Abstract
The number of lungs available for lung transplantation is far lower than the number of patients awaiting them. Consequently, there is a significant attrition rate while awaiting transplantation. Lung procurement rates are lower than those of other solid organs. Lungs are procured from only 15–20% of donors compared with 30% of decreased donors for hearts. The reason for this low retrieval rate is related to a number of factors. Brain death is associated with neurogenic pulmonary edema. Additionally, injury to the lung itself may occur before or after brain death. Aspiration of gastric contents, pneumonia, previous thoracic trauma, ventilator-associated injury, atelectasis, and pulmonary thrombosis/embolism may all contribute to lung injury before consideration for harvest. Donation after circulatory death (DCD) is one category of nontraditional organ donation now being performed in increasing numbers as a way to increase the number of lungs available for transplantation. In some studies, estimates show that utilization of DCD lung procurement could increase the number of lungs available by up to 50%.
Keywords: Donation after circulatory death (DCD), Lung procurement, Different scenarios, Checklist for DCD, Recent advances
Donation after circulatory death
From a legal perspective, individuals who are classified as brain dead are equal to patients that have already expired. In these cases, it is legal to procure organs regardless of whether or not the circulation remains intact. Patients who have suffered devastating and irreversible brain injury but who are not brain dead may still qualify as a potential organ donor if the patient, by advance directive or familial consent, decide that life support be withdrawn. In these cases, withdrawal of care is only offered if the patient is near death, if care is futile, or if the overall prognosis is poor. After withdrawal of support, death is usually declared after a 5–15-min window of no cardiac activity (varies depending on country). In order for donation after circulatory death (DCD) to formally take place, the cessation must be permanent but not necessarily irreversible. Cessation is considered permanent if cardiac activity will not resume on its own [1] .
DCD lung transplant history
The first lung transplant and first long-term successful human lung transplant both utilized DCD donors [2]. James Hardy performed the first human lung transplant on June 11, 1963, with a patient diagnosed with both a lung abscess and advanced lung cancer [3, 4], and a few days later, George Magovern and Adolph Yates reported the second human lung transplant at the University hospital in Pittsburgh [3, 5]. The combined survival of both patients was only 26 days. The first successful long-term lung transplantation was reported by Fritz Derom in Belgium in 1971. The patient survived a total of 10.5 months after the procedure. During that same time period, it was recognized that death resulted from irreversible damage to the brain stem; donation after brain death (DBD) become widely accepted, and DCD was largely abandoned [3].
In 1991, the concept of DCD lung transplantation was revisited in the canine model by Tom Egan [3, 6]. He demonstrated that animals could survive at least 8 h after transplanting a lung procured 1 h after cardiac arrest into a recipient with a ligated contralateral pulmonary artery. In 1995, D’Alessandro reported the first successful case of lung transplantation using a controlled DCD donor strategy [7], and since then, multiple studies have been performed utilizing DCD donors.
Maastricht classification
Donors after circulatory arrest are grouped by the Maastricht classification which was developed in the Netherlands in 1995 [8]. The first two categories in the original classification are uncontrolled DCD donors: type I—found dead, type II—witnessed cardiac arrest with unsuccessful resuscitation (Table 1). Category III donors—waiting cardiac death (planned withdrawal of life-sustaining therapy) and IV—cardiac arrest while brain dead are the next two categories in the Maastricht classification. They are considered controlled because pulmonary graft assessment can be made before withdrawal of life support (Table. 1).
Table 1.
Modified Maastricht classification of DCD [8] and the locations where mainly practiced. ICU, intensive care unit; ED, emergency department
| Category | Description | Type of DCD | Locations practiced |
|---|---|---|---|
| I | Dead on arrival | Uncontrolled | ED in a transplant center |
| II | Unsuccessful resuscitation | Uncontrolled | ED in a transplant center |
| III | Anticipated cardiac arrest | Controlled | ICU and ED |
| IV | Cardiac arrest in a brain-dead donor | Controlled | ICU and ED |
| V | Unexpected arrest in ICU patient | Uncontrolled | ICU in a transplant center |
Criteria regarding suitability for transplantation are the same as with DBD. In general, most groups using extended criteria reject organs from donors with age > 65, smoking to >20 pack years, ICU period <5 days, and a PO2/FiO2 ratio to less than 40 kPA [9]. Additionally, pre-procedural chest x-ray must be clear, and significant aspiration should be ruled out on bronchoscopic examination when possible (usually not possible due to restrictions pertaining to DCD donors). Warm ischemic time is limited to 10–15 min after death [2]. The allowed length of the agonal phase, heparin pre-treatment, timing of withdrawal of the endotracheal tube, timing of lung re-inflation, warm ischemic time, and use of ex vivo lung perfusion (EVLP) are variables specific to DCD donation that may affect graft function after transplantation [9].
