and was significantly different from MMN surfaces (Fig. 4I). There were no differences in alkaline phosphatase activity between all groups (Fig. 4J). OCN was increased by cells grown on MMN surfaces without abBMP2 treatment and treatment prevented this increase in production (Fig. 4K). OPN was also increased by cells grown on inserts grown in wells with MMN surfaces and treatment with abBMP2 decreased production of OPN to lower levels than MSCs cultured above MSCs on TCPS (Fig. 4L). Paracrine signaling factors OPG, BMP2, and VEGF-A were all increased by cells grown above MMN surfaces without abBMP2. Treatment with abBMP2 prevented the increase in production (Fig. 4M–O). IL6 was increased by MSCs cultured above MSCs on TCPS compared to insert control and untreated MMN surfaces, treatment of MMN surfaces with abBMP2 decreased IL6 (Fig. 4P).
Reciprocal paracrine signaling varies as a function of time.
At day 12, total DNA content was decreased in all MMN surface cultures compared to TCPS and treatment with abBMP2 did not alter DNA content (Fig. 5A). BMP2 and BMP4 were increased in the conditioned media produced by cells on all MMN surfaces and BMP2 production was unaffected by abBMP2 treatment (Fig. 5B,C). However, treatment with abBMP2 decreased BMP4 in conditioned media produced by cells on MMN, but was still greater than TCPS (Fig. 5C). This effect was the same for OCN, OPN, and VEGF-A (Fig. 5D,F,G). abBMP2 treatment completely prevented the MMN increase in OPG production (Fig. 5E). IL6 was not changed between TCPS and MMN surface groups but treatment with abBMP2 increased IL6 on MMN surfaces (Fig. 5H). IL10, an anti-inflammatory immune marker for biomaterials, was increased on both MMN surface groups and was unaffected by abBMP2 treatment (Fig. 5I).
Fig 5. MSCs Cultured on MMN Surfaces for 11 Days in a Co-Culture Model.
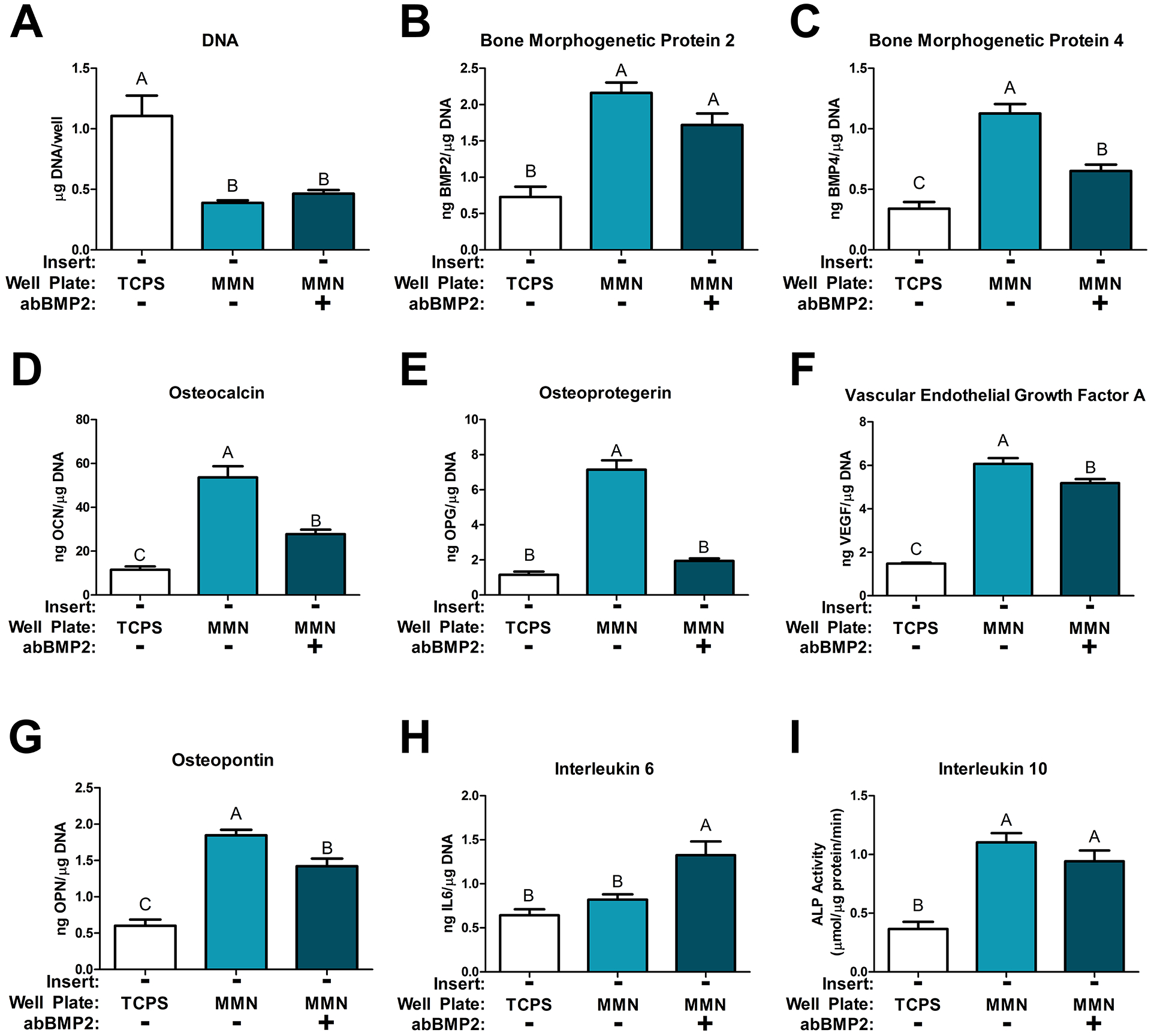
