Abstract
Background:
Full thickness burn wounds are lack of angiogenesis, cell migration, epithelialisation and finally scar tissue formation. Tissue engineered composite graft can provide sustained release of growth factor and promote the wound healing by cell migration, early angiogenesis and proliferation of extracellular matrix and wound remodeling. The objective of this study was to evaluate the gene embedded (pDNA-platelet-derived growth factor, PDGF-B) porcine acellular urinary bladder matrix with transfected mesenchymal stem cells (rBMSC) on healing of full thickness burn wound in rat model.
Methods:
Full thickness burn wound of 2 × 2 cm size was created in dorsum of rat model under general anesthesia. Burn wounds were treated with silver sulfadiazine; porcine acellular urinary bladder matrix (PAUBM); PAUBM transfected with pDNA-PDGF-B; PAUBM seeded with rBMSC; PAUBM seeded with rBMSC transfected with pDNA-PDGF-B in groups A, B, C, D and E respectively. The wound healing was assessed based on clinical, macroscopically, immunologically, histopathological and RT-qPCR parameters.
Results:
Wound was significantly healed in group E and group D with early extracellular matrix deposition, enhanced granulation tissue formation and early angiogenesis compared to all other groups. The immunologic response against porcine acellular matrix showed that PDGF-B gene activated matrix along with stem cell group showed less antibody titer against acellular matrix than other groups in all intervals. PDGF gene activated matrix releasing the PDGF-B and promote the healing of full thickness burn wound with neovascularization and neo tissue formation. PDGF gene also enhances secretion of other growth factors results in PDGF mediated regenerative activities. This was confirmed in RT-qPCR at various time intervals.
Conclusion:
Gene activated matrix encoded for PDGF-B protein transfected stem cells have been clinically proven for early acceleration of angiogenesis and tissue regeneration in burn wounds in rat models.
Graphic abstract
Evaluation of PDGF-B gene-activated acellular matrix and mesenchymal stem cell in full thickness skin burn wound in rat. 
Keyword: Acellular matrix, PDGF-B, Gene therapy, Stem cell, Burn wound
Introduction
Severe burn injury causes devastating damages with systemic complication. Full thickness burn wound will not heal spontaneously which need early removal and adequate treatment to prevent loss of fluids and risk of infection [1]. Major burn wound patient are lack of full skin coverage and the currently used autologous skin graft and transplanted donor skin graft are not an efficient option due to their non availability, donor site morbidity and graft rejection [2]. The novel tissue engineered bioactive materials present potential tissue regeneration and avoid the tissue deterioration.
The extracellular matrix (ECM) can be derived from various tissue or organs of different species but most commonly, small intestine and urinary bladder of porcine origin have been used successfully as scaffold for reconstruction [3, 4]. ECM is obtained by process of decellularization, during the process of decellularization, parenchymal cells and endothelial cells are destroyed/ removed [5]. The residual ECM conserves their biological activity, glycoproteins, proteoglycans and growth factors [6, 7]. These conserved bioactive molecules have great potential for repair of the tissues and hence decellularized matrix is a prospect for tissue engineering [8].
The burn wounds are likely to cause hyper tropic scaring when increasing contact time [9]. Various levels of burns injury may cause additional damage to vessels, which results in more tissue damage. Therefore, vascularization of burn wound is very tough compared to the incisional wounds.
Tissue engineering strategies will be successful depending on the extent of vascularization in to the scaffold [10]. The main role of blood vessel is to deliver the nutrients and oxygen to the transplanted cells in the scaffold and cells migration to the host tissue. So far, use of angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and platelet-derived growth factor beta (PDGF-B) etc. have been widely employed to establish angiogenic response [11, 12]. However, use of growth factor in practice is limited because of short half-life, protein instability, large dose required, the need for repeated administration and a high cost [13]. In order to overcome the disadvantages, slow release of the growth factors, incorporation of DNA into the matrices which is called as gene-activated matrices (GAMs), has been used to make depot for localized expression and also as a scaffold for new tissue growth [14]. The GAMs have been used widely to support restoration of several tissues such as skin, cartilage and bone [15, 16].
Cutaneous wound healing occur in precise and regulated manner with integration of multifaceted events movement and large number of cell, as well as collagen ingrowth, new vessel ingrowth and remodeling [17]. Bone marrow stromal cells can produce higher amounts of collagen, VEGF and bFGF, thus can be used for wound healing at a faster rate [18, 19].
Therefore, the study was intended to investigate the therapeutic efficacy of gene (pDNA-PDGF-B) transfected mesenchymal stem cells embedded porcine acellular matrix on healing of full thickness skin burn wound in rat model.
Materials and methods
Cloning of rat platelet-derived growth factorB (PDGF-B)
Total RNA was isolated from rat peripheral blood monolayer cells (PBMC) using TRIzol® reagent (Amresco, Cleveland, OH, USA) as per the manufacturers’ instructions. Purity and quantity of RNA was analyzed using Nano Drop2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Once the RNA was isolated it treated with DNAse 1(# ENo521, Thermo Scientific) to remove any DNA contamination and complementary DNA was prepared with cDNA synthesis kit (# K1622, Thermo Scientific). Using specific primers forward; 5′-TTAAGCTTGAGTCGGCATGAATCGCTG-3′ (HindIII) and reverse 5′ CTGAATTCTCACTCTCGCTGACAC-3′ (EcorI) PDGF-B gene was amplified from c DNA in polymerase chain reaction (PCR). The amplified PCR product was analyzed using 1% agarose gel electrophoresis with 1 kb ladder and viewed under UV transiluminator. PCR amplification of PDGF-B gene resulted in an amplicon of 751 bp in size. The PCR product was gel purified using Gene JET Gel extraction kit (#K0692, Thermo Scientific) as per the manufactures’ instructions. Then the pVAX1 vector and gel purified PDGF-B DNA fragment were digested with HindIII and EcoRI restriction enzymes and ligated with T4 DNA ligase enzyme. Transformation of ligation mixture in E. coli top 10 competent cells was done as per the method described by Green and Sambrook, [20] with certain modifications and the resulting recombinant plasmid was screened in colonies by colony PCR, restriction enzyme digestion and sequencing analysis.
Transfection of pVAX1-PDGF-B gene plasmid with rBMSC
Bone marrow was obtained from femur and tibia of adult wistar rat. Isolation, expansion, characterization and differentiation studies were performed as per the protocol developed in our lab [21, 22]. The 3rd passage rat bone marrow mesenchymal stem cells (rBMSC) were used after attaining 40–80% confluence. The volume of lipofectamine and DNA was standardized after trying various combinations. Briefly, 20 μl of lipofectamine 3000 reagent and 10 μg of pVAX1-PDGF-B reagent was added in 250 μl of serum free media (DMEM) in separate tube and mixed thoroughly. Subsequently, both lipofectamine and DNA was prepared by mixing and incubated for 30 min at RT to form DNA lipofectamine complexes. During the incubation period, the media was decanted from the cell culture flask and cells were rinsed with serum free DMEM. Then the DNA-lipofectamine complex was added at a regular pace to the each flask, and additionally desired volume of serum free DMEM was added to make up the total media in a well around 6 ml. Then the flask was incubated at 37 °C in a 5% CO2 for 4 h with occasional mixing by rotating the plate every 1 h interval. After 4 h of incubation the serum free media was poured out and growth media (DMEM with 10% FBS) was added. After 48, 96 and 120 h of incubation, flask was rinsed with DPBS and trypsinized with 0.25% Trypsin–EDTA solution. Then trypsin activity was blocked with complete media with serum and cells were centrifuged at 960 rpm for 4 min to make cell pellet. Cells treated with naked uncomplexed plasmids DNA were served as control. Protein was isolated from the transfected cell pellet and measured by brad ford method [23].