In centers where EVLP is unavailable, criteria for acceptable lungs are usually more stringent. More specifically, a PaO2/FiO2 cutoff ratio of 350 is utilized because real-time physiologic assessment of donor lungs is limited; additionally, a clear computed tomogram (CT) of the chest is preferable/desirable.
Controlled DCD lung donation
For category III donors, the length of the agonal phase, warm ischemic time, and timing of lung re-inflation are of variable of importance. The agonal phase is defined as the time period between withdrawal of life-sustaining therapy and cardiac arrest. There is no consensus about the optimal time period among centers, and the period varies from 30 to 180 min. Most transplant centers however utilize a 60–90-min window between withdrawal of therapy and cardiac death [2].
The definition of warm ischemic time remains debatable and varies among most centers [2, 10]. The interval period between withdrawal of life-sustaining therapy and either initiation of cold preservation solution or lung re-inflation is typically utilized as the most commonly used definition. Other centers use the interval period between asystole and initiation of preservation as their definition. In either case, most centers try to avoid warm ischemia times greater than 60 min [10]. The Melbourne group in Australia has recently introduced the concept of functional warm ischemia defined as a systolic blood pressure less than 50 mmHg as the mark for the beginning of warm ischemic time [10, 11]. Despite the multitude of definitions currently utilized, warm ischemic time has not been shown to affect either 30- or 365-day survival [11].
Most hospitals allow for withdrawal of life-sustaining therapy in the operating room. This approach is intended to minimize warm ischemia time which is thought to correlate with increased risk of graft dysfunction [10]. Once death is confirmed by asystole on the cardiac monitor, or absence of electrical activity, a defined “no-touch” period of 2–5 min is generally accepted as sufficient to exclude spontaneous return of circulation [10]. In Australia, a period of 10–15 min is utilized before ventilation is re-initiated [12]. The donor is then re-intubated and ventilation initiated. Bronchoscopy is then performed to assess the airway and evaluate for gastric contents.
We have put together a pictorial depiction of different phases of DCD procurement to give a visual impact (Fig. 1).
Fig. 1.
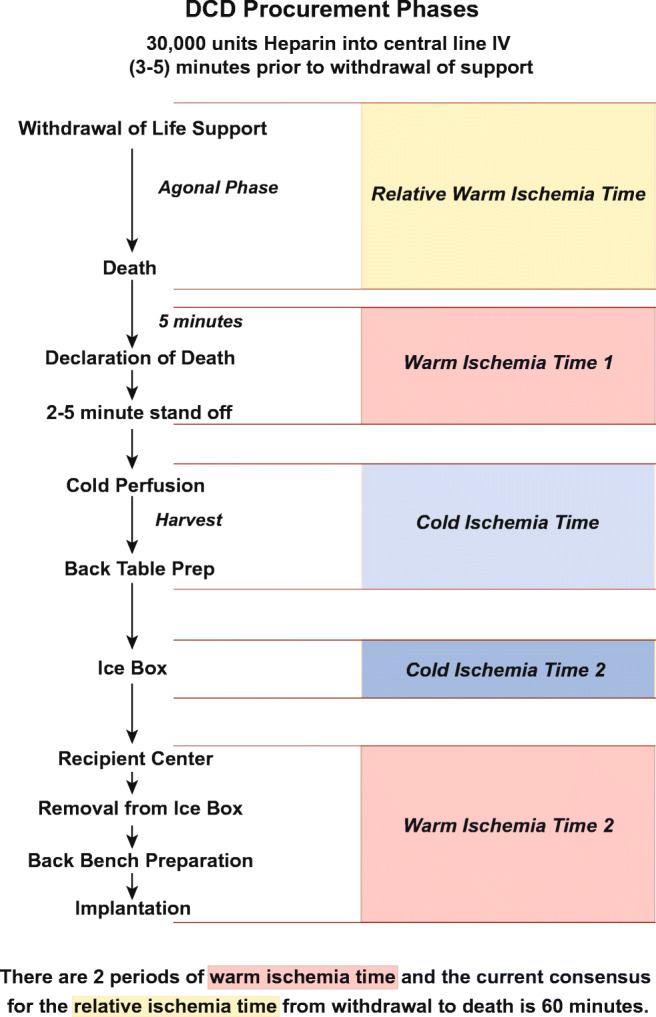
DCD Procurement Phases
We recommend giving 30,000 units of heparin into a central line intravenously 3–5 min prior to withdrawal of care to help allow the heparin to circulate evenly. If, however, the donor center has restrictions on giving heparin prior to death, we have an alternate strategy of adding 50,000 units of heparin to the first bag of pulmonary flush solution (Perfadex).
