Abstract
Early initial massive transfusion protocol and blood transfusion can reduce patient mortality, however accurately identifying the risk of massive transfusion (MT) remains a major challenge in severe trauma patient therapy. We retrospectively analyzed clinical data of severe trauma patients with and without MT. Based on analysis results, we established a MT prediction model of clinical and laboratory data by using the decision tree algorithm in patients with multiple trauma. Our results demonstrate that shock index, injury severity score, international normalized ratio, and pelvis fracture were the most significant risk factors of MT. These four indexes were incorporated into the prediction model, and the model was validated by using the testing dataset. Moreover, the sensitivity, specificity, accuracy and area under curve values of prediction model for MT risk prediction were 60%, 92%, 90% and 0.85. Our study provides an easy and understandable classification rules for identifying risk factors associated with MT that may be useful for promoting trauma management.
Keywords: Massive hemorrhage, Multiple trauma, Massive transfusion, Decision tree, Algorithm
Introduction
Trauma is a major global public problem and the leading cause of death [1–4]. About 50% of trauma deaths occur as a result of uncontrolled hemorrhage within the first 48 h after trauma [3, 4]. In recent years, with the clinical progress of damage control resuscitation (DCR) and massive transfusion protocol (MTP), the mortality of trauma patient has reduced [5, 6]; however, the mortality of trauma patients with massive hemorrhage remains high.
Previous studies have shown that the mortality of trauma patients was associated with an increase of blood transfusion; furthermore, the mortality of massive transfusion (MT) patients is significantly higher than non-MT patients [7–9]. For massive hemorrhage in trauma patients, MTP plays an important role in early DCR and improved survival [6, 10, 11]. MTP is defined as rapid hemorrhage control through early administration of blood products in a balanced ratio for the prevention and immediate correction of coagulopathy, and to minimize occurrence of increased use of crystalloid fluids [12–14]. Studies have shown that early start MTP could reduce the risk of MT and related complications. They have also been shown to improve outcomes [12–14]. However, it is still difficult to identify the MT risk early and accurately.
Decision tree (DT) is a machine learning method used as a powerful solution to classify and predict problems [15]. Several studies have demonstrated that the DT algorithm can classify and predict diseases or outcomes with high accuracy, sensitivity, and specificity [16–22]. However, so far, the DT algorithm has not been used to predict MT risk in multiple trauma patients. Thus, to define the variables that could identify individuals at a risk for MT among patients with multiple trauma, we aimed to construct a model for MT prediction using the DT algorithm. This established model may be useful to determine patients with a high MT risk, and help to improve clinical decision-making in the case of patients with multiple trauma.
Materials and Methods
Study Population
The present study is a retrospective study of patients treated from 1 January 2013 through 30 June 2017. Patients diagnosed with multiple trauma who were consecutively admitted to the First Affiliated Hospital of Nanchang University were enrolled in this study. The inclusion criteria were as follows: all patients diagnosed as multiple trauma and adult patients with age ≥ 18 years. The exclusion criteria were as follows: pregnant woman; diagnosed with traumatic brain injury; diagnosed with serious cardiovascular and cerebrovascular diseases; or diagnosed with serious hematologic disorders.
Base Characteristics and Clinical Data Collection
Of the 670 identified patients with multiple trauma, 478 patients were eligible for inclusion and were included in this study, which included 435 who did not receiving MT (≥ 10 units of packed red blood cells (RBCs) in 24 h or > 4 units RBCs 1 h with anticipation of continued need) and 43 who receiving MT. Clinical data of enrolled patients were obtained through review of medical records. The following data were selected for the decision tree analysis: sex; age; injury causes; injury type; vital signs, including body temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), shock index (SI); injury severity score (ISS); fracture, including rib fracture and pelvis fracture; Glasgow coma score (GCS); and levels of hemoglobin (Hb), platelets, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT). The clinical outcomes related to in-hospital mortality, 24 h mortality, complications (including infection, multiple organs dysfunction syndromes (MODS), and acute kidney failure), hospital length of stay (LOS), intensive care unit (ICU) LOS, and duration of mechanical ventilation.