Expression of PDGF-B by western blotting analysis
The expressed recombinant proteins were evaluated under denaturing conditions of SDS-PAGE, described by Laemmli [24] with certain modifications. The gel was documented and stored in 10% acetic acid solution. The protein was transferred to nitrocellulose membrane using Transblot-SD electrophoresis transfer cell (Bio-Rad) and the expression was confirmed by Western blotting as per the standard protocol explained by Manjunathachar, et al. [25].
Preparation and evaluation of porcine acellular urinary bladder (PAUB) scaffold
Preparation of acellular porcine bladder
The porcine bladders samples were collected from a local stockyard within 4 h of slaughter and adhered fat, luminal surface the urothelium, fascia of outside bladder was entirely removed. Remaining portions were subjected to incubation overnight in ice-cold hypotonic Tris buffer (10 mMTris, pH 8.0) containing 5 m—methylene diamine tetracetic acid (EDTA). After three rinses with ice-cold PBS, the specimens were incubated for 24 h at 37 °C with physical agitation (orbital shaker 300 rpm) in containing 1% Sodium dodecyl sulfate (SDS) ionic detergent. After washing with detergents, the bladder tissues were rinsed thrice with ice-cold PBS. Followed by, the samples were transferred to the enzymatic solutions containing 50 U/mL type I DNase, 1 U/mL RNase (Merck Bioscience, Darmstadt, Germany), 0.02% EDTA and 1% penicillin/ streptomycin/ fungizone (P/S/F), in PBS 3 hr at 37 °C with physical agitation to (Orbital shaker 300 rpm) remove any remaining cellular material. Bladder was sterilized with ethanol for 3h and washed in PBS 3 times. Finally the biomaterials were stored in PBS (PH 7.4) at − 20 °C for further use.
Histology
The acellular and native samples were stored in 10% formalin and stained with Haematoxylin and Eosin (H&E) stain as per the referred protocol [26]. Collagen staining was done using Masson’s trichrome stain [27].
DNA quantification
The acellular and native samples were rinsed with double distilled water for 5 minutes and powdered by pouring liquid nitrogen. DNA was extracted by using Genomic DNA isolation Kits (Thermo fisher) according to the instruction provided by the manufacturer.
Sterility assessment
Sterility of samples were tested by incubated in media containing (Dulbecco’s Modified Eagles Medium) 10% fetal bovine calf serum for 4 weeks at 37 °C. Any turbidity or discoloration of the medium was taken as contamination.
Fabrication of gene-activated matrix (GAM)
To assist the complete incorporation of pVAX1-PDGF-B gene transfected stem cell complex into porcine acellular matrix, scaffold was kept in serum free media for 4 h in 6 well plates. Once the serum free media was decanted, gene transfected stem cells complexes were seeded with 2.5 × 106 rBMSC with complete media on each side of the graft and kept for 15 min. Before implantation in to the animal’s gene transfected stem cell matrix was maintained for 3 days in complete media.
In vivo implantation of gene-activated matrix in rat full thickness burn wound model
The study was designed after getting permission from the Institute Animal Ethics Committee (IAEC). Seventy five clinically healthy male Wistar rats (Ratus norvegicus), weighing (200–300gm) were utilized for this procedure. Animals were procured from Laboratory Animal Resource (LAR) section of the institute. The animals were acclimatized to their environment 7 days prior to study. The animals were anaesthetized with xylazine at 5 mg/kg and ketamine HCl at 50 mg/kg intra peritoneal. Contact burn was inflicted by applying a heated custom-made steel bar (2 × 2 cm size) (Fig. 1) on the dorsum of the animal after aseptic preparation of the site. Briefly, the bar was heated in flame at constant temperature of 130 °C, placed on the dorsum of the animal for 30 s at constant pressure. Temperature was measured in thermocouples. After infliction of burn wound, the animals were received intraperitoneal injection of ringers lactate 60 ml/kg to resuscitate the fluid loss. To simulate the clinical condition, the necrosed tissue around the burn wound was removed up to the underlying muscular fascia 72 h of after injury. The normal size of the ensuing defects was 2 cm. The burn wound was replaced by the same size of the scaffold as shown in Table 1. After filling the wound with the predefined material, the edge of the scaffold was sutured with surrounding skin layer with 3–0 polyamide suture in interrupted pattern. To protect the burn wound from infection and sepsis non adhesive pressure bandage was applied. Antibiotic ceftriaxone 5 mg /10 ml of water and anti-inflammatory drugs butorphanol 0.5 mg/kg subcutaneous route was given for 5 days postoperatively. Dressing of suture line with povidone iodine was done after first week of surgery and in control group animals were dressed with silver sulfadiazine ointment. The efficacy of the porcine AUBM on renovation of 3rd degree burn wound defects was assessed based on the following parameters.
Fig. 1.
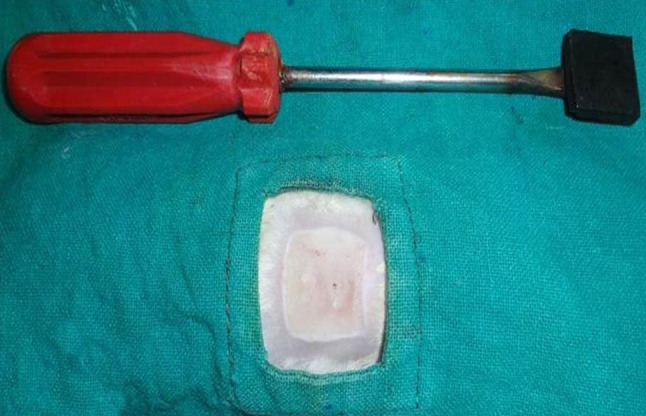
Custom made iron rod (2 × 2 cm) used to produce standard burn
Table 1.
Experimental protocol for in-vivo testing of gene-activated matrices (GAM)
| S. No | Group | Number of animals | Experimental model | Type of scaffold |
|---|---|---|---|---|
| 1 | Group A (control) | 15 | 2 × 2 Full thickness burn wound | Silver sulfadiazine ointment |
| 2 | Group B | 15 | 2 × 2 Full thickness burn wound | Porcine acellular urinary bladder matrix (PAUBM) |
| 3 | Group C | 15 | 2 × 2 Full thickness burn wound | PAUBM fabricated with pDNA-PDGF-B |
| 4 | Group D | 15 | 2 × 2 Full thickness burn wound | PAUBM seeded with rBMSC |
| 5 | Group E | 15 | 2 × 2 Full thickness burn wound | Gene pDNA-PDGF-B transfected rBMSC embedded in PAUBM |
Colour digital imaging
A colour photograph was taken on 0, 3, 7, 14, 21 and 28 days and was analyzed for healing status.
Evaluation of wound size
It was performed on same intervals 3, 7, 14, 21 and 28 days and percentage wound size was measured as per the standard procedure described by Schalleberger et al. [28].