Procurement technique (DCD lung only/heart not placed for transplantation)
Following consent, suitability testing, cross matching, and acceptance, the patient is taken to the operating room along with the organ retrieval teams (Table 2). It is preferable to have 2 surgeons on the procurement team. Withdrawal of life-sustaining therapy is initiated by removal of the endotracheal tube. In certain cases when there is extensive airway edema due to potential difficulty in securing airway, the ventilator is disconnected after discussion with the anesthesiology team.
Table 2.
Procurement check list prior to proceeding
Of note, it is important to have the nasogastric tube (NG) maintained on continuous suction during withdrawal of life-sustaining therapy. This action prevents aspiration of gastric contents and also facilitates surgical dissection near the esophagus. It is also important to communicate the importance of the NG to the anesthesia team before extubation. Investigation of the NG/OG (orogastric tube) prior to extubation is typically performed by at least one procurement team member. The utmost importance must be given to prevent accidental removal of the NG tube at the time of withdrawal of the endotracheal tube.
The surgical team is then scrubbed and ready in the adjacent operating room or sterile corridor; the patient is prepped and draped in sterile fashion; a hand left out for the family if they are coming in to the operating room to pay their last respects and a sterile sleeve is made available for the stethoscope for auscultation/declaration of death. The flush solution is prepared by injecting prostaglandin into the first bag of Perfadex. The lines are then passed off the sterile field, bags spiked and either left to hang on an IV pole or alternatively the flush bags can be kept in the ice box at the side of the table.
Death is declared on cessation of circulation for a period of 5 min, or as evidence by absence of pulse on arterial trace. There is a mandatory “stand-off” period based on institutional protocol following the surgical team is allowed to start. Median sternotomy is performed and pericardiotomy completed. Maximal attention should be paid towards quick entry to facilitate the organ harvest without injuring vital structures. Notably the majority of dissection is performed using scissors and electrocautery is seldomly used to save time. The patient is then re-intubated by the anesthesiology team and ventilation is commenced 15 min after cardiac arrest. The inferior vena cava (IVC) is then vented by partial transection in a location previously discussed with the liver procurement team.
At this juncture, the liver team will also ask for the thoracic team’s help with clamping the descending aorta in the chest in order to perfuse the liver/abdominal organs with cold perfusate. The thoracic team will open the left pleura, release the inferior pulmonary ligament, and loop the descending thoracic aorta using blunt finger dissection. The cross clamp is placed immediately to help start perfusing the abdominal organs. Focus is then returned to perfusing the lungs.
Techniques of pulmonary artery flush:
Often times we have found in our experience that it is easy to use a straight, stiff arterial cannula (a femoral arterial cannula) especially when there is a single surgeon or there is insufficient help; using a #15 blade a small nick is made in the right ventricular outflow tract (RVOT) just enough to permit the cannula entry and the cannula is introduced into the pulmonary artery (PA) after advancing across the pulmonary valve; this effectively delivers equitable antegrade PA flush. This obviates the need for a purse string and effectively holds the cannula in place.