Decision Tree Development and Internal Validation
Enrolled patients were randomly divided into a training dataset and a testing dataset with a ratio of 7:3. Of the 478 patients with multiple trauma, 332 and 146 patients were assigned to the training dataset and the testing dataset, respectively. The training dataset was used for predictor discovery and supervised classification to generate a plausible model. The testing dataset was used to test the performance of the model, which was generated in the training sample.
Statistical Analysis
Statistical analyses were performed using R-program (version 3.5.1, The R Foundation for Statistical Computing). All tests were two-sided, and a p-value less than 0.05 was considered statistically significant. Continuous variables are presented as mean ± standard deviation or medians and quartiles, Data were analyzed with t-test or Mann-Whitney U-test, as appropriate. Categorical variables are presented as frequency and percentages and were analyzed with Chi-squared test or Fisher’s exact test, as appropriate. The DT model was performed using classification and regression trees (CART) [23], based on the Gini impurity index using the rpart package in the R-program.
Results
Base Characteristics and Outcomes of Patients with Multiple Trauma
As shown in Table 1, the MT group had significantly higher in-hospital mortality, 24 h mortality, LOS, ICU LOS, duration of mechanical ventilation, and incidence of complications. No significant differences in sex, age, causes of injury, rib fracture, or body temperature were observed between the MT and non-MT group. In addition, the MT group had a significantly higher ISS, HR, SI, PT, APTT, and INR, but it had a lower GCS, SBP, DBP, and Hb levels compared with the non-MT group (Table 1).
Table 1.
Patient characteristics and outcomes of patients with massive transfusion (MT) and non-massive transfusion (non-MT) groups
| Variables | Overall (n = 478) | Non-MT group (n = 435) | MT group (n = 43) | p value |
|---|---|---|---|---|
| Male (n, %) | 356 (74.5) | 328 (75.4) | 28 (65.1) | 0.196 |
| Age (years) | 45.21 ± 13.82 | 45.15 ± 13.85 | 45.84 ± 13.64 | 0.756 |
| Injury cause (n, %) | 0.561 | |||
| Traffic injury | 265 (55.4) | 242 (55.6) | 23 (53.5) | |
| Mechanical injury | 4 (0.8) | 4 (0.9) | 0 (0.0) | |
| Sharp injury | 34 (7.1) | 29 (6.7) | 5 (11.6) | |
| Falling injury | 146 (30.6) | 135 (31.0) | 11 (25.6) | |
| Others | 29 (6.1) | 25 (5.8) | 4 (9.3) | |
| Injury type (n, %) | < 0.001 | |||
| Blunt injury | 328 (68.6) | 311 (71.5) | 17 (39.5) | |
| Penetrating injury | 150 (31.4) | 124 (28.5) | 26 (60.5) | |
| Rib fracture (n, %) | 154 (32.2) | 142 (32.6) | 12 (27.9) | 0.643 |
| Pelvis fracture (n, %) | 61 (12.8) | 46 (10.6) | 15 (34.9) | < 0.001 |