Histological observations
The biopsy specimen from 3 animals in each group was collected on 7, 14, 21 and 28 post implantation days after humane method of euthanizing using thiopentone sodium and decapitation for histopathological evaluation. The biopsy specimens were fixed in 10% neutral buffer formalin (NBF). The 5 micron sections were stained by hematoxylin and eosin to evaluate the healing process. The section was observed under light microscope for epithelialisation, inflammation, fibroplasia and new vessels ingrowth. The scoring was done according to Borena et al. [29] as mentioned in Table 2. The section was also put through to Masson’s Trichrome staining to note the orientation of collagen.
Table 2.
Scoring system for histopathological observations
| No | Parameters | Score |
|---|---|---|
| 1 | Epithelialization |
1—Present 2—Partially present 3—Absent |
| 2 | Inflammation |
1—Resembling normal skin 2—Mild 3—Moderate 4—Severe |
| 3 | Fibroplasia |
1—Resembling normal skin 2—Mild 3—Moderate 4—Severe |
| 4 | Neovascularization |
1—Resembling normal skin (0–1 new blood vessel) 2—Mild (2–5) 3—Moderate (6–10) 4—Severe (> 10) |
| 5 | Collagen fiber density |
1—Denser 2—Dense 3—Less dense |
| 6 | Collagen fiber thickness |
1—Thicker 2—Thick 3—Thin |
| 7 | Collagen fiber arrangement |
1—Best arranged 2—Better arranged 3—Worse arranged 4—Worst arranged |
Indirect ELISA
Hyper immune serum was raised for native porcine bladder antigen. The pre immune (day 0) serum sample from implanted rat was kept as a negative control and 45th day sample was kept as positive serum by which the standard curve was drawn and the antigen antibody concentration was fixed for further analyzing the implanted animal serum. Around 0.5 ml blood was collected from the orbital sinus of the test animals at days 0, 3, 7, 14, 21 and 28 in sterile vials. ELISA was performed to estimate antibodies that specifically interact with native porcine bladder antigen, wherein the humoral response of the host was assessed exact to the biomaterials. The test was done as per the standard protocol.
Real-time quantitative PCR (RT-qPCR) analysis for angiogenesis
Total RNA was isolated from tissue using TRIzol® reagent in tissue homogenizer and cDNA was amplified using RevertAid First Strand cDNA Synthesis Kit using oligo (dT) 18 primers as described earlier. Real-time PCR of various genes (Table 3) was carried out using Maxima SYBR Green/ROX qPCR Master Mix (2 ×) (#K0222, Thermo Scientific) on 7500 Real Time PCR System (ABI USA Group, Vernon, CA, USA) for 40 cycles. To rule out genomic contamination if any, non-template control (NTC) was included in the reaction for each pair of primers. GAPDH was used as an endogenous (housekeeping gene) control against which different template values were normalized.
Table 3.
Gene specific primers used in the real time quantitative polymerase chain reaction RT-qPCR
| Name of the gene | Sequence | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| VEGF |
For 5′-GGCTTTACTGCTGTACCTCCACCAT-3' Rev 5′-CGGGGTACTCCTGGAAGATGTC-3' |
161 | 60 |
| PDGF |
For-5′-TGGAGTCGAGTCGGAAAGCT-3' Rev 5′-GAAGTTGGCATTGGTGCGAT-3 |
148 | 60 |
| α-SMA |
For 5′-CCAGGGAGTGATGGTTGGAATG-3' Rev 5′-GCCGTGTTCTATCGGATACTTCAG-3 |
98 | 62 |
| GAPDH |
For 5′-GTAGCCATATTCATTGTCATACCAG-3' Rev 5′-CTTCAACAGCAACTCCCATTC-3' |
100 | 60 |
Statistical analysis
The data was analyzed using Statistical Program for Social Sciences (SPSS) software. To compare the means at different time intervals between groups, Duncan’s multiple range test (DMRT) was done. Student’s paired t test was performed for comparing the mean values between different time intervals within a group. Kurskal Wallis test was done for subjective data of various group compared between groups. Wilcoxan signed rank test was used to compare subjective data from scoring of various parameters between the groups [30] to find out the amount of significance. A value of p < 0.05 was considered to be statistically significant.
Results
Development of gene constructs
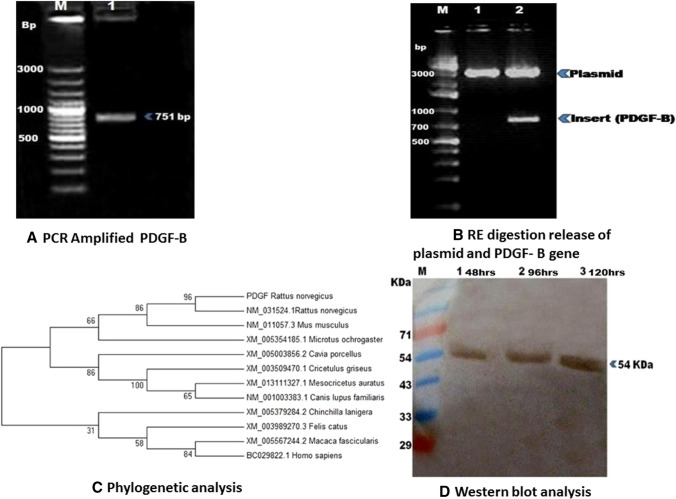
The PDGF-B gene was synthesized from cDNA and amplified product (751 bp in size) was confirmed on 1% agarose gel electrophoresis (Fig. 2A). The gel purified PDGF-B cDNA fragment and pVAX1 vector were digested with restriction enzymes (RE) and ligated. Recombinant clones were verified by colony PCR (751 bp product) and also insert release using the RE enzymes (Fig. 2B). Further, sequencing results showed that insert was in proper orientation and in frame with the vector. The sequencing results were submitted to Gen-Bank, NCBI and accession number was obtained (KX646428). Rattus norvegicus and homologous sequence available in the Gen-Bank were aligned and phylogenetic tree was constructed using Mega 7 software. Phylogenetic analysis revealed that PDGF-B has > 99% sequence identity with published gene of Rattus norvegicus at nucleotide and protein level (Fig. 2C).
Fig. 2.
A Agarose gel electrophoresis of platelet-derived growth factor beta, PDGF-beta (751 bp) was amplified from rat (Rattus norvegicus) peripheral blood mononuclear cells. M, 100 bp plus DNA ladder; Lane 1, PDGF-beta DNA fragment. B Confirmation of recombinant pVAX1-PDGF-B insert release by RE digestion: M, 1 kb plus DNA ladder; Lane 1, Intact Pvax1-PDGF-B, Lane 2, RE digestion with HindIII and EcoRI releases specific 751 bp insert. C Phylogenetic analysis of rat PDGF-B. D Western blotting of purified PDGF-B. Purified recombinant PDGF-B (954 kDa) were confirmed using polyclonal PDGF-B antibodies. M, Pre-stained marker (250 KDa); Lane 1: 48 h; Lane 2: 96 h and Lane 3:120 h recombinant PDGF-B
In-Vitro expression of PDGF-B Gene on rat BMSCs cells
Expression of PDGF-B protein was confirmed by western blot analysis at 48, 96 and 120 h (Fig. 2D). pVAX1-PDGF-B vector encodes 241 amino acid 27 kDa molecular weight and under non denaturing conditions there was dimer formation of 54 kDa.
Decellularization of porcine acellular urinary bladder (PAUB)
Histological evaluation of PAUB
Cells were completely removed leaving the extracellular matrix such as collagen and glycosaminoglycan’s. Matrices were devoid of urothelium when compared to native bladder (Fig. 3A), and tissue was free from cells (Fig. 3B). The collagen fibers bundles were uniformly and closely packed in the native tissue (Fig. 3C). Cell removal was further confirmed absence of cytoplasmic staining by Masson’s trichrome stain (cytoplasm stains pink and collagen blue) (Fig. 3D).