When qualified help is available a 4-0 polypropylene purse string suture is placed on the main pulmonary artery just below the bifurcation/more towards the RVOT. Using a #11 blade an arteriotomy is performed; this is then gently dilated with a tonsil forceps/Schnidt and a right-angled/straight cannula is inserted; if angled then ensure the bevel is pointing towards the pulmonary valve. The cannula is connected to the Perfadex flush solution. The ascending aorta is cross clamped, the left atrial appendage is amputated and 60–70 ml/kg of Perfadex is then infused in antegrade fashion. Both pleural cavities are widely opened with blunt finger dissection and the chest cavity is packed with soft ice. After the patient is re-intubated by the anesthesiology team/second surgeon, the lungs are then ventilated with tidal volume of 6–8 cc/kg and fiO2 of 50%. The second surgeon then proceeds with bronchoscopy while the first surgeon continues with the flush. If only one surgeon is available, then this is the time to re-intubate if insufficient help is available, ventilate, and perform bronchoscopy while the flush runs and the chest is adequately packed in ice.
Following instillation of the Perfadex solution, the cannula is removed. The lungs are then examined and any areas of atelectasis recruited with short inspiratory holds at 30 cm H2O pressure and both lungs are sequentially examined.
The heart is then excised in standard fashion; in certain instances, there is consent for homograft valves and sufficient care needs to be exercised while excising the heart.
Dissection begins first by dividing the inferior pulmonary ligament (bilateral) and the posterior pericardium. The main and right pulmonary arteries are dissected away from the aorta. Bilaterally, the inferior pericardium is the divided to the level of the esophagus. Then, the posterior pericardium is divided in a horizontal fashion just above the esophagus to the level of the pulmonary veins. The aortic arch is then transected. The superior aspect of the arch is then exposed and divided as distal as possible along the arch vessels so that a portion of the wall of the arch and descending aorta are left as a cuff to prevent dividing the ligamentum arteriosum.
Attention is then turned to the trachea which is isolated just above the carina with blunt dissection. A TA-30 stapler is passed around the trachea 3 rings above the carina. The lungs are inflated to 60% tidal volume—to avoid baro-trauma (especially being flown to recipient center), and the endotracheal tube is withdrawn. The stapler is used to divide the trachea. The lung bloc is removed from the donor after division of any remaining attachments.
The lung bloc is taken to the back table. Retrograde flush is performed using cold Perfadex solution infusing 250–500 cc/pulmonary vein. One technique is to use a Foley catheter with an inflated bulb at the tip inserted into each vein sequentially as the flush is administered. The caveat with this is that one may inadvertently injure the pulmonary vein ostium (which is delicate) by excessively distending it. The other technique is to use a retrograde cardioplegia catheter which has a self-inflating balloon, so it is much more elegant and less traumatic [13]. The only problem with this is it is not part of the standard kit, so the procurement surgeons must remember to bring it with them. Yet another technique is to use the rubber tubing that comes on the end of line. Insert the tube into the pulmonary vein and then pinch the vein to provide a tight seal. Whichever technique is used, it is important to have the Perfadex solution only 30 cm above the table and run it by gravity; avoid delivering it at excessive pressure which can lead to pulmonary edema. If lot of clots/thrombi are seen exiting the PA during retrograde flush, one can take a call regarding quality; small clots are not uncommon.
The double lung bloc is then examined for compliance, color, any areas of inadequate perfusion, atelectasis etc.; once the procurement surgeon is satisfied that the lungs are suitable for transplantation the recipient surgeon is called, the lungs are then split on the back table and packed in standard fashion. The main PA is divided at carina of the PA. The atrial cuff is then created approximately 1 cm from insertion of the pulmonary veins in to the left atrium. The atrium is then divided in the midline with scissors. The left mainstem bronchus is divided with a TA-30 stapler near the tracheal carina. The lungs are packed first in the first bag containing Perfadex only, surrounded by 2 bags containing ice-cold saline slurry. The organs are subsequently tagged and then transported to their respective transplant centers.
Alternate scenarios
Only 1 surgeon and no help available to intubate/perform bronchoscopy
If only 1 surgeon is available on the procurement team, the steps occur as described until flush occurs with the Perfadex solution. The pleural cavities are then packed with ice. The surgeon will scrub out and intubate the patient, initiate ventilation, and perform bronchoscopy. The surgeon will then scrub back in and continue to harvest the lung/s in the previously described fashion.
