Abstract
LKB1 is a significant tumor suppressor and epigenetic regulator playing a vital role in different types of cancers. SHMT1 and GLDC are two critical genes of the epigenetic pathway influenced by LKB1. As epigenetic is the major cause of AML pathogenesis, this study aimed at investigating LKB1, SHMT1, and GLDC gene expression levels in acute myeloid leukemia patients. The present study was conducted on LKB1, SHMT1, and GLDC gene expression levels in 60 de novo AML samples and 30 normal controls using real-time RT-PCR. The results showed that LKB1 and SHMT1 have respectively a significantly lower (P < 0.05) and higher (P < 0.05) expression level than that of normal controls. Furthermore, the correlation between LKB1 with SHMT1 and GLDC was significant and positive (P value: 0.015, r: 0.299). Positive findings confirm that metabolic pathways alongside the LKB1 association drive the epigenetic axis and its substrate production. Therefore, it can be concluded that the newly-discovered pathway in the pathogenesis of this disease provides new insights into the design of therapeutic targets.
Keywords: LKB1, SHMT1, GLDC, AML
Introduction
Considering that the tumorigenesis process of acute myeloid leukemia (AML) is a very complex procedure involving several gene aberrations [1–3], epigenetic dysregulation is now regarded as the major cause of AML [4]. In addition, hypermethylation has shown to be a potential and crucial factor in AML pathogenesis [4].
Encoded by serine/threonine kinase gene (STK11), the liver kinase B1 (LKB1) is known as a critical tumor suppressor in cell growth and proliferation which its downregulation has been identified in various solid tumors including breast, colon, and prostate cancers as well as hematologic malignancies [5, 6]. Over the years, this function of LKB1 has been demonstrated through the regulation of the LKB1-AMPK-mTOR axis which inhibits cell proliferation and tumorigenesis [7]. Recently, however, studies have suggested that the epigenetic pathway by metabolic enzymes is also regulated through this tumor suppressor [8]. In other words, there exists a network linking metabolism and epigenetic alterations wherein LKB1 functions as their master regulator. In fact, LKB1 impedes hypermethylation by suppressing methyl producer enzymes in metabolic pathways containing the serine-threonine pathway which leads to the production of SAM as a universal methylation substrate [8]. Due to the fact that patients’ DNMTs are mutated in AML and help DNA hypermethylation, it is required to increase the production of SAM provided from the serine-threonine metabolic axis [9, 10]. Therefore, LKB1 downregulation and induced abnormal methylation might result in genome instability and increased inactivation of tumor suppressor genes or a higher expression of oncogenes, which correlates closely with tumor initiation and progression.
Located on chromosome 17, serine hydroxymethyltransferase 1 (SHMT1) is a critical enzyme involved in the serine metabolism which negatively regulated by LKB1 and catalyzes serine and tetrahydrofolate to glycine and 5,10 methylenetetrahydrofolate (as one carbon group carrier) [11]. Therefore, hypermethylation and DNA synthesis is more induced by this gene overexpression, providing a one-carbon group for purine and methionine synthesis. Consequently, SHMT1 overexpression can lead to the growth of cell and tumorigenesis which has been reported in some malignancies including lung cancer [12].
In addition, Glycine decarboxylase (GLDC), located on chromosome 9, is known as a potential metabolic oncogene regulated by LKB1, and play a role in serine-glycine metabolism pathway involvement in tumorigenesis. GLDC catalyzes glycine into ammonia, carbon dioxide, and methylenetetrahydrofolate which carry the methylene group entering the threonine cycle and are assisted in SAM (methylation substrate) production [6]. So, GLDC overexpression contributes to cell growth throughout the genome hypermethylation. In this regard, studies suggested that GLDC is essential for tumor-initiating cells in non-small cell lung cancer (NSCLC) and has a higher level of expression in glioma [13, 14].
Therefore, given the epigenetic importance in AML trigger and promotion and due to the lack of data regarding these gene expression statuses in AML patients, this study aimed at determining the gene expression level of LKB1 as an epigenetic regulator similar to a tumor suppressor as well as that of SHMT1 and GLDC as metabolic enzymes, which play a major role in the epigenetic pathway throughout SAM production.