Fig. 3.
Photomicrographs showing H&E and Masson’s trichrome staining of native and decellularized PAUB. A Native porcine bladder, nuclei present in the cells; B complete decellularization of porcine bladder with intact matrix (Scale bar 100 µm); C, D Masson’s Trichrome stain, C native D decellularized tissue showing collagen fibers were thin and arranged regularly
DNA quantification
Native tissue prepared using PBS showed the greatest concentration of DNA (p < 0.05) with 85.73 ± 4.19 ng /μl compared to decellularization with ionic detergent 4.4 ± 0.70 (Fig. 4), implying that ionic detergent resulted in high efficiency of decellularization.
Fig. 4.
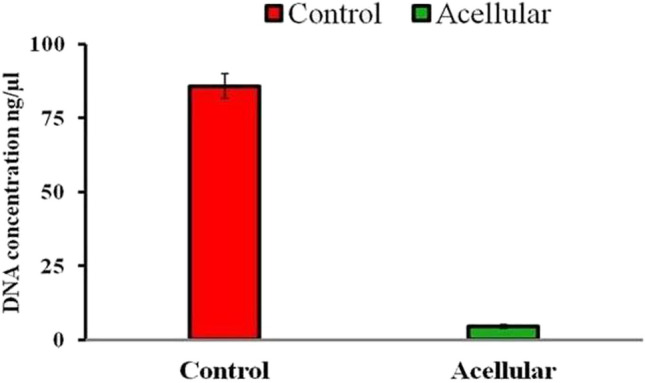
Mean ± SE values of DNA content (ng/µl) after decellularization porcine urinary bladder
Sterility assessment
There was no bacterial and fungal contamination noticed after 5 days incubation in complete media. Further, there was no change in media pH as indicated by absence of turbidity.
Clinical evaluation
The anaesthetic regimen, xylazine and ketamine combination provide safer surgical anaesthesia for about 20–25 min in rats. All the group animals were depressed on the first day assuming a hunched back posture while sitting on their cages. All the animals of different groups had limited quantity of feed and water within 24 h of post-surgery and became normal by 3rd post-operative day. Four animals died due to dehydration and were excluded from the study.
Evaluation of wound size
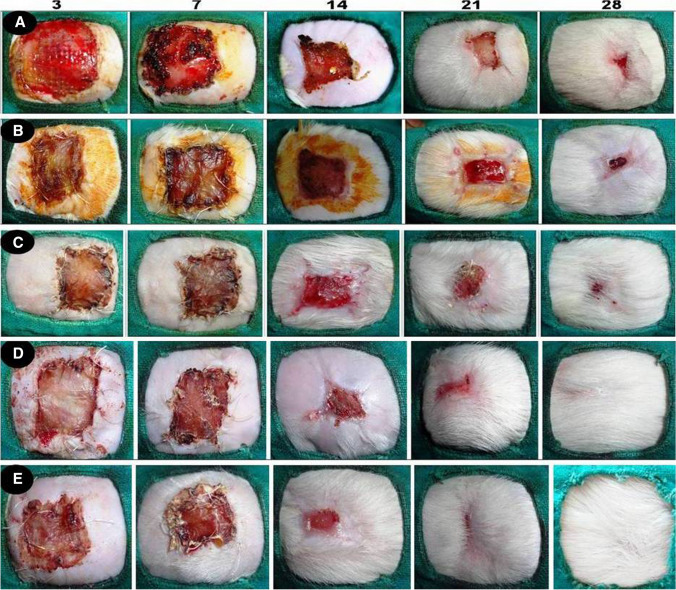
Mean ± SE values of wound size percentage in different groups of animals are presented in Table 4. Significant increase in wound healing was observed after 7 days in all the groups. In groups D and E, wound was almost healed at day 21 but complete healing was noticed at day 28. In other groups, complete healing was not observed even after day 28 post-surgery. There was significant difference noticed between groups of A and B compared to groups C,D and E on 28th day.
Table 4.
Mean ± SE values of wound size (%) in different groups at different time intervals (days)
| Groups | 3Day | 7 Day | 14 Day | 21 Day | 28 Day |
|---|---|---|---|---|---|
| Group A | 19.29 ± 2.70aA | 33.02 ± 2.99bA | 70.44 ± 2.34cAB | 88.72 ± 1.51dA | 93.64 ± 0.49dA |
| Gorup B | 23.30 ± 7.28aA | 38.90 ± 4.85abA | 51.73 ± 7.10bA | 82.23 ± 3.28cA | 92.90 ± 1.50cA |
| Group C | 32.73 ± 5.69aA | 39.20 ± 5.03abA | 58.63 ± 8.88bAB | 84.02 ± 3.26cA | 98.70 ± 0.92cB |
| Group D | 17.30 ± 4.18aA | 43.93 ± 9.47bA | 83.70 ± 4.38cB | 90.20 ± 4.50cA | 100.00 ± 0.00cB |
| Group E | 23.17 ± 7.97aA | 49.53 ± 1.80bA | 75.30 ± 1.45cAB | 94.43 ± 1.44dA | 100.00 ± 0.00dB |
abcd—means bearing superscript in a row indicate significant difference (p < 0.001) in wound size of particular graft for different time intervals. AB—means bearing different superscript in a column indicate the significant difference (p < 0.001) in the wound size at same time intervals of particular graft
Color digital imaging
In all the 5 groups, there was no evident of inflammatory reaction like infection, swelling around the wound margin. At first week of post-operative days graft was looking pink and moist in groups C, D and E, compared to other groups where the implanted graft edges were dry (Fig. 5). At second week post implantation, the wound contraction was increased gradually and granulation tissue growth was noticed under the graft. This was more progressive in groups C, D and E compared to A and B. Further graft merged with granulation tissue and superficial layers of graft shrinked exposing the boundaries of healing margins in groups D and E. From 3rd to 4th week the wound was completely healed and there was no scar formation noticed in groups D and E. This process was not obvious in other groups where the graft was dry and sloughed off. The wound was completely healed and became indistinguishable between normal and grafted one in groups D and E (Fig. 5).
Fig. 5.