More than one organ procured
If a heart is also being procured, the case is considered both a DCD heart and DCD lung. The heart, liver, lung, and kidney are typically procured in respective order of priority.
The heart procurement surgeon will perform the harvest first. In short, after the sternotomy, the pericardium is opened and the right atrium cannulated and 1100 cc of blood drained into a blood collecting system (if using the OCS device); a cardioplegia cannula is then placed in the ascending aorta; the aorta cross clamped and cardioplegia infused; the left atrial appendage is vented; the pulmonary artery is then cannulated and the lung procurement surgeon will initiate the Perfadex lung flush as described earlier. The heart procurement will then proceed in standard fashion while the lung team performs bronchoscopy. During the donor cardiectomy, it is important for both heart and lung teams to communicate during division of the left atrium and pulmonary artery. More specifically, both teams need to ensure that they have adequate left atrial cuff and PA length prior to division.
Ex vivo lung perfusion
Ex vivo lung perfusion (EVLP) is a widely expand technology used to assess and prepare lungs that are considered marginal for transplantation. During EVLP, the lungs remain viable without additional injury. In a retrospective review from Toronto, extension of graft preservation beyond 12 h with EVLP does not affect early graft outcomes [14]. In other words, EVLP is attractive because preserving lungs longer makes transplant more elective than emergent.
The EVLP system consists of a ventilator, endotracheal tube, perfusion solution, reservoir, oxygenator, air filter, O2 sensor, and pump [15]. Currently, there are 4 commercialized devices for clinical EVLP available: The Organ Care System™ (OCS); XPS™ (XVIVO Perfusion AB); Lung Assist® (Organ Assist); and the Vivoline® LS1 [15].
If DCD lung procurement is being performed with the Transmedics OCS device, then they are not divided on the back table. Instead they are attached to the OCS device by cannulating the PA, and then connecting the airway using the supplied tracheal connector. The lungs are then ventilated. At least 3 units of uncross matched type O blood is required for the reservoir which is carried by the procurement team having issued it out of the recipient institution.
For the other available devices, most centers follow either the Toronto or Lund protocol. Both protocols are similar in that after cold pulmonary flush and lung harvest, the lungs are kept in static cold storage during transportation and then connected to the ex vivo device once they have arrived at the recipient institution [15]. The XVIVO system from Toronto requires a silicone cuff to be anastomosed to the left atrium (LA) (closed system) prior to initiation, while the other devices use an open circuit. The lungs are then re-animated, and then more fully evaluated regarding suitability for transplant.
Currently there are some centers experimenting with EVLP in combination with the proprietary CytoSorb adsorber (CytoSorbent, Monmouth Junction, NJ) in animal models. This is performed by introducing a solute adsorption membrane into the EVLP circuit which removes pro-inflammatory cytokines thought to be associated with primary graft dysfunction [16]. While there are some concerns that the adsorption membrane may also remove beneficial components or the perfusate, it is likely that additional studies will be utilized in the future [17].
Normothermic regional perfusion
During DCD procurement, organs are subjected to variable degree of hypotension and hypoxia during the agonal phase after withdrawal of life-sustaining therapy. While used mainly for procurement of the abdominal organs and the heart, normothermic regional perfusion is a technique that utilizes pre-mortem insertion of peripheral veno-arterial extracorporeal membrane oxygenation cannulae to prevent injury sustained during warm ischemic time.
In short, donors are transferred to the operative suite, intubated, and sedated. The chest and abdomen are prepared and draped. A bolus of heparin is administered before femoral venous-arterial cannulation is initiated. The patient is then connected to the extra corporeal membrane oxygenation (ECMO) circuit, and the circuit clamped. Withdrawal of life-sustaining treatment is then initiated. After the arrest period, a rapid sternotomy is performed, the pericardium opened, and the 3 aortic arch vessels clamped to exclude cerebral perfusion. Full flow veno-arterial-ECMO is then initiated for approximately 30 min before weaning [18].