MSCs were cultured for 7 days on TCPS and MMN surfaces then placed in a co-culture model for 4 days and cultured separately 24 hours prior to harvest. One MMN group received abBMP2 treatment. (A) DNA content was determined in the cell layer lysate. Osteoblast differentiation markers (D) osteocalcin, (G) osteopontin, as well as paracrine signaling factors (E) osteoprotegerin, (B) bone morphogenetic protein 2, (C) bone morphogenetic protein 4, (F) vascular endothelial growth factor A, (H) interleukin 6, (I) interleukin 10 were determined in the collected media from the 24 well plate. The data are means ± SEM & cultures/variable via one-way ANOVA and Tukey post-test. Groups not sharing letters are considered statistically significant at a p-value < 0.05 and n = 6.
Assessment of the cells cultured in co-culture inserts above groups on the 24 well plate was conducted with the same cell response markers. Total DNA was decreased by MMN surface co-culture compared to all other groups (Fig. 6A). Both BMP2 and BMP4 were increased by co-culture with MMN surfaces and was inhibited by blocking BMP2 during co-culture (Fig. 5B, C). OCN and OPG were increased similarly on MMN co-cultures. Treatment with abBMP2 decreased production of both proteins to levels not statistically different from insert control and TCPS co-culture (Fig. 5D, E). VEGF-A was increased on both MMN co-culture groups but treatment with BMP2 decreased VEGF-A compared to untreated MMN co-culture (Fig. 6F). OPN was not changed between insert control and TCPS co-culture with MMN cells but there was a difference between the MMN groups. Blocking BMP2 signaling partially prevented OPN production induced by MMN surfaces (Fig. 6G). IL6 was increased compared to all groups in the MMN abBMP2 group and IL10 was increased by MSCs cultured above untreated MMN surfaces (Fig. 6H, I).
Fig 6. MSCs Cultured on Mesh Inserts Distal to MSCs Cultured on MMN Surfaces for 4 Days in a Co-Culture Model.