Digital photograph showing the gross morphology of the burn healing wounds in different groups (A, B, C, and D, E) at different time interval
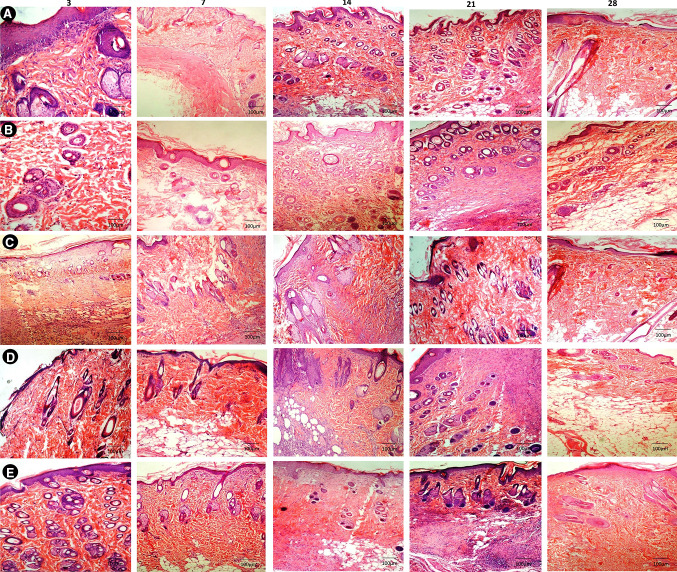
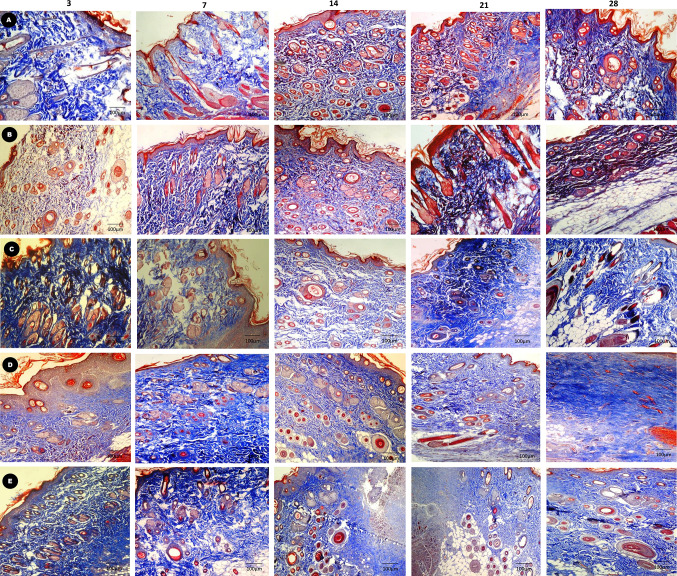
Histological observations
The histopathological examinations of various graft implanted groups are shown in Fig. 6 and 7. On day 3–7 of implantation, graft was easily remarkable from the host tissue in all the groups. Moderate amount of inflammatory cells such as neutrophil and macrophages were noticed at this stage. In groups D and E, papillary layer and reticular cell formation were noticed from edge of the wound margin and graft epidermal margin was thicker. There was moderate to severe fibroplasia and neovascularization which was mainly restricted to the periphery of acellular matrix and the extracellular matrix deposition was observed in the wound margin (Fig. 6D, E). Collagen fibers were present near the graft side and thicker irregularly arranged in nature compared to group A, B and C where the collagen deposition was thin and sparsely arranged (Fig. 7D, E). Total score was 16 in group D and 15 in group E. In groups A, B & C, moderate amount of inflammatory cells and fibroplasias were noticed. Fibroblast was mainly seen in the wound margin and less collagen deposition noticed (Fig. 6A–C). On day 14, the degree of inflammatory cells were reduced more prominently and graft was closely integrated with the host tissue except in groups A, B & C (Fig. 6 A–C). The four layers (horny layer, basal layer, granular layer and germinal layer) and keratosis area of epidermis was observed clearly. The thickness and shape of them seems to be normal. Progressive increase in vascularity by appearance of more newly formed capillaries together with deposition of more collagen rather than inflammatory cells was observed in groups D and E (Fig. 6 D, E). Collagen fiber deposition was noticed throughout the scaffold in groups D and E and near the surface in other groups (Fig. 7 D, E). Total histological score was 14 in group D and 12 in group E. On day 21, group E showed cells filling the ECM (Extra cellular matrix) and newly formed capillaries were more prominent and which was not clear in other groups (Fig. 6D). The papillary layer and reticular layer became thicker continuously in groups D and E and epithelialization was not complete in other groups. Collagen fibrils were arranged more homogenous and ordered in groups D and E compare to other groups (Fig. 7 D, E). Total histological score was 10 and 12 in groups D and E respectively (Table 5).
Fig. 6.
Photomicrograph showing H&E staining of groups (A, B, C, and D, E) burn healing wounds at different time interval (scale bar 100 µm)
Fig. 7.
Photomicrograph showing Masson’s trichrome staining of groups (A, B, C, and D, E) burn healing wounds at different time interval (Scale bar 100 µm)
Table 5.
Histopathological scores in different groups at different time intervals
| Treatment groups | 3 Day | 7 Day | 14th Day | 21st Day | 28th day |
|---|---|---|---|---|---|
| Group A | 19 | 17 | 16 | 15 | 12 |
| Group B | 19 | 18 | 16 | 13 | 11 |
| Group C | 19 | 18 | 17 | 14 | 10 |
| Group D | 18 | 16 | 14 | 12 | 9 |
| Group E | 15 | 14 | 12 | 10 | 8 |
From day 28th onwards, the gene matrix at this stage completely resorbed and replaced with more abundant newly formed tissue. The regeneration of hair follicle and sebaceous gland was observed at this stage in group E and less in group D compared to all other groups. There was clear demarcation between epidermis and dermis in groups E and D and outline were difficult to discriminate from host tissue (Fig. 6D, E). Especially in group E newly formed tissue was well integrated with host surrounding tissue. Collagen was more abundant and regularly oriented in groups E and D whereas heterogeneous deposition was noticed in all other groups (Fig. 7A–E). The histological score was reduced to 8 at this stage in group E and high in all other group.
Indirect ELISA
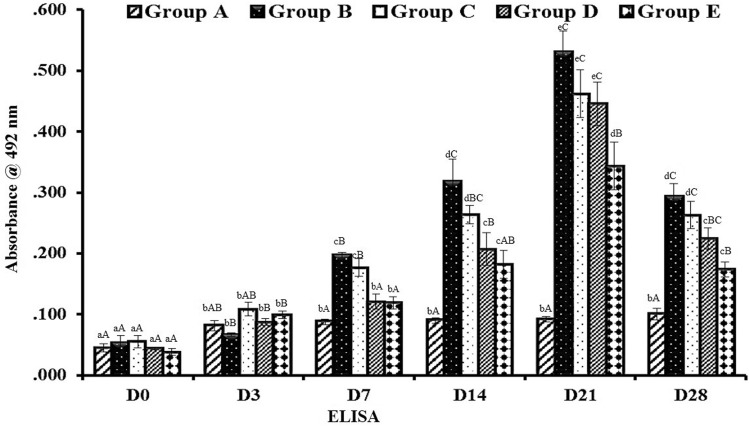
The concentration of antigen and antibody was selected after standardization and it was 1:40 and 1:320 dilutions, respectively. The implant specific antibodies were expressed as mean ± SE of absorbance values at 495 nm wavelength (OD 495) for various graft which is presented in (Fig. 8). In group A (control), absorbance value remained unchanged throughout the period of observation because no grafting was done in this group. In all the other groups, there was comparable rise in antibody titer from 0 to day 21 post implantation. Thereafter, progressive decline was noticed. The antibody titer of groups B, C, D and E significantly increased from day 7 to day 21. Comparison between the groups at different time intervals revealed that group B animals showed a relatively higher antibody titer (p < 0.05) from day 14th up to the end of observation period as compared to all other groups. However, group E animals showed an antibody production at the lowest level as compared to all other groups in different time intervals up to the end of observation period. It showed significantly (p < 0.005) lower values in group E from 7th day to day 28 as compared to groups B, C and D.
Fig. 8.