Harvest for bronchial arterial revascularization (BAR)
Bronchial arterial revascularization is performed in some centers in order to reduce airway ischemia and improve airway healing [19]. In brief, the cold lung perfusate is administered in antegrade fashion through the pulmonary artery. The lungs are removed en bloc with the trachea and accompanying thoracic esophagus and descending aorta. This is done by stapling both the trachea and esophagus superior to the azygous vein. Inferiorly, the aorta is divided at the level of the diaphragm and the esophagus stapled at the level of the diaphragm after taking care to withdraw the nasogastric tube. The tissue block is removed with any accompanying tissue including paraspinal tissue to minimize any potential injury to the bronchial arteries [19]. On the back table in addition to the standard 1 l of Perfadex retrograde flush via the pulmonary veins, an additional 1 l is perfused via the descending thoracic aorta after clamping the upper part of the attached aorta—to help flush the bronchial arteries.
After arrival to the recipient operating room, the esophagus is removed sharply from surrounding mediastinal tissue. The descending aorta is then opened vertically in the midline on its pleural covered surface. Bronchial artery orifices are then identified and the lungs are implanted as described by Yun et al. [19].
Uncontrolled DCD lung donation
Uncontrolled DCD lung donation has been primarily reported in Spain with type II donors [20–22]. In short, after a witnessed cardiac arrest, the out-of-hospital emergency unit starts resuscitation maneuvers during transportation the emergency unit. The on call intensive unit personnel certifies death, and legal permission for organ preservation is requested from the judge on duty. The patient is then heparinized and connected to a veno-arterial ECMO circuit—some form of aortic clamping/endo-balloon is also usually inserted to prevent re-animation of the heart. Simultaneously bilateral chest tubes are inserted, and Perfadex solution is perfused at 4 °C for topical cooling while both mechanical ventilation and resuscitation are ceased. After the decision is made to proceed with organ retrieval, the topical cooling solution is drained, and ventilation is restarted. Bronchoscopic examination of the lungs to rule out injury is then performed. Next, the chest is opened and the lungs undergo antegrade perfusion with 5 to 6 l of Perfadex solution directed through the pulmonary artery. The lungs are further evaluated by instilling 300 cc of the donor’s blood through the pulmonary artery and performing blood gas analysis of the patient’s pulmonary veins. The lungs are then harvested. Centers in Spain accept a warm ischemic time of 120 min and maximum preservation time of 240 min from topical cooling to harvest [22]. Interestingly, approximately 40% of preserved uncontrolled DCD lungs that were previously harvested are discarded at the time of transplant due to poor gas exchange or sub-optimal macroscopic appearance [22]. The Madrid group retrospectively demonstrated a similar 1-year survival and increased percentage of primary graft dysfunction compared to controlled DCD lung donation [20].
DCD results
Over the last 1–2 decades, multiple centers have reported single-center experience associated with controlled DCD. In general, most centers have reported similar outcomes between lungs obtained from DCD donors and DBD donors. A summarized review of the results of multiple single-center studies has been completed by Ceulemans et al. [23].
In 2015, a meta-analysis and review of the literature demonstrated that there was no significant difference in 1-year survival between recipients of DCD and DBD donors. Similarly, there also appeared to be no difference in primary graft dysfunction rates [24]. The study was limited, however, by the fact that all of the included works were retrospective observational cohort studies. Additionally, even when pooled, the total number of DCD lungs included in the analysis was only 271.
A follow-up meta-analysis conducted in 2020 including 403 patients who received a graft from DCD donors and 2570 patients who received a graft from DBD persons demonstrated no difference in 1-year survival, grade 2–3 primary graft dysfunction, or chronic lung allograft dysfunction [25]. Conversely, the analysis also demonstrated an increased number of airway complications and lower pooled 5-year survival in the DCD group. The airway result was limited however by varying institutional definitions of airway complications and limited cohort size. Pooled 5-year survival analysis was likely influenced as a consequence of bias in lung allocation with the possibility that long-term survival was more likely influenced by pre-existing recipient condition [25].