Materials and Methods
Patients and Healthy Volunteers
60 AML cases [Peripheral blood (PB)] and 30 control samples (PB) from healthy volunteers were obtained to conduct the experiment. The cases were in the 3–87 age range and the patients were classified into M3/NM3 (Non-M3) subtypes based on the FAB/WHO classification (32% and 68% cases were M3/NM3 respectively, and there were no cases of M7 and M6 in this study). Written informed consent was obtained from all participants including adult healthy volunteers, adult patients, and the parents/legally authorized representatives of the minor participants. In addition, the ethics committee approval was requested from Shahid Beheshti University of Medical Sciences Ethics Committee (IR.SBMU.RETECH.REC.1396.976).
RNA Isolation, cDNA Synthesis, Real-Time PCR
RNA extraction was performed using the RNasy Kit (Qiagen, Germany). The purity of the sample was evaluated by the Nanodrop (OD 260/280 nm ratio > 1.8) (Thermo Scientific, USA). cDNA was subsequently produced using a TaKaRa kit (Japan). Primer sequences used in this study were demonstrated in Table 1, and the ABL primer was considered as a housekeeping gene. The total reaction in qRT-PCR (Rotor-Gene 6000, Qiagen) was 15 µL, which consisted of 0.5 µL for both forward and reverse primers, 4.5 µL water, 7.5 µL of RealQ Plus 2x Master Mix Green- Low ROX (Amplicon, Denmark), and 2 µL of template cDNA. The amplification of cDNA products in thermal cycling conditions for each reaction (LKB1, SHMT1 and GLDC and ABL) was demonstrated in Table 2. All the experiments were performed in duplicate with a negative control. Gene expression levels were calculated using 2−ΔΔCt and Livak formula.
Table 1.
Nucleotide sequences of primers used for ABL, LKB1 and SHMT1 and GLDC qRT-PCR reactions
| Gene | Forward | Reverse |
|---|---|---|
| ABL | AGTCTCAGGATGCAGGTGCT | TAGGCTGGGGCTTTTTGTAA |
| LKB1 | ATGGCACTCTGGTCACTG | TTAAATCTTGCAACCTGG |
| SHMT1 | ACCGGCGCACAGAGGAAGAGAA | TGGGGAGAGGAGCTGGTGTTGT |
| GLDC | AAACCAGGGAGCAACACATTCG | GCAGCCATATTCGCCAAGAGG |
Table 2.
The Program time and temperature of Real-time PCR for target genes and reference gene
| Primers | Initial hold | Denaturation | Annealing/extension | Final extension |
|---|---|---|---|---|
| ABL | 95 °C for 10 min | 95 °C for 10 s | 62 °C for 15 s | 72 °C for10 min |
| LKB1 | 95 °C for 10 min,40 cycles | 95 °C for 45 s | 61.7 °C for 9 s | 72 °C for 5 min |
| SHMT1 | 95 °C for 10 min,40 cycles | 95 °C for 15 s | 60 °C for 15 s | 72 °C for 5 min |
| GLDC | 62 °C for 15 s,40 cycles | 95 °C for 15 s | 60 °C for 15 s | 72 °C for 5 min |
Data Analysis
Statistical analysis was performed by SPSS (version 16.0) and GraphPad Prism 6.07 software. The normal distribution of the genes was examined by the Shapiro–Wilk and Kolmogorov–Smirnov. The t-test or Mann–Whitney samples from parametric and nonparametric tests were used for the analysis of gene expression differences between patients and controls, and two-state variables were used in order to determine the significant levels. Pearson´s and Spearman Chi squared samples were applied for the evaluation of Gene expression correlation between M3/NM3 AML subtypes and the normal controls. P < 0.05 was considered as the significance level.
Results
Profile of Patient Sample Specifications
The demographic and clinical information of 60 patients with de novo AML and 30 normal controls from healthy volunteers were indicated in Table 3.
Table 3.