Mean ± SE of absorbance values (OD492nm) of different groups in response to native porcine bladder tissue (Indirect ELISA). abcd—Superscript indicate, significant difference (p < 0.05) in absorbance value of particular graft at different time intervals. ABC—Superscript between groups indicate the significant difference (p < 0.05) in absorbance value at same time intervals
Real-time quantitative PCR analysis for angiogenesis
Group E contain pDNA-PDGF-B and rBMSC complex up regulated the level of angiogenic markers such as PDGF-B, VEGF, and α-SMA gene in wound area at different time period when compared to other groups (Figs. 9, 10, 11). This group showed significantly (p < 0.05) elevated level of gene expression in all intervals compare to all other groups. Next to this, the level of expression was more in group D which was significantly higher expression compare to other groups A, B and C but lesser than group E (Figs. 9, 10, 11). Generally, gene expression in groups A, B, and C vary regardless of the genes, whereas there was higher expression in group D and E at different time points. PDGF-B, α-SMA and VEGF expressions was higher in group D and E at different implantation periods.
Fig. 9.
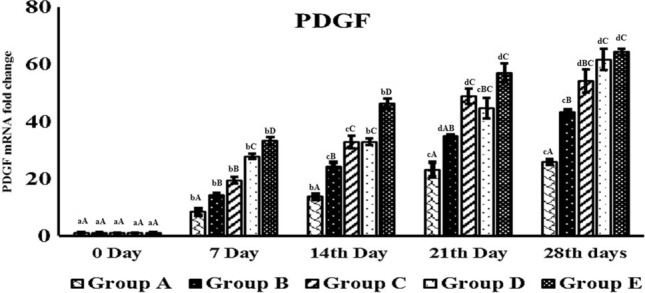
Real time quantitative analysis of PDGF-B expression on day 7, 14, 21 and 28 days post-burn injury. abcd—superscript indicate, significant difference (p < 0.05) in expression level of PDGF-B mRNA fold change of particular graft at different time intervals. ABCD—different superscript between groups indicate the significant difference (p < 0.05) in PDGF-B mRNA fold change value at same time intervals
Fig. 10.
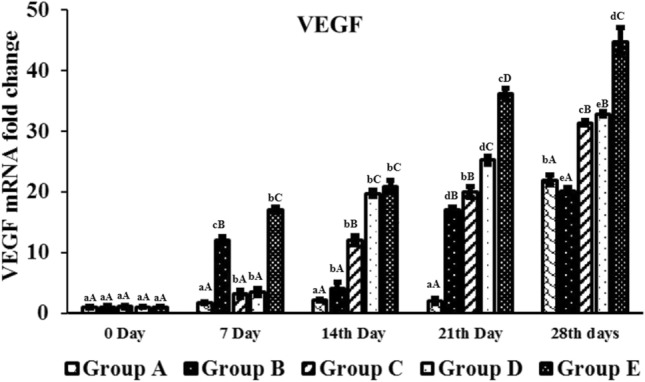
Real time quantitative analysis of VEGF expression on day 7, 14, 21 and 28 days post-burn injury. abcd—Superscript indicate, significant difference (p < 0.05) in expression level of VEGF mRNA fold change of particular graft at different time intervals. ABCD—Superscript between groups indicate the significant difference (p < 0.05) in VEGF mRNA fold change value at same time intervals
Fig. 11.
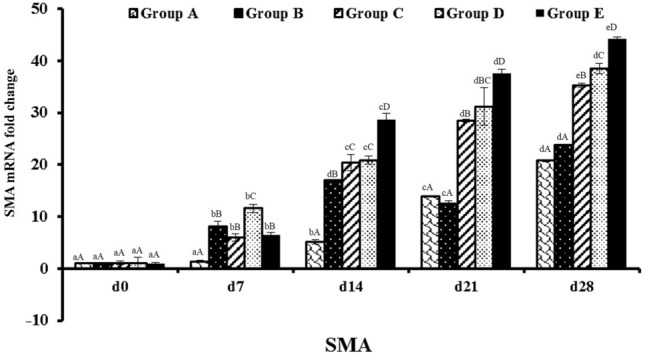
Real time quantitative analysis of SMA (Smooth muscle actin) expression on day 7, 14, 21 and 28 days post-burn injury. abcd—Superscript indicate, significant difference (p < 0.05) in expression level of SMA mRNA fold change of particular graft at different time intervals. ABCD—Superscript between groups indicate the significant difference (p < 0.05) in SMA mRNA fold change value at same time intervals
Discussion
Most of the researchers had used PDGF-B gene for dermal ulcer, diabetic ulcer model through adeno viral vector mediated delivery [31, 32]. The major disadvantage of adenoviral vectors is the cytotoxicity caused by the viral proteins and inflammatory response of the host cell to the adenoviral proteins. Further, Liechty et al. [33] showed that even topical use of adenoviral vector expressing lacZ gene which impaired re-epithelization result of inflammatory response caused by the adenovirus proteins or as a result of cytotoxicity. Hence, in the present study, non-viral gene delivery method was chosen. Non-viral gene delivery does not cause inflammation and the method were simple and cheap [34]. Earlier reports show that non-viral approach can be used for stable transfection [35]. In this study, recombinant pVAX1 clones with PDGF-B gene was generated and confirmed by restriction enzyme digestion which was used for studying the wound healing in rat model.
Mesenchymal stem cell (MSCs) has been used successfully for treatment of large spectrum of diseases. MSCs have not induced host immunity when administered either locally or systemically. Reports shows that lipofection reagents successfully introduced plasmid DNA into MSC and MSCs maintained their proliferation capability and capacity to differentiate into mesodermal lineages [36]. In the present study, lipofectamine 3000 was used as transfecting agent for rat bone marrow mesenchymal stem cells. This was correlated with study of Curtin et al. [36] where they have found, lipofectamine mediated transfection of rat mesenchymal stem cells showed 40% transfection efficiency compare to nHA transfection. In another study, goat mesenchymal transfected with lipofectamine and DNA at 1.2 μg: 2.2 μl showed highest transfection efficiency compare to other combinations [37]. It is also to be noted that at certain circumstances lesser transfection efficiency will be beneficial as higher transfection can lead to death of cells [38]. Lipofectamine has lower toxicity of less than 20% hence it is useful for transfection studies [39].
The expression of pVax1-PDGF-B gene in rat bone marrow derived stem cells was sustaining up to 120 h, and expression was evident from 48 h post-infection. This correlates well with study of Lin et al. [40]. Another report shows that there was PDGF-ά and PDGF-B gene expression for 10 days in human gingival fibroblast post gene transfection [41]. In addition, exogenously expressed PDGF-B translated and secreted as dimer and trimer in rBMSC cell medium, which was confirmed by western blot analysis. Lin et al. [40] similarly found that Ad-PDGF-B was secreted as dimer.
One of the primary goals of a decellularization is to remove cellular materials completely to prevent immune mediated rejection of implant [42]. The urothelium of bladder forms a waterproof barrier preventing the decelluar ionic detergent from infiltrating the sub epithelial layers [43]. Therefore, to remove the smooth muscle layer cells prior removal of urothelium is essential. The procedure used in this study to delaminate, decellularized, disinfect, and terminally sterilize the extracellular matrix (ECM) scaffolds does not affect the integrity of the basement membrane in urinary bladder matrix (UBM). The protocol described in this study completely decellularized the matrix which was apparent on light microscopy and confirmed by DNA quantification. Hyper-osmolar saline was reported to be effective de-epithelialisation agent, but was ineffective in this study. SDS 1% was highly effective decellularization agent with minimal effect on mechanical integrity and preserved biological activity of remaining ECM.
The size of wound showed uniform average distribution of 20 ± 1 mm in diameter. It is not always possible to create a uniform burn wound in rats [44]. The animals of the entire group remain dull and hunched back posture for first few days of after postoperative period of surgery. Thereafter, animals started to take feed and water normally from day 3 onwards [45].