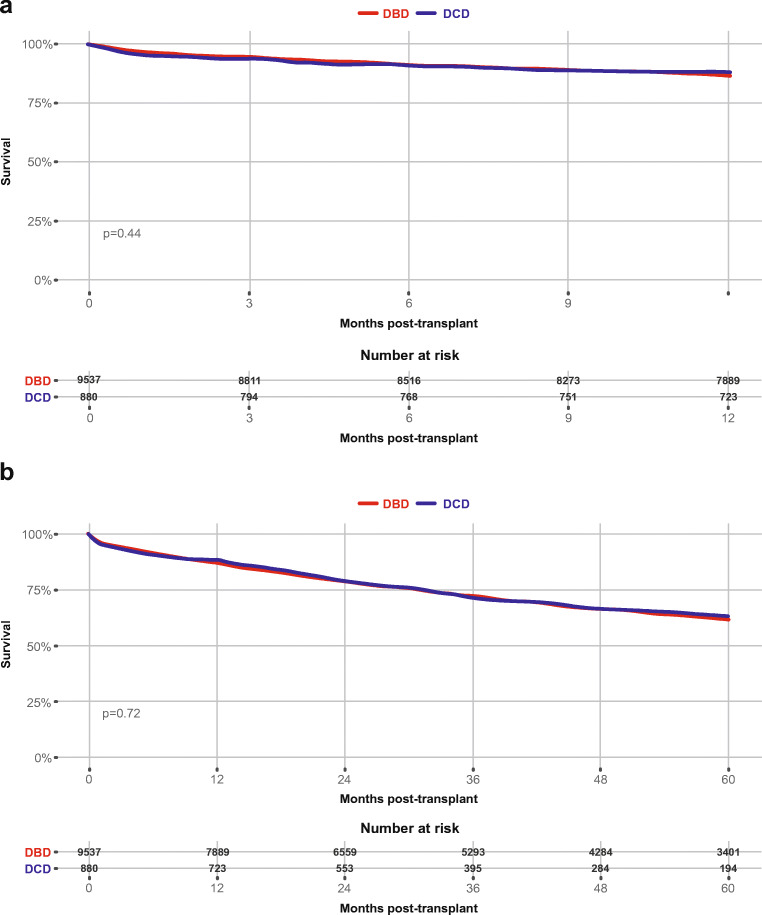
Van Raemdonck et al. [26] recently reported a 5-year follow-up analysis of the International Society for Heart and Lung Transplantation (ISHLT) registry. All patients transplanted between January 2003 and June 2017 from 22 participating centers across multiple continents were included. The study cohort included 11,516 lung transplants, of which 1090 (9.5%) were DCD (Fig. 2). There was no significant difference found in 5-year survival rates between controlled DCD and DBD lung recipients.
Fig. 2.
a One year and b 5-year post transplant survival in DCD vs. DBD groups. Transplants performed between January 2003 and June 2016 are used for survival calculations. Only Maastricht category III donors induced in the DCD group. Used with permission from Elsevier [26]
Summary
Donation after circulatory death for lung transplantation is now being performed to increase the number of lungs available. Most centers perform DCD lung procurement with controlled category III donors. Communication between the lung procurement surgeon, anesthesiology team, and other procurement teams is necessary to avoid potential pitfalls during their procurement. Multiple devices such as ECMO and EVLP are currently being investigated to help facilitate organ retrieval and reduce warm ischemic time. DCD organ donation is non-inferior in terms of survival, or primary graft dysfunction.
Funding
Nil.
Declarations
Ethical approval
NHR (non-human research) determination letter from UKY attached.