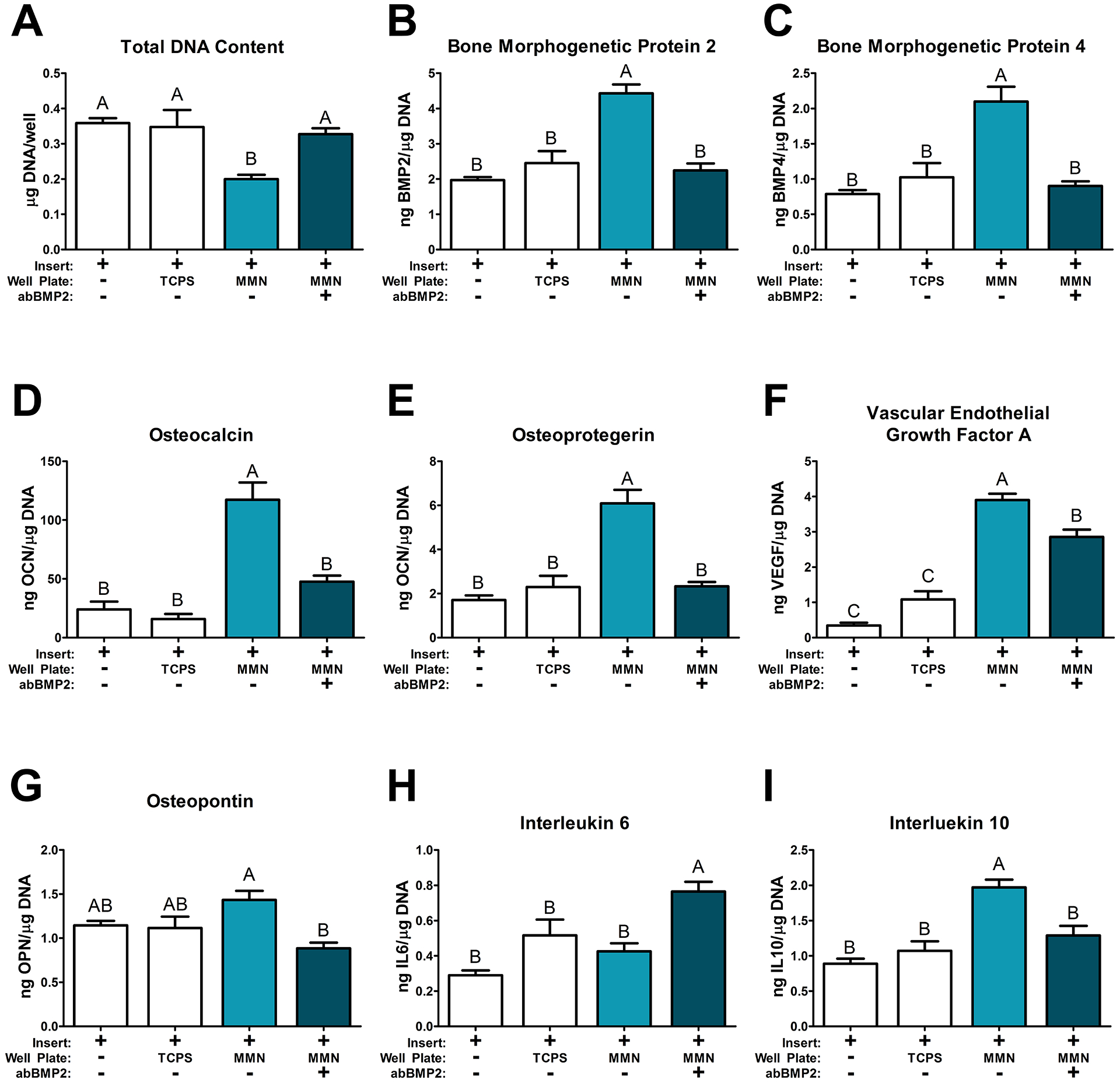
MSCs were cultured on mesh inserts and placed in a co-culture model where the baseplate contained MSCs cultured on TCPS and MMN surfaces for 7 days prior to plating cells on the inserts. The combined insert and baseplate were cultured together for 4 days and separated 24 hours prior to harvest. (A) DNA content was determined in the cell layer lysate. Osteoblast differentiation markers (D) osteocalcin, (G) osteopontin, as well as paracrine signaling factors (E) osteoprotegerin, (B) bone morphogenetic protein 2, (C) bone morphogenetic protein 4, (F) vascular endothelial growth factor A, (H) interleukin 6, (I) interleukin 10 were determined in the collected media from the 24 well plate. The data are means ± SEM & cultures/variable via one-way ANOVA and Tukey post-test. Groups not sharing letters are considered statistically significant at a p-value < 0.05 and n = 6.