| ISS | 16.00 (9.00, 22.00) | 14.00 (9.00, 22.00) | 22.00 (17.00, 27.00) | < 0.001 |
| GCS | 14.40 ± 2.11 | 14.46 ± 2.05 | 13.81 ± 2.60 | 0.058 |
| Body temperature (°C) | 36.82 ± 0.56 | 36.82 ± 0.56 | 36.75 ± 0.62 | 0.418 |
| HR (beats/min) | 93.29 ± 18.45 | 91.44 ± 17.22 | 112.07 ± 20.13 | < 0.001 |
| SBP (mmHg) | 118.17 ± 19.77 | 120.27 ± 18.22 | 96.88 ± 22.32 | < 0.001 |
| SI | 0.82 ± 0.28 | 0.78 ± 0.22 | 1.23 ± 0.44 | < 0.001 |
| DBP (mmHg) | 71.87 ± 13.47 | 73.16 ± 12.58 | 58.84 ± 15.29 | < 0.001 |
| Hb (g/L) | 106.35 ± 30.79 | 110.43 ± 28.61 | 65.05 ± 19.74 | < 0.001 |
| PT (s) | 11.90 (11.20, 13.50) | 11.80 (11.10, 13.00) | 16.20 (14.60, 18.00) | < 0.001 |
| APTT (s) | 28.60 (24.20, 34.95) | 27.90 (23.95, 32.40) | 52.60 (39.45, 69.10) | < 0.001 |
| INR | 1.07 (1.00, 1.19) | 1.05 (0.99, 1.15) | 1.47 (1.31, 1.69) | < 0.001 |
| RBC transfusion (U) | 0.00 (0.00, 2.00) | 0.00 (0.00, 0.00) | 10.00 (8.00, 13.50) | < 0.001 |
| Plasma transfusion (mL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 950.00 (600.00, 1325.00) | < 0.001 |
| Complication (n, %) | < 0.001 | |||
| Infections | 62 (13.0) | 50 (11.5) | 12 (27.9) | |
| MODS | 10 (2.1) | 4 (0.9) | 6 (14.0) | |
| Acute kidney failure | 2 (0.4) | 1 (0.2) | 1 (2.3) | |
| Duration of mechanical ventilation (days) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 1.00 (0.00, 6.00) | < 0.001 |
| ICU LOS(days) | 0.00 (0.00, 4.00) | 0.00 (0.00, 3.00) | 5.00 (2.00, 12.50) | < 0.001 |
| Hospital LOS (days) | 18.00 (11.00, 28.75) | 18.00 (10.00, 27.00) | 32.00 (16.00, 50.00) | < 0.001 |
| 24 h mortality (n, %) | 9 (1.9) | 5 (1.1) | 4 (9.3) | 0.002 |
| Hospital mortality (n,%) | 19 (4.0) | 11 (2.5) | 8 (18.6) | < 0.001 |
Data are present as mean ± standard deviation or median (quartile)
ISS injury severity score, GCS glasgow coma score, HR heart rate, SBP systolic blood pressure, SI shock index, DBP diastolic blood pressure, Hb hemoglobin, PT prothrombin time, APTT activated partial thromboplastin time, INR international normalized ratio, RBC red blood cells, MODS multiple organs dysfunction syndromes, length of stay LOS, ICU intensive care unit
Establishment Decision Tree Model
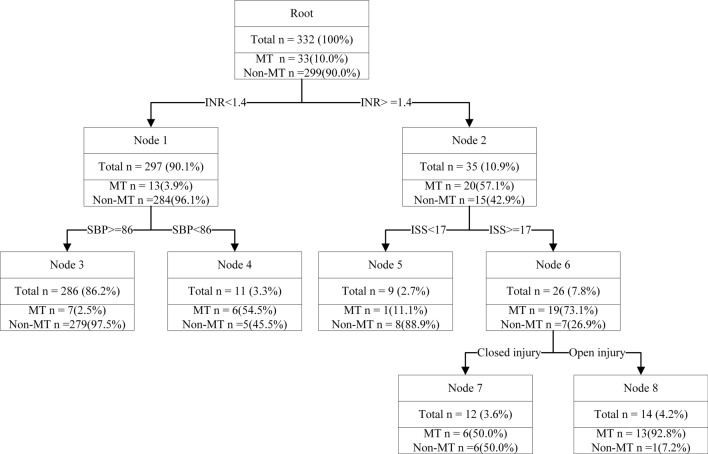
In this model, a decision tree was built on training dataset (332 records). Testing datasets (146 records) were used to evaluate the model. There were no differences in clinical characteristics between the training dataset and testing dataset (Table 2). The algorithm used the Gini index to select the variables. In the training model, 12 variables were used as input variables. The INR, SBP, ISS, and injury type remained in the model. The final decision tree is shown in Fig. 1.
Table 2.