Informed consent
Not required.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sade RM. Brain death, cardiac death, and the dead donor rule. J S C Med Assoc. 2011;107:146–149. [PMC free article] [PubMed] [Google Scholar]
- 2.Inci I. Donors after cardiocirculatory death and lung transplantation. J Thorac Dis. 2017;9:2660–2669. doi: 10.21037/jtd.2017.07.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panchabhai TS, Chaddha U, McCurry KR, Bremner RM, Mehta AC. Historical perspectives of lung transplantation: connecting the dots. J Thorac Dis. 2018;10:4516–4531. doi: 10.21037/jtd.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy JD, Webb WR, Dalton ML, Jr, Walker GR., Jr Lung homotransplantation in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 5.Magovern GJ, Yates AJ. Human homotransplantation of left lung: report of a case. Ann N Y Acad Sci. 1964;120:710–728. doi: 10.1111/j.1749-6632.1965.tb30696.x. [DOI] [PubMed] [Google Scholar]
- 6.Egan TM, Lambert CJ, Jr, Reddick R, Ulicny KS, Jr, Keagy BA, Wilcox BR. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg. 1991;52:1113–1120. doi: 10.1016/0003-4975(91)91290-C. [DOI] [PubMed] [Google Scholar]
- 7.D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Controlled non-heart-beating donors: a potential source of extrarenal organs. Transplant Proc. 1995;27:707–709. [PubMed] [Google Scholar]
- 8.Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893–2894. [PubMed] [Google Scholar]
- 9.Erasmus ME, van Raemdonck D, Akhtar MZ, et al. DCD lung donation: donor criteria, procedural criteria, pulmonary graft function validation, and preservation. Transpl Int. 2016;29:790–797. doi: 10.1111/tri.12738. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez PG, Rouse M, Pratt DL, et al. Lung donation after controlled circulatory determination of death: a review of current practices and outcomes. Transplant Proc. 2015;47:1958–1965. doi: 10.1016/j.transproceed.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Levvey B, Keshavjee S, Cypel M, et al. Influence of lung donor agonal and warm ischemic times on early mortality: Analyses from the ISHLT DCD Lung Transplant Registry. J Heart Lung Transplant. 2019;38:26–34. doi: 10.1016/j.healun.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Oto T, Levvey B, McEgan R, et al. A practical approach to clinical lung transplantation from a Maastricht Category III donor with cardiac death. J Heart Lung Transplant. 2007;26:196–199. doi: 10.1016/j.healun.2006.11.599. [DOI] [PubMed] [Google Scholar]
- 13.Nemeh HM, Madoun N. Donor procurement. In: Vigneswaran WT, Garrity ER, Odell JA, editors. Lung transplantation: principles and practice. Boca Raton: CRC Press; 2016. pp. 105–109. [Google Scholar]
- 14.Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med. 2017;5:119–124. doi: 10.1016/S2213-2600(16)30323-X. [DOI] [PubMed] [Google Scholar]
- 15.Makdisi G, Makdisi T, Jarmi T, Caldeira CC. Ex vivo lung perfusion review of a revolutionary technology. Ann Transl Med. 2017;5:343. doi: 10.21037/atm.2017.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iskender I, Arni S, Maeyashiki T, et al. Perfusate adsorption during ex vivo lung perfusion improves early post-transplant lung function. J Thorac Cardiovasc Surg. 2021;161:e109–e121. doi: 10.1016/j.jtcvs.2019.12.128. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Krupnick AS. Commentary: Tis the season to filter your perfusate. J Thorac Cardiovasc Surg. 2021;161:e127–e128. doi: 10.1016/j.jtcvs.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Tsui SSL, Oniscu GC. Extending normothermic regional perfusion to the thorax in donors after circulatory death. Curr Opin Organ Transplant. 2017;22:245–250. doi: 10.1097/MOT.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 19.Yun JJ, Unai S, Pettersson G. Lung transplant with bronchial arterial revascularization: review of surgical technique and clinical outcomes. J Thorac Dis. 2019;11:S1821–S18s8. doi: 10.21037/jtd.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26:529–534. doi: 10.1016/j.healun.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-de-Antonio D, Varela A. Non-heart-beating donation in Spain. Gen Thorac Cardiovasc Surg. 2011;59:1–5. doi: 10.1007/s11748-010-0661-4. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant. 2012;31:349–353. doi: 10.1016/j.healun.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Ceulemans LJ, Inci I, Van Raemdonck D. Lung donation after circulatory death. Curr Opin Organ Transplant. 2019;24:288–96. [DOI] [PubMed]
- 24.Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34:675–684. doi: 10.1016/j.healun.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Palleschi A, Rosso L, Musso V, Rimessi A, Bonitta G, Nosotti M. Lung transplantation from donation after controlled cardiocirculatory death. Systematic review and meta-analysis. Transplant Rev (Orlando). 2020;34:100513. [DOI] [PubMed]
- 26.Van Raemdonck D, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant. 2019;38:1235–1245. doi: 10.1016/j.healun.2019.09.007. [DOI] [PubMed] [Google Scholar]