DISCUSSION
Topographical cues from cell-surface interactions can change the conformation of the adsorbed proteins. This results in an activation of cytoskeletal-based signal cascades, usually acting through phosphorylation of integrin-linked kinase (ILK) and focal adhesion kinase (FAK), and their respective downstream signals.19,20 Related studies in our lab have shown that changes in the physical microscale structure of a Ti surface can modulate FAK activity and alter osteoblast shape and differentiation.21,22 Here, we show that biomimetic macro/micro/nanoscale surface topography can promote MSCs to rapidly express an osteoblast phenotype.23–25 Moreover, factors produced by these cells can modulate osteoblast differentiation in MSCs not in direct contact with the surface.
Grit-blasting and acid-etching Ti and Ti alloys are capable of producing implant surface properties that mimic the native bone surfaces at the macro/micro/nanoscale and match previous literature quantifying bone biomimetic surface properties.23,26 Qualitative SEM analysis of the MMN substrates used in the present study demonstrated large osteoclast resorption pit-like craters created during grit-blasting, and at higher magnifications a surface texture similar to that of exposed demineralized collagen fibers.23
The overlay of nanoscale surface features on the macro/microscale features generated by grit blasting may have resulted in altered adsorption of proteins, leading to the shift in cell phenotype we observed. In the literature, increases in the number of nanostructures has been shown to alter protein adsorption and fibrin polymerization. These alterations extend clot formation and increase the available extracellular matrix for MSC migration to occur.27,28
Our results using MMN substrates with biomimetic surface properties confirm our previous observations using Ti substrates showing osteoblastic differentiation of MSCs by 7 days of culture in vitro without the use of osteogenic media supplements, as shown previously for Ti substrates.8,21,23,29–31 MMN surface-mediated differentiation was much more rapid than osteogenic differentiation on TCPS, shown by reduced alkaline phosphatase specific activity and increased production of OCN and OPN at a much earlier time-point than reported literature using osteogenic media, which can take anywhere from 14 to 21 days post-confluence.32–35 Thus, any change in MSC fate due to treatment with conditioned media produced by MSCs on the MMN surface, or in co-culture with MSCs on MMN surfaces, is due to factors produced by the MMN cells, clearly demonstrating their ability to generate an osteogenic microenvironment via paracrine signaling.
Although most studies examining the effects of osteogenic compounds on MSCs grown on TCPS use osteogenic media, including dexamethasone and beta-glycerophosphate, our results show that the physical properties of TCPS were sufficient to initiate expression of osteoblast phenotypic markers at 21 days of culture in growth media. TCPS is a stiff substrate compared to hydrogels and protein coatings, but not as stiff as Titanium. However, the stiffness provided by the TCPS can account for the partial shift of the MSCs towards an osteoblast cell fate.36,37
Experiments using conditioned media from cultures of osteocytes and osteoblasts have demonstrated that progenitor cells are capable of receiving soluble signals in the form of growth factors from more mature cell types and respond robustly to these signals.38,39 This local regulation of progenitor cells by mature osteoblast lineage cells is more likely local vs systemic in native bone tissue, and is similar to that of peri-implant signaling during osseointegration of a titanium based implant.34 Treatment of MSCs cultured on TCPS with conditioned media produced by MSCs on MMN surfaces stimulated production of osteoblast markers osteocalcin and osteopontin, as well as factors that modulate osteoblast differentiation like BMP2 and osteoclast activity like osteoprotegerin. These data support the hypothesis that there is a complex interaction between MSCs that are directly on the implant surface with MSCs in the peri-implant environment.