Patient characteristics and outcomes of patients in training dataset and testing dataset
| Variables | Training dataset (n = 332) | Testing dataset (n = 146) | p value |
|---|---|---|---|
| Male (n, %) | 241 (72.6) | 115 (78.8) | 0.189 |
| Age (years) | 44.82 ± 13.30 | 46.10 ± 14.94 | 0.350 |
| Injury cause (n, %) | 0.992 | ||
| Traffic injury | 183 (55.1) | 82 (56.2) | |
| Mechanical injury | 3 (0.9) | 1 (0.7) | |
| Sharp injury | 23 (6.9) | 11 (7.5) | |
| Falling injury | 102 (30.8) | 44 (30.1) | |
| Others | 21 (6.3) | 8 (5.5) | |
| Injury type (n, %) | 0.999 | ||
| Penetrating injury | 104 (31.3) | 46 (31.5) | |
| Blunt injury | 228 (68.7) | 100 (68.5) | |
| Rib fracture (n, %) | 106 (31.9) | 48 (32.9) | 0.922 |
| Pelvis fracture (n, %) | 47 (14.2) | 14 (9.6) | 0.219 |
| ISS | 15.00 (9.00, 22.00) | 17.00 (9.00, 22.00) | 0.568 |
| GCS | 14.35 ± 2.22 | 14.51 ± 1.84 | 0.426 |
| Body temperature (°C) | 36.85 ± 0.58 | 36.74 ± 0.51 | 0.068 |
| HR (beats/min) | 93.92 ± 18.47 | 91.86 ± 18.39 | 0.262 |
| SBP (mmHg) | 117.79 ± 19.84 | 119.04 ± 19.65 | 0.523 |
| SI | 0.83 ± 0.29 | 0.80 ± 0.25 | 0.260 |
| DBP (mmHg) | 71.93 ± 13.59 | 71.73 ± 13.24 | 0.879 |
| HB (g/L) | 104.70 ± 31.92 | 110.10 ± 27.80 | 0.077 |
| PT (s) | 12.00 (11.30, 13.62) | 11.80 (11.20, 13.20) | 0.199 |
| APTT (s) | 28.70 (24.50, 35.15) | 28.15 (23.63, 34.22) | 0.294 |
| INR | 1.08 (1.00, 1.21) | 1.05 (0.99, 1.18) | 0.366 |
| RBC transfusion (U) | 0.00 (0.00, 2.00) | 0.00 (0.00, 0.00) | 0.252 |
| Plasma transfusion (mL) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.274 |
| Massive transfusion (n,%) | 33 (9.9) | 10 (6.8) | 0.361 |
| Complication (n, %) | 0.700 | ||
| Infections | 44 (13.2) | 18 (12.3) | |
| MODS | 8 (2.4) | 2 (1.4) | |
| Acute kidney failure | 2 (0.6) | 0 (0.0) | |
| Duration of mechanical ventilation (days) | 0.00 (0.00, 0.00) | 0.00 (0.00, 1.00) | 0.823 |
| ICU LOS(days) | 0.00 (0.00, 4.25) | 1.00 (0.00, 4.00) | 0.565 |
| Hospital LOS (days) | 18.00 (11.00, 28.00) | 19.00 (10.25, 29.00) | 0.975 |
| 24 h mortality (n, %) | 7 (2.1) | 2 (1.4) | 0.856 |
| Hospital mortality (n,%) | 13 (3.9) | 6 (4.1) | 0.999 |
Data are present as mean ± standard deviation or median (quartile)
ISS injury severity score, GCS glasgow coma score, HR heart rate, SBP systolic blood pressure, SI shock index, DBP diastolic blood pressure, Hb hemoglobin, PT prothrombin time, APTT activated partial thromboplastin time, INR international normalized ratio, RBC red blood cells, MODS multiple organs dysfunction syndromes, length of stay LOS, ICU intensive care unit
Fig. 1.
Decision tree model for the occurrence of MT with training dataset. MT: massive transfusion; INR: international normalized ratio; SBP: systolic blood pressure; ISS: injury severity score
Evaluation of the Decision Tree Model
The evaluation of the model was undertaken using a confusion matrix on a training and testing dataset and is shown in Tables 3 and 4. The present decision tree model had an accuracy of 90%. Of the 136 individuals without MT in testing datasets, 122 were classified correctly using the decision-tree, with a specificity of 90%. For the 10 cases of MT in the testing dataset, the decision tree correctly classified 8 individuals with a sensitivity of 80%.