Sample and patient characteristics
| Characteristics | Data |
|---|---|
| Age |
Median (range) 38 years (3–78) |
|
Specimen type PB |
% 100 |
| Classification (FAB) | % |
| M3 | 32 |
| NM3 | 68 |
| Blast percentage |
Median (range) 70 (35–96) |
LKB1 and SHMT1 and GLDC Expression in AML Patients and Healthy Subjects
In normal samples, the mean Ct values (± SD) of ABL, LKB1, SHMT1, and GLDC were 25.74 ± 1.61, 24.50 ± 1.53, 33.64 ± 1.96, and 34.84 ± 1.11, respectively. The obtained mean Ct values (± SD) in 60 de novo AML samples were 23.87 ± 2.83, 24.82 ± 3.28, 29.39 ± 2.92, and 32.53 ± 2.79 for ABL, LKB1, SHMT1, and GLDC, respectively. Subsequently, the Ct values for LKB1, SHMT1, and GLDC were normalized against ABL as the housekeeping gene for both patients and the control samples. The normalized values of AML-positive and the normal control group were compared statistically in terms of the level of expression in the range of a 95% confidence interval. The average expression level for LKB1, SHMT1, and GLDC in the healthy population was considered intermediate at 1.1–10.9, 0.001–0.021 and, − 0.0003–0.01, respectively. This analysis reveals that 28%, 45%, and 28% of AML positive patients carried intermediate LKB1, SHMT1, and GLDC expression respectively. Expression levels that fell below the threshold of the intermediate ranges for LKB1, SHMT1, and GLDC were defined as low expression levels and were observed in 69% of LKB1 in AML-positive patients (P-value = 0.00, OR 0.77, 95% CI 0.6–0.9). However, low expression levels were not observed in the SHMT1 and GLDC in AML-positive patients. Conversely, high expression levels were detected in 53% and 13% of SHMT1 and GLDC in AML positive patients, respectively (P-value = 0.00, OR 5.7, 95% CI 1–7.9/P-value = 0.4, OR 4.0, 95% CI 0–2.4). Overall, significant low expression of LKB1 and high expression of SHMT1 was confirmed whilst there was not a significant alteration in GLDC gene expression (Fig. 1).
Fig. 1.
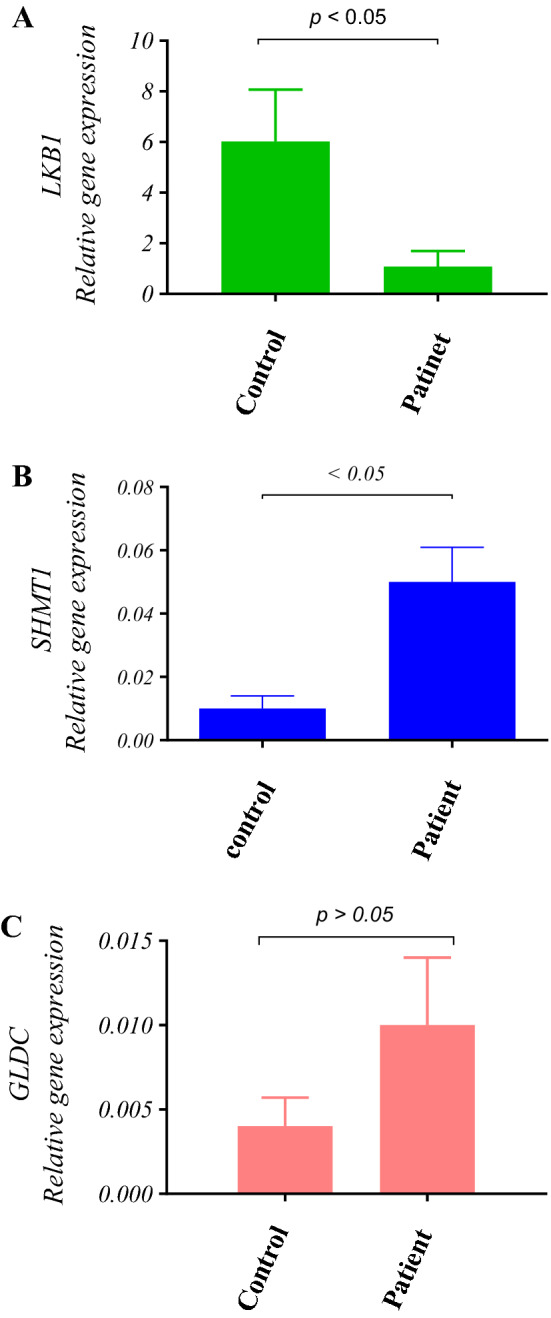