Healing was complete in group D and E on day 28 when compared to other groups. Wound size markedly reduced in the group E from day 7, 14 and 21. Rapid wound closure and reduced healing time in groups D and E might be due to improved epithelialization [46]. Further, this data was validated by color digital imaging, where more wound healing was seen in control wound, where it slowly progress into scar formation. However, in other groups wound healing was less but wound area reduced from original size. On day 28, wound was completely healed without scar in groups E and D compare to other groups grossly. Wound healing starts with granulation tissue which later covered with neo-epidermis. Certain portion of the wound fibroblasts will transform into myofibroblasts, which will aid in reducing the size of the wound and strengthening the defect. Myofibroblasts are loaded with F-actin bundles, initiating cell–cell and cell-ECM linkages thus creating the force for closure of wound [47].
Post operatively the acellular bladder matrix has shown minimal antibody reaction at 21 days. Wu et al. [48] also established that there was immunogenicity and inflammatory reaction in the initial stages of xenogeneic acellular matrix applications. Even after complete removal of cells in extracellular matrix can also elicit immune response at times [49]. Collagen is a poor antigen and significance of immunogenicity is very less [50]. Further, acellular matrix was verified by quantifying DNA content which indirectly implied the amount of cells present in the matrix. In this study, the level of DNA content was significantly (p < 0.05) very less compared to native porcine urinary bladder. Many commercial biologic scaffolds can contain different amounts of residual DNA [51]. The residual DNA can typically have lesser bases as low as 300 bp and this DNA can be degraded enzymatically [52].
Burn wound differ from other wounds because there is widespread enhancement in capillary permeability which is not a phenomenon in other wounds. In burn wound, first phase healing is inflammatory phase in which there is proliferation of neutrophil and monocyte are the first cells to migrate at the site of inflammation. Due to the mitogenic activity of the released growth factor there was increase in cellular proliferation contributing to healing. Group E showing less histological score compare to all other groups. This was correlated with study of Gu et al. [53] where they have found that increase in wound bed and cellularity following treatment with AdPDGF-GAM. Noteworthy, VEGF was secreted comparatively more quantities with no significant differences between groups on day 7. VEGF is a main pro-angiogenic factors in accelerating early phase of wound healing by synthesizing neo vascularization [54]. On histological examination, more amount of new vessel formation, fibroblast and granulation tissue progression was noticed in groups D and E compared to other groups. The PDGF-B is a known chemo attractant that can induce migration of repair cells to wound bed from wound margins [55] PDGF-B can also stimulate proliferation of repair cell there by increasing the amount of granulation tissue [56]. It is documented that there was interlinked relationship between fibroblast growth factor, VEGF and PDGF-B. Increased production of PDGF-B gene simultaneously increase production of VEGF contributed in mural cell recruitment and new vessel formation [11]. Expression of PDGF-B and VEGF was higher in group D and group E on day 14. The level of expression was significantly higher in groups E than D. The fibroblast proliferation was much more in groups D and E which indirectly increase the deposition extracellular matrix and collagen. More amount of collagen deposition is relatively increase the fibroblast function in the local microenvironment [57]. This was confirmed on Masson’s trichrome staining where deposition of new collagen was observed. The entire scaffold integrated well with host tissue. Specifically in group E, there was no gap between newly formed tissue and implanted tissue.
On day 21, the histological score was least in group E followed by group D. The epithelization was almost complete in both the groups and was nearly similar to the normal tissues. Wound healing is related to wound size reduction and re-epithelization which has shown in group E. The wound healing started with gap filling and the contractile forces attract the wound edges together. Due to tension proto-myofibroblasts discriminate into highly contractile myofibroblasts expressing α-SMA [58]. In this study, it was observed that wound contraction was minimal in group E suggesting that scar formation is minimum when PDGF-B was used in the scaffold [59]. Endothelial cell proliferation was rapid in wound area of group E compare to other groups which directly correlated with the new vessel formation in the burn wound area. On histological examination fully formed vasculature was noticed in group E compared to other groups. PDGF-B recruits pericytes which is essential for stabilization and maturation of vascular structure [60]. Highest mRNA expression of VEGF, α-SMA and PDGF-B was noted in group E, gradually increasing from weeks 1 to 4. On day 28, wound of groups D and E were completely covered with epidermis. Arrangement and orientation of collagen fibers were best in group D and group E signifying a better healing of burn wound in these groups. Based on the results it confirmed that in group E wound healing was faster with no scar tissue formation, induce cell infiltration, neo-tissue formation, and promote blood vessel in-growth compared with all other groups.
In conclusion, this study demonstrated that, gene transfected stem cells matrix (pDNA-PDGF-B-rMSC) clinically proven for early acceleration of angiogenesis and tissue regeneration in burn injured animals. This study confirms the potency of gene-activated matrices (GAMs) on where a prolonged application of growth factor/protein delivery is required in clinical conditions. Hope, burn wound regeneration will not remain the only application of GAMs in regenerative medicine.
Acknowledgements
The authors wish to thank Dr K.P. Singh, Principal Scientist, CADRAD, IVRI, Izatnagar (UP), India for his technical assistance in evaluation of histological sections. The authors also wish to thank to the Director, Indian Veterinary Research Institute, Izatnagar, India and Head, Division of Surgery, Indian Veterinary Research Institute for providing necessary facilities for this study. SKM and TP conceived and designed the study, analyzed data and prepared the manuscript. TP, DM, PS and RS performed the experiment. HVM and KK conducts the experiment related to cloning and its expression and real time PCR. KN contributed in data analysis and editing the manuscript. All authors have given approval to the final version of the manuscript. There were no any external funding agencies supported to carry out this research.
Compliance with ethical standards
Conflict of interest
All the authors confirmed that, there was no conflict of interest regarding publication of this manuscript.