Table 3.
The confusion matrix obtained as a result of training the rpart decision tree
| Predicted | ||
|---|---|---|
| MT | Non-MT | |
| Actual | ||
| MT | 28 | 5 |
| Non-MT | 8 | 291 |
MT massive transfusion
Table 4.
The confusion matrix obtained as a result of testing the rpart decision tree
| Predicted | ||
|---|---|---|
| MT | Non-MT | |
| Actual | ||
| MT | 8 | 2 |
| Non-MT | 14 | 122 |
MT massive transfusion
A ROC curve was obtained by applying the decision tree to test the dataset model that is shown in Fig. 2. The sensitivity, specificity, accuracy and area under the ROC curve (AUC) values for model were 80%, 90%, 89% and 0.86, respectively, for the testing dataset. We also repeated the analysis for the dataset when there was a partition of 50% in the training dataset and 50% in the testing dataset, or a partition of 80% in the training dataset and 20% in the testing dataset. The confusion matrix outcomes are shown in Table 5.
Fig. 2.
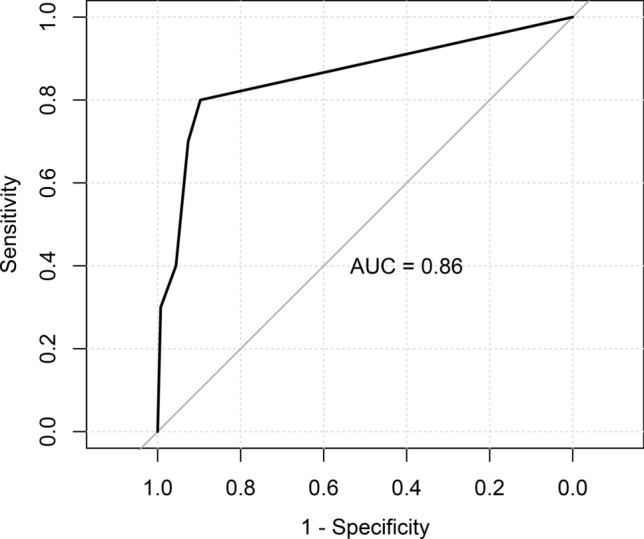
Roc curve of the decision tree model in testing dataset
Table 5.
The confusion matrix obtained as a result of testing the rpart decision tree
| Predicted (using 50% as testing dataset) | Predicted (using 20% as testing dataset) | |||
|---|---|---|---|---|
| MT | Non-MT | MT | Non-MT | |
| Actual | ||||
| MT | 12 | 10 | 4 | 2 |
| Non-MT | 12 | 198 | 6 | 81 |
MT massive transfusion
Discussion
Massive hemorrhage is a major potential preventable cause of deaths [3, 24]. Recent studies have suggested that an improvement of survival in trauma patients can be achieved by implementing MTP and by resuscitating with a balanced proportion of platelets, plasma, and packed red blood cells [12, 14, 25, 26]. Early and accurate prediction for trauma patients who required MT is necessary to increase the mortality benefits of early administration of blood transfusion. However, one of the major challenges of improving the outcome of trauma patients is the early identification of patients in need of MT. Several scoring systems [27–30], including the trauma associated severe hemorrhage (TASH), prince of wales hospital (PWH) and assessment of blood consumption (ABC) score, have been introduced to predict the risk of MT in trauma patients. Brockamp et al. [31] validated 6 scoring systems and algorithms related to calculating the risk of MT and concluded that the TASH score was the highest (AUC of 0.889).
In the present study, the decision tree (DT) algorithm was used to screen the risk factors related to MT and constructed a prediction model in the training dataset and we further validated the testing dataset. The variables of INR, SBP, ISS, and injury type were entered in the prediction model. Wang et al. [32] set up an early blood transfusion needs score and Nunez et al. [28] set up an ABC score; both concluded that penetrating injury and SBP were independent risk factors of massive transfusion in trauma patients. Lui et al. [33] incorporated INR to the dynamic MBT score, and found that ISS and INR differed from MT and non-MT. The results obtained from our model were similar to the results of other studies [28, 32, 33].