The relative expression of LKB1, SHMT1, and GLDC in 60 AML patients and 30 normal controls was measured using the 2−ΔΔct approach by considering ABL as a housekeeping gene. a There was a significant difference (P < 0.05) for LKB1 expression between AML patients and the normal controls. The LKB1 relative expression level of 1.85 ± 0.617 (Mean, SEM) was obtained in AML patients as opposed to 6.02 ± 2.04 (Mean, SEM) in the normal controls. In comparison with the normal controls, the fold change of the LKB1 expression level was 0.18. b Also, there was a significant difference (P < 0.05) for SHMT-1 expression between AML patients and the normal controls. The SHMT-1 relative expression level of 0.05 ± 0.011 (Mean, SEM) was obtained in AML patients as opposed to 0.01 ± 0.004 (Mean, SEM) in the normal control group. In comparison with the normal controls, the fold change for the SHMT1 expression level was 4.68. c There was no significant difference (P > 0.05) for GLDC expression between AML patients and the normal controls. A relative GLDC expression level of 0.010 ± 0.004 (Mean, SEM) was measured in AML patients as opposed to 0.004 ± 0.0017 (Mean, SEM) in the normal controls
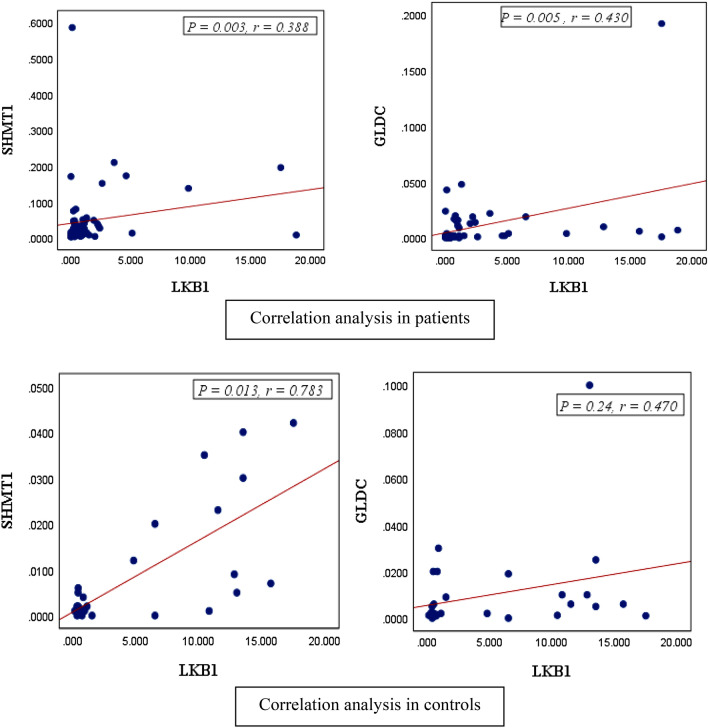
Correlation Between the Expression Levels of LKB1 and SHMT1 and GLDC
To determine whether the expression of LKB1, SHMT1, and GLDC was dependent or independent, statistical analysis was performed. The analysis verified a positive and significant correlation between LKB1 and SHMT1 in both AML patients (0.003, r = 0.388) and the normal controls (P = 0.013, r = 0.783). In addition, it was evident that the correlation in the control group was stronger than that of the AML samples. The gene expression of LKB1 and GLDC revealed a significant correlation between the 60 AML patients (P = 0.005, r = 0.430). The correlation between LKB1 and GLDC gene expression was not statistically significant in the normal controls (P = 0.24, r = 0.470) (Fig. 2a, b).
Fig. 2.