Ethical statement
Protocols for this study were approved by the Institute Animal Ethics Committee for Animal Care and Animal Experimentation (IAEC, No-F. 26–1/2015–16-JD (Research).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Herndon DN, Nguyen TT, Gilpin DA. Growth factors: local and systemic. Arch Surg. 1993;128:1227–1233. doi: 10.1001/archsurg.1993.01420230055009. [DOI] [PubMed] [Google Scholar]
- 2.Gajiwala K, Gajiwala AL. Evaluation of lyophilised gamma-irradiated amnion as biological dressing. Cell Tissue Bank. 2004;5:73–80. doi: 10.1023/B:CATB.0000034076.16744.4b. [DOI] [PubMed] [Google Scholar]
- 3.Merguerian PA, Reddy PP, Barrieras DJ, Wilson GJ, Woodhouse K, Bagli D, et al. Acellular bladder matrix allografts in the regeneration of functional bladders: evaluation of large-segment (> 24 cm) substitution in a porcine model. BJU Int. 2000;85:894–898. doi: 10.1046/j.1464-410x.2000.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal sub mucosa as a scaffold. J Surg Res. 2001;99:352–358. doi: 10.1006/jsre.2001.6199. [DOI] [PubMed] [Google Scholar]
- 5.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, et al. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653–2659. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Middelkoop E, van den Bogaerdt AJ, Lamme EN, Hoekstra MJ, Brandsma K, Ulrich MM. Porcine wound models for skin substitution and burn treatment. Biomaterials. 2004;25:1559–67. [DOI] [PubMed]
- 10.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug-and cell-delivery systems. J Biomater Sci Polymer Ed. 2004;15:701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 11.Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 12.Ehrbar M, Zeisberger SM, Raeber GP, Hubbell JA, Schnell C, Zisch AH. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29:1720–1729. doi: 10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9:S5–S15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 14.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Chandler LA, Gu DL, Ma C, Gonzalez AM, Doukas J, Nguyen T, et al. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000;8:473–479. doi: 10.1046/j.1524-475x.2000.00473.x. [DOI] [PubMed] [Google Scholar]
- 16.Endo M, Kuroda S, Kondo H, Maruoka Y, Ohya K, Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489–497. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 18.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55:414–419. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Maiti SK, Ravindran NA, Balwada AK, Mathew DD, Kumar N. The effects of topical mesenchymal stem cell therapy in full thickness skin wound healing in a rat model. Trends Biomater Artif Organs. 2014;28:65–73. [Google Scholar]
- 20.Green MR, Sambrook J. The Hanahan method for preparation and transformation of competent Escherichia coli: high-efficiency transformation. Cold Spring Harb Protoc. 2018 doi: 10.1101/pdb.prot101188. [DOI] [PubMed] [Google Scholar]
- 21.Paramasivam T, Maiti SK, Palakara S, Sashmi, Singh P, Kumar N, et al. Culture, characterization and differention potential of rat bone marrow derived mesenchymal stem cells. J Stem Cell Res Ther. 2016;1:193–9. [Google Scholar]
- 22.Palakara S, Maiti SK, Mohan D, Shivaraju S, Raghuvaran R, Rafee MA, et al. Mesenchymal stem cells derived from rat bone marrow (rBM MSC): techniques for isolation, expansion and differentiation. J Stem Cell Res Ther. 2017;3:272–277. [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Manjunathachar HV, Saravanan BC, Kumar B, Ghosh S. Expression and determination of immunization dose of recombinant tropomyosin protein of Hyalomma anatolicum for the development of anti-tick vaccine. Indian J Anim Sci. 2017;87:275–279. [Google Scholar]
- 26.Munro BH. Manual of histologic staining methods of the Armed Forces Institute of Pathology. Pathology. 1971;3:249. [Google Scholar]
- 27.Masson P. Some histological methods. Trichrome staining and their preliminary technique. J Tech Methods. 1929;12:75–90. [Google Scholar]
- 28.Schallberger SP, Stanley BJ, Hauptman JG, Steficek BA. Effect of porcine small intestinal submucosa on acute full-thickness wounds in dogs. Vet Surg. 2008;37:515–524. doi: 10.1111/j.1532-950X.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 29.Borena BM, Pawde AM, Amarpal, Aithal HP, Kinjavdekar P, Singh R, et al. Autologous bone marrow-derived cells for healing excisional dermal wounds of rabbits. Vet Rec. 2009;165:563–568. doi: 10.1136/vr.165.19.563. [DOI] [PubMed] [Google Scholar]
- 30.Snedecor GW, Cochran WG. Statistical methods, 8thEdn. Ames: Iowa State Univ. Press Iowa; 1989. [Google Scholar]
- 31.Tyrone JW, Mogford JE, Chandler LA, Ma C, Xia Y, Pierce GF, et al. Collagen-embedded platelet-derived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J Surg Res. 2000;93:230–236. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 32.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF down regulates gas gene product PDGFαR and prolongs ERK and Akt/PKB activation. Am J Physiol Cell Physiol. 2002;282:C538–C544. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wliechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated over expression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J Invest Dermatol. 1999;113:375–383. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 34.Vogel JC. Nonviral skin gene therapy. Hum Gene Ther. 2000;11:2253–2259. doi: 10.1089/104303400750035780. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz-Urda S, Thyagarajan B, Keene DR, Lin Q, Fang M, Calos MP, et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med. 2002;8:1166–1170. doi: 10.1038/nm766. [DOI] [PubMed] [Google Scholar]
- 36.Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mat. 2012;24:749–754. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 37.Kumar M, Yasotha T, Singh RK, Kumar K, Ranjan R, Meshram CD, Das B, Bag S. Generation of transgenic mesenchymal stem cells expressing green fluorescent protein as reporter gene using no viral vector in caprine. Indian J Exp Biol. 2013;51:502–509. [PubMed] [Google Scholar]
- 38.Ye L, Haider HK, Esa WB, Law PK, Zhang W, Su L, et al. Nonviral vector-based gene transfection of primary human skeletal myoblasts. Exp Biol Med (Maywood) 2007;232:1477–1487. doi: 10.3181/0706-RM-175. [DOI] [PubMed] [Google Scholar]
- 39.Santos JL, Oramas E, Pêgo AP, Granja PL, Tomás H. Osteogenic differentiation of mesenchymal stem cells using PAMAM dendrimers as gene delivery vectors. J Control Release. 2009;134:141–148. doi: 10.1016/j.jconrel.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Lin Z, Sugai JV, Jin Q, Chandler LA, Giannobile WV. Platelet-derived growth factor-B gene delivery sustains gingival fibroblast signal transduction. J Periodontal Res. 2008;43:440–449. doi: 10.1111/j.1600-0765.2008.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–756. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Staack A, Hayward SW, Baskin LS, Cunha GR. Molecular, cellular and developmental biology of urothelium as a basis of bladder regeneration. Differentiation. 2005;73:121–133. doi: 10.1111/j.1432-0436.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 44.Meyer TN, Silva ALD. A standard burn model using rats. Acta Cir Bras. 1999. 10.1590/S0102-86501999000400009.
- 45.Sandeep K, Maiti SK, Naveen K, Sams-uz-Zama MM, Ravindran NA, Balwada AK, et al. Effect of medical grade chitosan powder in full thickness skin wound healing in rat model. Adv Anim Vet Sci. 2014;5:270–276. [Google Scholar]
- 46.Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethno pharmacol. 2002;83:193–199. doi: 10.1016/s0378-8741(02)00195-2. [DOI] [PubMed] [Google Scholar]
- 47.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 48.Wu Q, Yao M, Pan Y, Qing C, Cao Q, Xu W. Experimental study on the inflammatory and immune responses of xenogenic acellular dermal matrix transplantation combined with thin split-thickness skin autograft. Zhonghua Zheng Xing Wai Ke Za Zhi. 2002;18:266–268. [PubMed] [Google Scholar]
- 49.Singh J, Kumar N, Sharma AK, Maiti SK, Goswami T, Sharma AK. Acellular biomaterials of porcine origin for the reconstruction of abdominal wall defects in rabbits. Trends Biomater Artif Organs. 2008;22:34–44. [Google Scholar]
- 50.Paul RG, Bailey AJ. Chemical stabilization of collagen as a biomimetic. ScientificWorldJournal. 2003;3:138–155. doi: 10.1100/tsw.2003.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert TW, Freund JW, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 53.Gu D, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, bio distribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Gauglitz GG, Jeschke MG. Combined gene and stem cell therapy for cutaneous wound healing. Mol Pharm. 2011;8:1471–1479. doi: 10.1021/mp2001457. [DOI] [PubMed] [Google Scholar]
- 55.Deuel TF, Kawahara RS, Mustoe TA, Pierce AF. Growth factors and wound healing: platelet-derived growth factor as a model cytokine. Ann Rev Med. 1991;42:567–584. doi: 10.1146/annurev.me.42.020191.003031. [DOI] [PubMed] [Google Scholar]
- 56.Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, Thomason A, et al. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988;167:974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien FJ, Harley B, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 58.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, Nguyen T. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gen Ther. 2001;12:783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 60.Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]