We used two co-culture systems to better understand this complex interaction. In the first model, porous plastic inserts containing MSCs were introduced by suspension into the culture system after 24 hours of MSC growth on MMN surfaces, enabling us to assess early paracrine signaling immediately following implant placement. Co-culture with MSCs on inserts above MMN surfaces altered the autocrine signaling of MSCs in contact with the MMN surfaces, decreasing the production of OPG. Inhibiting BMP2 signaling further reduced the production of OPG, and prevented surface mediated differentiation, including upregulation of OCN, OPN, BMP2, and VEGF.
BMP2 signaling has been shown to be tightly correlated with RUNX2 activation, and osteoblast differentiation. RUNX2 is also activated by dexamethasone in osteogenic media. In this case we use unmodified growth media so activation of Runx2 both on the surface and distally on cell culture inserts was due to the topography inducing cytoskeletal reorganization and upregulation of osteoblast transcription growth factors.32 The use of the antibody against BMP2 likely removed the propagation of this effect in both an autocrine and paracrine manner. Interestingly, signaling factors like IL6 and OPG were either decreased or unchanged by addition of this antibody, suggesting other methods of differentiation and activation were being activated in parallel with surface mediated differentiation. Literature has tied BMP2 activation to IL6 upregulation and low basal levels of IL6 are necessary to recruit MSCs into the implant microenvironment.40–43 However, prolonged increases in IL6 can lead to activation of M1 inflammatory macrophages and fibrous encapsulation.44 IL6 production on MMN surfaces was decreased compared to MSCs cultured on TCPS, suggesting that MMN surfaces are capable of maintaining low basal levels of IL6 necessary for MSC recruitment but reduce production to prevent M1 macrophage induction.
The soluble signaling factors produced by MSCs on MMN surfaces are required for differentiation of cells distal to the implant surfaces. Results from the second co-culture model confirm this. In this model, MSCs were cultured on the test surfaces for 6 days prior to beginning co-culture. BMP2 blocking did not change BMP2 or IL10 production but decreased concentrations of osteogenic proteins like BMP4, OPN, OCN, and VEGF-A compared to untreated MSCs on MMN surfaces.42,45 Decreases in overall local factor production in response to antibody blocking of BMP2 after surface mediated differentiation shows that BMP2 is a more potent regulator of osteogenesis during implant integration, and that the other local factors produced by MSCs on MMN surfaces rely on BMP2 signaling to initiate production.
On cell culture inserts, MSCs cultured in wells with MSCs on MMN surfaces increased production of osteogenic proteins after co-culture, but blocking BMP2 action arrested this effect completely, showing that the distal stimulation of osteoblastic differentiation relies on BMPs produced by MSCs on the implant surface. Angiogenic potential may not be affected as VEGF-A signaling was still upregulated so alternative signaling pathways may be involved, such as partial oxygen pressure in the co-cultures. The blockage of BMP2 signaling increased IL6 production, which can be a response by MSCs to recruit additional stromal cells in response to decreased BMP2 peri-implant.40,42 These results must be confirmed in vivo using an osteoinductive ectopic assay because it is important to evaluate these data in vivo to account for the variety of cell types that will colonize the implant microenvironment during the complex signaling cascade that occurs immediately after the implants are placed.