Statistical analysis employing Spearman Chi squared sample suggests a relationship between the expression of LKB1 with SHMT1 and GLDC in AML patients. a The correlation between LKB1 and SHMT1 in 60 AML patients was determined to be positive and significant (P = 0.003, r = 0.388), and the correlation between LKB1 and SHMT1 gene expression was stronger in normal controls than in AML samples (P = 0.013, r = 0.783). b The correlation between LKB1 and GLDC in 60 AML patients was determined to be positive and significant (P = 0.005, r = 0.430), and there was no significant correlation between LKB1 and GLDC gene expression in normal controls (P = 0.24, r = 0.470)
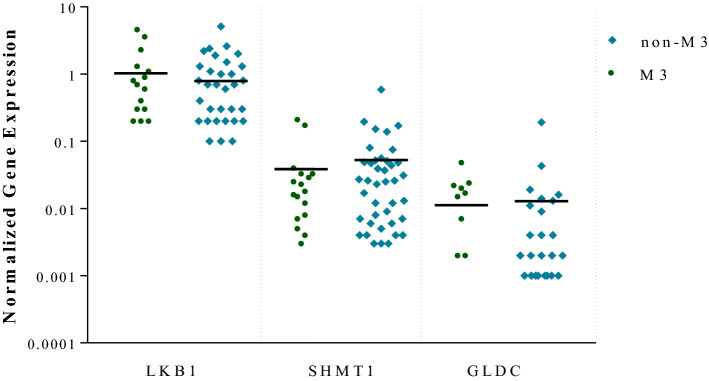
Differential Expression of LKB1 and SHMT1 in M3/NM3 AML Subtypes
The differential expression of LKB1, SHMT1, and GLDC between M3/NM3 FAB subtypes was analyzed using nonparametric samples. The expression level of LKB1 mRNA was not significantly different between M3/NM3 subtypes (P > 0.05). However, in contrast to the normal controls, a significant change was observed in the LKB1 expression (Fig. 3). There was no significant SHMT1 mRNA expression level between M3/NM3 subtypes (P > 0.05). Nevertheless, a significant change was obtained in the SHMT1 mRNA expression level between the patients and the normal group (Fig. 3).
Fig. 3.
The analysis of the relative expression of LKB1 and SHMT1 and GLDC using the Man-Whitney U test in 60 AML patients sorted by M3/NM3 FAB subtypes revealed no significant difference in mRNA expression between these subtypes (P > 0. 05)
Discussion
DNA methylation in the promoter region of tumor suppressors and oncogenes plays a vital role in the development of hematological malignancies, especially in acute myeloid leukemia, however, its mechanism has long been remained obscure [10, 15]. In addition, not many investigations have been conducted on aberrancies in epigenetic modifying enzymes and their expression level in AML patients. Recently, researchers have identified an impressive pathway to drive the leukemogenesis, which is associated with DNA methylation signature, through the affording methyl donor group. This metabolic axis contains LKB1 as the main regulator and serine-glycine pathway enzymes as methylation substrate supporters [8].
In this study, we analyzed the gene expression level of LKB1, SHMT1, and GLDC, which have recently been known as epigenetic axis components. Our results suggested a significantly lower expression of LKB1 and higher expression of SHMT1 (fold change: 0.18 and 4.68 respectively) in AML samples than that in normal controls. However, no significant alterations were observed in the GLDC gene expression. Moreover, there was a meaningful correlation between LKB1 and SHMT1 gene expression in both AML samples and the normal controls. On the other hand, despite the absence of statistical overexpression for GLDC gene expression level (fold change: 2.41), there was a positive correlation in LKB1 and GLDC gene expression in AML samples. Nevertheless, the normal controls were not taken into consideration since it may express their pathway importance in this type of malignancy.
Furthermore, the evaluation of LKB1, SHMT1, and GLDC expression levels in M3/NM3 patients did not reveal important changes in this study, which might be due to similar involvement mechanisms in these pathways in AML subtypes. However, further studies must be conducted in this regard.
Altogether in agreement with our findings, Kottakis et al. [8] report a low expression for LKB1 and overexpression for SHMT1 and GLDC in tumor cell lines that the examination of gene expression relevance indicated a significant relationship between LKB1 and the epigenetic axis through metabolic enzymes.