Our MT prediction model demonstrated an 80% sensitivity, 90% specificity, and 89% accuracy. A major strength of the present study was the application of the decision tree for investigating predictors associated with MT in multiple trauma. The study presented may provide a new insight into exploring MT risk prediction with trauma patients.
Despite our promising findings, there are still some certain limitations in our study. Firstly, the small sample size of this single-center retrospective study is the major limitation. This MT prediction model has not been validated in a prospective study. Secondly, this study did not use other machine learning algorithms such as a support vector machine (SVM), random forest (RF) for analysis. The best machine learning prediction model is still worth further exploring. In future studies, multi-center and larger-scale researches still need to be performed to develop a machine learning prediction model with greater sensitivity and specificity, which can be used to more accurately determine the risk of MT.
Conclusion
A MT prediction model is established using the decision tree algorithm and evidently has a good predictive performance. This study provides an easy and understandable classification of rules that helps to identify risk factors associated with MT that may be useful to develop programs for trauma management.
Funding
The study was funded by the Jiangxi Major Science and Technology Project (No. 20144BBG70001).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 2.Sise RG, Calvo RY, Spain DA, Weiser TG, Staudenmayer KL. The epidemiology of trauma-related mortality in the United States from 2002 to 2010. J Trauma Acute Care Surg. 2014;76(4):913–919. doi: 10.1097/TA.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 versus a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. doi: 10.1001/jamasurg.2014.940. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha B, Holcomb JB, Camp EA, Del Junco DJ, Cotton BA, Albarado R, et al. Damage-control resuscitation increases successful nonoperative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78(2):336–341. doi: 10.1097/TA.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Sun Y, Xu CX, Dang QL, Li L, Xu YG, et al. Correlation between red blood cell transfusion volume and mortality in patients with massive blood transfusion: a large multicenter retrospective study. Exp Ther Med. 2015;9(1):137–142. doi: 10.3892/etm.2014.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra B, Gabbe BJ, Kaukonen KM, Olaussen A, Cooper DJ, Cameron PA. Long-term outcomes of patients receiving a massive transfusion after trauma. Shock. 2014;42(4):307–312. doi: 10.1097/SHK.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 9.Stanworth SJ, Davenport R, Curry N, Seeney F, Eaglestone S, Edwards A, et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br J Surg. 2016;103(4):357–365. doi: 10.1002/bjs.10052. [DOI] [PubMed] [Google Scholar]
- 10.Pohlman TH, Walsh M, Aversa J, Hutchison EM, Olsen KP, Lawrence Reed R. Damage control resuscitation. Blood Rev. 2015;29(4):251–262. doi: 10.1016/j.blre.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 11.West N, Dawes R. Trauma resuscitation and the damage control approach. Surgery (Oxford) 2015;33(9):430–436. doi: 10.1016/j.mpsur.2015.07.007. [DOI] [Google Scholar]
- 12.Bawazeer M, Ahmed N, Izadi H, McFarlan A, Nathens A, Pavenski K. Compliance with a massive transfusion protocol (MTP) impacts patient outcome. Injury. 2015;46(1):21–28. doi: 10.1016/j.injury.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Curry N, Davis PW. What's new in resuscitation strategies for the patient with multiple trauma? Injury. 2012;43(7):1021–1028. doi: 10.1016/j.injury.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury. 2013;44(5):587–592. doi: 10.1016/j.injury.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Safavian SR, Landgrebe D. A survey of decision tree classifier methodology. IEEE Trans Syst Man Cybern. 2002;21(3):660–674. doi: 10.1109/21.97458. [DOI] [Google Scholar]