Traditional methods of cellular differentiation in vitro use osteogenic media, which pulse the MSCs with high doses of phosphates and other media supplements that alter cellular response and can cause mineralization after 21 days of culture. High concentrations of beta glycerol phosphate are supraphysiologic and these increases in the local concentration of organic phosphates can result in dystrophic calcification and therefore a limited model of cell differentiation.35,46 Additionally, osteogenic media often include ascorbic acid and dexamethasone, which act in a two-way manner to activate Runx2 and β-Catenin/Wnt signaling, and by super charging enzymes that hydroxylate proline and lysine in pro-collagen to increase collagen 1 production around cells.32 MMN surfaces provide a physical microenvironment that can serve as an improved model for MSC differentiation in vitro without the need of osteogenic media supplements.23
BMP signaling activates SMAD signaling pathways and induces RUNX2 transcription factor and osteoblastic gene expression.47 BMP signaling is not only SMAD dependent, but additional signaling pathways via ERK/MAPK can also be upregulated to enhance expression of osteoblastic genes. Downstream targets of these pathways are alkaline phosphatase, osteopontin, osteocalcin and collagen type I.48,49 Blocking of BMP signaling in this study by epitope binding antibody most likely prevented both activation of SMAD signaling and ERK signaling demonstrated by complete abrogation of osteopontin, osteoprotegerin, and osteocalcin. This effect was especially evident during the first co-culture experiment where BMP2 was blocked immediately after cell attachment and the ILK and FAK effects were blunted especially for cells not in contact with the MMN surfaces. The lack of surface cues and the diminished production of soluble signaling factors from cells on MMN surfaces decreased the differentiation of MSCs on inserts in wells that received abBMP2 treatment.
Complex MMN Ti6Al4V implant surfaces differentiate MSCs into osteoblasts without any additional exogenous osteogenic growth factors. MSCs cultured on MMN Ti6Al4V implant surfaces increased the production of multiple local osteogenic factors necessary for angiogenesis, immune regulation, and bone formation. These local factors are able to regulate MSCs distal to MMN surfaces through paracrine signaling to induce osteoblast differentiation. BMP2 is one of the most important signaling factors and reduction of BMP2 signaling by a BMP2 blocking antibody prevents MSCs differentiation in contact and distal to the MMN implant surfaces. This in vitro study indicated that these microtextured implants possessing nanoroughened topography can increase the local factor production necessary for bone regeneration and implant integration by promoting the activation of multiple signaling pathways. Collectively, these results with MMN surfaces further indicate that the biomimetic effect is similar to that of exogenous osteoanabolic agents like BMP2, supporting their use clinically.
Acknowledgments
Medtronic/Titan Spine (Mequon, WI) provided the MMN surfaces and partial support for these studies. NIH Awards Numbers R01AR052102 and R01AR072500 and CIT grant MF19-005-LS provided additional support for this research.
Footnotes
Disclosures
BDB is an unpaid consultant for Institut Straumann AG (Basel, Switzerland) and Spineology Inc. (St. Paul, MN), and a paid consultant for Titan Spine LLC (Mequon, WI).
References
- 1.Turri A, Rossetti P, Canullo L, et al. 2016. Prevalence of peri-implantitis in medically compromised patients and smokers: a systematic review. Int. J. Oral Maxillofac. Implants 31(1):111–118. [DOI] [PubMed] [Google Scholar]
- 2.Christodoulou S, Goula T, Ververidis A, Drosos G. 2013. Vitamin D and bone disease. Biomed Res. Int. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leboff M, Hawkes W, Glowacki J, et al. 2008. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos. Int 19(9):1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gittens R a., Olivares-Navarrete R, Schwartz Z, Boyan BD. 2014. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 10(8):3363–3371Available from: 10.1016/j.actbio.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rupp F, Gittens R a., Scheideler L, et al. 2014. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 10(7):2894–2906Available from: 10.1016/j.actbio.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gittens R a., Scheideler L, Rupp F, et al. 2014. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 10(7):2907–2918Available from: 10.1016/j.actbio.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. 2007. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater 23(7):844–854. [DOI] [PubMed] [Google Scholar]