Furthermore, there are lots of studies that explain LKB1 overexpression concerning different networks. For instance, Green et al. [16] introduced a tumor suppressor activity of LKB1 in AML through the repression of mTOR mRNA translation. Zhang et al. [17] suggested that LKB1 deletion increases angiogenesis and tumor growth via the vascular endothelial growth factor, which confirms the tumor suppressor function of LKB1. In another study, Ma et al. [18] revealed that LKB1 inhibits the proliferation of gastric cancer cells by suppressing the nuclear translocation of Yap and β-catenin. All these studies from different points of view are inconsistent with our hypothesis in the tumor suppressor role of LKB1. Accordingly, considering the results of this study, it can be concluded that LKB1 plays its suppressor role differently as a master regulator whose main mechanism in AML patients requires further examinations.
On the other hand, in a study conducted by Yi-Wei Wang et al. and Zhao et al., the SHMT1 polymorphisms are potentially associated with a malignant state, which is consistent with the results of this study as well as similar studies revealing an important change in SHMT1 expression in AML samples [11, 19]. Paone et al. [20] point out the vital role of SHMT1 in lung cancer patients. They indicated SHMT1 overexpression in the tissue samples of lung cancer patients and lung cancer cell lines. The results of this, which are associated with different types of malignancies, provide new data for the SHMT1 oncogenic role.
In a study performed by Maybelle Kho Go et al., glycine decarboxylase was considered an unusual enzyme involved in tumorigenesis [21]. The study was conducted by Noh et al. [13] on the serine/glycine metabolism-related protein level of gene expression, including SHMT1 and GLDC, in triple-negative breast cancer tissues which have proved major alterations. According to a study carried out by Xiangdong, the expression of both GLDC mRNA and protein increased in the B cell lymphoma tissue of the p53 protein-positive group. Therefore, the overexpression of the GLDC gene expression level can cause important changes in glycine/serine metabolism leading to tumor initiation and development [14]. In the present study, however, no statistically critical alterations were observed in the GLDC gene expression level, but it may be due to substrate consumption by SHMT1 overexpression.
Finally, correlation analysis showed that there was an inverse and significant relationship between the intended genes. This finding supports the hypothesis of this study, as well as evidence from other studies on the effect of LKB1 regulation and its association with metabolic pathways gene expression. Although it is not possible to make a precise claim because of lack of data about the protein level of these enzymes and rate of SAM production, it can be proposed that, given the role of metabolic pathway enzymes in methylation substrate production, the LKB1 decreasing gene expression, and serine metabolism increasing gene expression are associated with elevated SAM production and facilitates the epigenetic abnormalities spectrum in AML. So, they can be considered as therapeutic targets and drug design in this group of patients.
Acknowledgements
We would like to express our gratitude to the honorable administrators, authorities and employees of the Hematology Laboratory and Blood Banking Department of the School of Allied Medical Sciences and Hematopoietic Stem Cell Transplantation Research Center of Shahid Beheshti University of Medical Sciences.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nezhad HA, Mohammadi MH, Khosravi MR, Salari S, Hajifathali A, Farsani MA. The evaluation of p21 and p27 expression in HLA-DR negative AML patients. Middle East J Fam Med. 2018;7(10):253. [Google Scholar]
- 2.Salarpour F, Goudarzipour K, Mohammadi MH, Ahmadzadeh A, Faraahi S, Allahbakhshian A, et al. Evaluation of growth factor independence 1 expression in patients with de novo acute myeloid leukemia. J Cancer Res Ther. 2018;16(1):23. doi: 10.4103/jcrt.JCRT_129_17. [DOI] [PubMed] [Google Scholar]