- 16.Bamber JH, Evans SA. The value of decision tree analysis in planning anaesthetic care in obstetrics. Int J Obstet Anesth. 2016;27:55–61. doi: 10.1016/j.ijoa.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Hostettler IC, Muroi C, Richter JK, Schmid J, Neidert MC, Seule M, et al. Decision tree analysis in subarachnoid hemorrhage: prediction of outcome parameters during the course of aneurysmal subarachnoid hemorrhage using decision tree analysis. J Neurosurg. 2018;129:1–12. doi: 10.3171/2017.7.JNS17677. [DOI] [PubMed] [Google Scholar]
- 18.Kasbekar PU, Goel P, Jadhav SP. A decision tree analysis of diabetic foot amputation risk in indian patients. Front Endocrinol (Lausanne) 2017;8:25. doi: 10.3389/fendo.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Kim MJ, Shin HJ, Yoon H, Han SJ, Koh H, et al. MRI-based decision tree model for diagnosis of biliary atresia. Eur Radiol. 2018;28(8):3422–3431. doi: 10.1007/s00330-018-5327-0. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadzadeh F, Noorkojuri H, Pourhoseingholi MA, Saadat S, Baghestani AR. Predicting the probability of mortality of gastric cancer patients using decision tree. Ir J Med Sci. 2015;184(2):277–284. doi: 10.1007/s11845-014-1100-9. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Voigt MD. Decision tree analysis to stratify risk of de novo non-melanoma skin cancer following liver transplantation. J Cancer Res Clin Oncol. 2018;144(3):607–615. doi: 10.1007/s00432-018-2589-5. [DOI] [PubMed] [Google Scholar]
- 22.Tayefi M, Esmaeili H, Saberi Karimian M, Amirabadi Zadeh A, Ebrahimi M, Safarian M, et al. The application of a decision tree to establish the parameters associated with hypertension. Comput Methods Programs Biomed. 2017;139:83–91. doi: 10.1016/j.cmpb.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Loh WY. Classification and regression trees. Wiley Interdiscip Rev Data Mining Knowl Discov. 2011;1(1):14–23. doi: 10.1002/widm.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson PI, Stensballe J, Oliveri R, Wade CE, Ostrowski SR, Holcomb JB. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3058. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 25.McQuilten ZK, Crighton G, Engelbrecht S, Gotmaker R, Brunskill SJ, Murphy MF, et al. Transfusion interventions in critical bleeding requiring massive transfusion: a systematic review. Transfus Med Rev. 2015;29(2):127–137. doi: 10.1016/j.tmrv.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Waters JH. Role of the massive transfusion protocol in the management of haemorrhagic shock. Br J Anaesth. 2014;113(Suppl 2):3–8. doi: 10.1093/bja/aeu379. [DOI] [PubMed] [Google Scholar]
- 27.Maegele M, Lefering R, Wafaisade A, Theodorou P, Wutzler S, Fischer P, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox Sang. 2011;100(2):231–238. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 28.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma Injury Infect Crit Care. 2009;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 29.Rainer TH, Ho AM, Yeung JH, Cheung NK, Wong RS, Tang N, et al. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011;82(6):724–729. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Umemura T, Nakamura Y, Nishida T, Hoshino K, Ishikura H. Fibrinogen and base excess levels as predictive markers of the need for massive blood transfusion after blunt trauma. Surg Today. 2016;46(7):774–779. doi: 10.1007/s00595-015-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockamp T, Nienaber U, Mutschler M, Wafaisade A, Peiniger S, Lefering R, et al. Predicting on-going hemorrhage and transfusion requirement after severe trauma: a validation of six scoring systems and algorithms on the trauma register DGU. Crit Care. 2012;16(4):R129. doi: 10.1186/cc11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Umejiego J, Robinson RD, Schrader CD, Leuck J, Barra M, et al. A derivation and validation study of an early blood transfusion needs score for severe trauma patients. J Clin Med Res. 2016;8(8):591–597. doi: 10.14740/jocmr2598w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui CT, Wong OF, Tsui KL, Kam CW, Li SM, Cheng M, et al. Predictive model integrating dynamic parameters for massive blood transfusion in major trauma patients: the dynamic MBT score. Am J Emerg Med. 2018;36(8):1444–1450. doi: 10.1016/j.ajem.2018.01.009. [DOI] [PubMed] [Google Scholar]