- 8.Lotz EM, Berger MB, Boyan BD, Schwartz Z. 2020. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 134(February):115260.Available from: 10.1016/j.bone.2020.115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyan BD, Schwartz Z, Lohmann CH, et al. 2003. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J. Orthop. Res 21(4):638–647. [DOI] [PubMed] [Google Scholar]
- 10.Olivares-Navarrete R, Raz P, Zhao G, et al. 2008. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc. Natl. Acad. Sci. U. S. A 105(41):15767–15772Available from: http://www.ncbi.nlm.nih.gov/pubmed/18843104%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2564982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripamonti U, Roden LC, Renton LF. 2012. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 33(15):3813–3823Available from: 10.1016/j.biomaterials.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Ripamonti U, Duarte R, Ferretti C. 2014. Re-evaluating the induction of bone formation in primates. Biomaterials 35(35):9407–9422Available from: 10.1016/j.biomaterials.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 13.Davison NL, Su J, Yuan H, et al. 2015. Influence of surface microstructure and chemistry on osteoinduction and osteoclastogenesis by biphasic calcium phosphate discs. Eur. Cells Mater 29:314–329. [DOI] [PubMed] [Google Scholar]
- 14.Villavicencio AT, Burneikiene S, Nelson EL, et al. 2005. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein–2. J. Neurosurg. Spine 3:436–443. [DOI] [PubMed] [Google Scholar]
- 15.Oetgen ME, Richards BS. 2010. Complications associated with the use of bone morphogenetic protein in pediatric patients. J. Pediatr. Orthop 30(2):192–198Available from: http://www.ncbi.nlm.nih.gov/pubmed/20179569. [DOI] [PubMed] [Google Scholar]
- 16.Crawford CH, Carreon LY, Mcginnis MD, et al. 2009. Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine (Phila. Pa. 1976) 34(13):1390–1394. [DOI] [PubMed] [Google Scholar]
- 17.Hyzy SL, Olivares-Navarrete R, Sarah O, et al. 2017. Bone morphogenetic protein 2 alters osteogenesis and anti-inflammatory profiles of mesenchymal stem cells induced by microtextured titanium in vitro. Tissue Eng Part A 23(19–20):1132–1141Available from: https://www.ncbi.nlm.nih.gov/pubmed/28351289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smoljanovic T, Cimic M, Bojanic I. 2010. Aggressive end plate decortication as a cause of osteolysis after rhBMP-2 use in cervical spine interbody fusion. Spine J. 10(2):187–188Available from: 10.1016/j.spinee.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Askari JA, Buckley PA, Mould AP, Humphries MJ. 2009. Linking integrin conformation to function. J. Cell Sci 122(2):165–170Available from: http://jcs.biologists.org/cgi/doi/10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barczyk M, Carracedo S, Gullberg D. 2010. Integrins. Cell Tissue Res. 339(1):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keselowsky BG, Wang L, Schwartz Z, et al. 2007. Integrin a5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. J. Biomed. Mater. Res. - Part A 80:700–710. [DOI] [PubMed] [Google Scholar]
- 22.Lai M, Hermann CD, Cheng A, et al. 2015. Role of A2B1 integrins in mediating cell shape on microtextured titanium surfaces. J. Biomed. Mater. Res. - Part A 103(2):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares-Navarrete R, Hyzy SL, Berg ME, et al. 2014. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann. Biomed. Eng 42(12):2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo F, Guo W, Wu H, et al. 2018. Fabrication of biomimetic resorption lacunae-like structure on titanium surface and its osteoblast responses. Appl. Surf. Sci 436:11–21Available from: 10.1016/j.apsusc.2017.11.282. [DOI] [Google Scholar]
- 25.Boyan BD, Lotz EM, Schwartz Z. 2017. (*) Roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng Part A 23(23–24):1479–1489Available from: https://www.ncbi.nlm.nih.gov/pubmed/28793839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster TJ, Ejiofor JU. 2004. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 25(19):4731–4739. [DOI] [PubMed] [Google Scholar]
- 27.Khang D, Lu J, Yao C, et al. 2008. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 29(8):970–983. [DOI] [PubMed] [Google Scholar]