- 3.Amiri V, Mohammadi M, Farsani MRK, Gharehbaghian A, Hajifathali A, Khazaei Z, et al. Evaluation of UHRF1 and P16INK4A expression levels in newly diagnosed AML patients. Biomed Res Ther. 2018;5(9):2658–2663. doi: 10.15419/bmrat.v5i9.475. [DOI] [Google Scholar]
- 4.Eriksson A, Lennartsson A, Lehmann S. Epigenetic aberrations in acute myeloid leukemia: early key events during leukemogenesis. Exp Hematol. 2015;43(8):609–624. doi: 10.1016/j.exphem.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Gan R-Y, Li H-B. Recent progress on liver kinase B1 (LKB1): expression, regulation, downstream signaling and cancer suppressive function. Int J Mol Sci. 2014;15(9):16698–16718. doi: 10.3390/ijms150916698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green AS, Chapuis N, Lacombe C, Mayeux P, Bouscary D, Tamburini J. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle. 2011;10(13):2115–2120. doi: 10.4161/cc.10.13.16244. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Marcus AI, Vertino PM. Dysregulation of mTOR activity through LKB1 inactivation. Chin J Cancer. 2013;32(8):427. doi: 10.5732/cjc.013.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kottakis F, Nicolay BN, Roumane A, Karnik R, Gu H, Nagle JM, et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016;539(7629):390. doi: 10.1038/nature20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 2015;22(5):861–873. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoofs T, Berdel W, Müller-Tidow C. Origins of aberrant DNA methylation in acute myeloid leukemia. Leukemia. 2014;28(1):1. doi: 10.1038/leu.2013.242. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y-W, Zhang S-D, Xue W-J, Zhu M-L, Zheng L-Z. SHMT1 C1420T polymorphism contributes to the risk of non-Hodgkin lymphoma: evidence from 7309 patients. Chin J Cancer. 2015;34(3):60. doi: 10.1186/s40880-015-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T, Shen L, Zhang X, Gu D, Zhang Q, Huo X, et al. Clinical significance of SHMT1 rs1979277 polymorphism in Asian solid tumors: evidence from a meta-analysis. Genet Mol Res. 2015;14:5602–5614. doi: 10.4238/2015.May.25.12. [DOI] [PubMed] [Google Scholar]
- 13.Noh S, Kim DH, Jung WH, Koo JS. Expression levels of serine/glycine metabolism-related proteins in triple negative breast cancer tissues. Tumor Biol. 2014;35(5):4457–4468. doi: 10.1007/s13277-013-1588-z. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Cui C, Guo Y, Yang G. Glycine decarboxylase expression increased in p53-Mutated B cell lymphoma mice. Oncol Res Treat. 2015;38(11):586–589. doi: 10.1159/000441595. [DOI] [PubMed] [Google Scholar]
- 15.Toyota M, Kopecky KJ, Toyota M-O, Jair K-W, Willman CL, Issa J-PJ. Methylation profiling in acute myeloid leukemia. Blood. 2001;97(9):2823–2829. doi: 10.1182/blood.V97.9.2823. [DOI] [PubMed] [Google Scholar]
- 16.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C et al (2010) The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood 2010:blood-2010-02-269837 [DOI] [PubMed]
- 17.Zhang W, Ding Y, Zhang C, Lu Q, Liu Z, Coughlan K, et al. Deletion of endothelial cell-specific liver kinase B1 increases angiogenesis and tumor growth via vascular endothelial growth factor. Oncogene. 2017;36(30):4277. doi: 10.1038/onc.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L-G, Bian S-B, Cui J-X, Xi H-Q, Zhang K-C, Qin H-Z, et al. LKB1 inhibits the proliferation of gastric cancer cells by suppressing the nuclear translocation of Yap and β-catenin. Int J Mol Med. 2016;37(4):1039–1048. doi: 10.3892/ijmm.2016.2494. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Takeuchi S, Hofmann WK, Ikezoe T, van Dongen JJ, Szczepański T, et al. Aberrant methylation in promoter-associated CpG islands of multiple genes in acute lymphoblastic leukemia. Leuk Res. 2006;30(1):98–102. doi: 10.1016/j.leukres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Paone A, Marani M, Fiascarelli A, Rinaldo S, Giardina G, Contestabile R, et al. SHMT1 knockdown induces apoptosis in lung cancer cells by causing uracil misincorporation. Cell Death Dis. 2014;5(11):e1525. doi: 10.1038/cddis.2014.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go MK, Zhang WC, Lim B, Yew WS. Glycine decarboxylase is an unusual amino acid decarboxylase involved in tumorigenesis. Biochemistry. 2014;53(5):947–956. doi: 10.1021/bi4014227. [DOI] [PubMed] [Google Scholar]