- 28.Di Iorio D, Traini T, Degidi M, et al. 2005. Quantitative evaluation of the fibrin clot extension on diffferent implant surfaces: An in vitro study. J. Biomed. Mater. Res. - Part B Appl. Biomater 74(1):636–642. [DOI] [PubMed] [Google Scholar]
- 29.Lotz EM, Olivares-Navarrete R, Berner S, et al. 2016. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J. Biomed. Mater. Res. - Part A 104(12):3137–3148. [DOI] [PubMed] [Google Scholar]
- 30.Zhao G, Raines AL, Wieland M, et al. 2007. Requirement for both micron and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 28(18):2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotz EM, Berger MB, Schwartz Z, Boyan BD. 2018. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater. 68:296–307Available from: 10.1016/j.actbio.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenbach F, Handschel J. 2013. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther 4:117.Available from: Stem Cell Research & Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brauer A, Pohlemann T, Metzger W. 2016. Osteogenic differentiation of immature osteoblasts : Interplay of cell culture media and supplements. Biotech. Histochem 91(3):161–169. [DOI] [PubMed] [Google Scholar]
- 34.Birmingham E, Niebur GL, Mchugh PE, et al. 2012. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater 23:13–27. [DOI] [PubMed] [Google Scholar]
- 35.Boyan BD, Bonewald LF, Paschalis EP, et al. 2002. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int 71(6):519–529. [DOI] [PubMed] [Google Scholar]
- 36.Olivares-Navarrete R, Lee EM, Smith K, et al. 2017. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One 12(1):e0170312.Available from: https://www.ncbi.nlm.nih.gov/pubmed/28095466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferlin KM, Prendergast ME, Miller ML, et al. 2016. Influence of 3D printed porous architecture on mesenchymal stem cell enrichment and differentiation. Acta Biomater. 32:161–169Available from: 10.1016/j.actbio.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Hall BK. 2015. Chapter 24 - Osteoblast and osteocyte diversity and osteogenesis in vitro. In: Hall BKBT-B and C (Second E, editor. San Diego: Academic Press. p 401–413 Available from: http://www.sciencedirect.com/science/article/pii/B9780124166783000240. [Google Scholar]
- 39.Xiong J, Almeida M, O’Brien CA. 2018. The YAP/TAZ transcriptional co-activators have opposing effects at different stages of osteoblast differentiation. Bone 112(December 2017):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshitake F, Itoh S, Narita H, et al. 2008. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J. Biol. Chem 283(17):11535–11540. [DOI] [PubMed] [Google Scholar]
- 41.Hyzy SL, Olivares-Navarrete R, Sarah O, et al. 2017. Bone Morphogenetic Protein 2 Alters Osteogenesis and Anti-Inflammatory Profiles of Mesenchymal Stem Cells Induced by Microtextured Titanium In Vitro. Tissue Eng Part A 23(19):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyzy SL, Olivares-navarrete R, Hutton DL, et al. 2013. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous BMP-2. Acta Biomater. 9(3):5821–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotchkiss KM, Ayad NB, Hyzy SL, et al. 2016. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin. Oral Implants Res 28(4):1–10Available from: 10.1111/clr.12814. [DOI] [PubMed] [Google Scholar]
- 44.Hotchkiss KM, Clark NM, Olivares-navarrete R. 2018. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 182(March):202–215Available from: 10.1016/j.biomaterials.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 45.Abe E 2006. Function of BMPs and BMP antagonists in adult bone. Ann. N. Y. Acad. Sci 1068(1):41–53. [DOI] [PubMed] [Google Scholar]
- 46.Beck GR, Zerler B, Moran E. 2000. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci 97(15):8352–8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linkhart TA, Mohan S, Baylink DJ. 1996. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone 19(1 SUPPL.). [DOI] [PubMed] [Google Scholar]
- 48.Lai CF, Chaudhary L, Fausto a, et al. 2001. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem 276(17):14443–14450Available from: http://www.ncbi.nlm.nih.gov/pubmed/11278600. [DOI] [PubMed] [Google Scholar]
- 49.Greenblatt MB, Shim J-H, Glimcher LH. 2013. Mitogen-activated protein kinase pathways in osteoblasts. Annu. Rev. Cell Dev. Biol 29(1):63–79Available from: http://www.annualreviews.org/doi/10.1146/annurev-cellbio-101512-122347. [DOI] [PubMed] [Google Scholar]


