Abstract
T cell therapy represents a new class of immunotherapies garnering considerable attention. T cell receptor beta chain constant region 1 (TRBC1) is partially expressed in subsets of normal T cells. However, the immunotherapy of T lymphocyte tumors is rarely validated in clinical trials. Here, we aim to explore whether TRBC1 is a promising target for the immunotherapy of T lymphocyte tumors. This study examined TRBC1 expression in 25 healthy bone marrow samples, 39 patients with T-lineage acute lymphocytic leukemia (T-ALL), 4 patients with mature T cell neoplasms, and 5 patients suspected with mature T cell neoplasms with evidence of T cell neoplasia. Moreover, the expression of TRBC1 was evaluated by flow cytometry and through PCR detection of TCR gene rearrangements. The expression of monophasic TRBC1 was identified in all 25 normal bone marrows (23.83% ± 2.74% positive rate). The expression of TRBC1 was positive in 5 patients (12.8%) among the 39 T-ALL patients. TRBC1 was partially expressed in 1 patient (25%) with T cell non-Hodgkin's lymphoma (T-NHL) and in 1 patient (20%) suspected to have T-NHL. Healthy donors showed a pattern of partial expression and patients with T-lymphocyte tumors showed a polytypic TRBC1 expression pattern. Thus, TRBC1 may be a diagnostic and therapeutic marker for T lymphocyte tumors.
Keywords: T lineage acute lymphocytic leukemia, T cell receptor β chain constant region 1, Flow cytometry, T cell receptor rearrangement
Introduction
T-lineage acute lymphoblastic leukemia (T-ALL) comprises up to 25% of all acute lymphoblastic leukemia (ALL) cases [1, 2], including early cortical, late cortical, and mature T cell stages. In 2016, the World Health Organization updated the classification of lymphoid neoplasms to include 27 mature T cell neoplasms [3]. Peripheral T cell lymphoma-not otherwise specified (PTCL-NOS; 26%) is the most common subgroup of T cell lymphoma, followed by angioimmunoblastic lymphoma (18%). Natural killer/T cell lymphoma (NKTCL) and adult T cell leukemia/lymphoma (ATL) represent 12% and 10% of cases, respectively [4]. PTCL is a heterogeneous group of tumors [5], accounting for 15%-20% of aggressive non-Hodgkin’s lymphomas (NHL) in Western countries [6–8], and is more common in middle-aged/elderly patients with stage III to IV disease, with nodal and/or extranodal locations [9–11], who die rapidly despite aggressive therapies [12, 13]. Malignant NHL tumors arise from the clonal expansion of B-, T-, or natural killer (NK) cells. T-NHL accounts for 10–15% of all NHL [14]. T cell lymphoma PTCL makes up mature T cell neoplasms that represents 5–10% of all lymphomas in the United States [15, 16]. Lymphoid malignancies with the T cell immunophenotype are associated with unique biologic behaviors and clinical features, where patients have a worse prognosis than those with B-progenitor lymphoblastic diseases [7]. However, the diagnostic and therapeutic targets of T lymphocyte tumors remain to be determined.
By genomic recombination, variable domains of the α and β subunits are assembled from discrete V, D, and J gene elements. The β chain includes the D region and two junctional regions (V–D and D–J), while the α chains only have one (V–J), therefore, the β chain is inherently more complex than the α chain [17, 18]. Most T-ALL tumors do not express the surface T cell receptor (TCR), while many lymphoma cells are TCR-positive [19]. T cells exclusively express only one of the two genes encoding a constant region of the TCR β chain: TCR β chain constant region 1 (TRBC1) and TCR β chain constant region 2 (TRBC2) [20]. Under normal circumstances, the expression of TRBC1 and TRBC2 genes showed a certain proportion of scattered expression. Recently, Maciocia et al. indicated that a normal population of T cells consist of a mixture of TCRB1+ and TCRB2+ cells. It showed that an antibody targeting TRBC1 was developed for a chimeric antigen receptor T cell therapy for T cell lymphomas [13]. Shimin et al. reported that monophasic (clonal) TRBC1 expression was identified in 17 cases of TRBC1‐positive events, and 3 cases with monophasic homogenous TRBC1‐dim expression [21]. Non-neoplastic T-cell populations are polyclonal for TCR gene rearrangements, whereas PTCLs are typically monoclonal [22]. However, the expression patterns of TRBC1 on the T lymphocyte tumors in clinical settings remain rare.
In present study, the expression of TRBC1 in was detected by flow cytometry. Moreover, the TRBC1-positive patients were validated by TCR rearrangement to evaluate whether this marker can be a target for T lymphocyte tumor chimeric antigen receptor T cell therapy.
Materials and Methods
Patient Selection
Samples were collected prospectively at different time points from October 2018 to March 2019 (48 samples), from patients who had their bone marrows submitted for routine flow cytometric analysis at the Hebei Yanda LU Dao-Pei Hospital. Control samples were from 15 men and 10 women, with a median age of 37 years (range 16–53 years). There were also 39 T-ALL patients, comprised of 5 patients with early precursor T-ALL (Pro-T-ALL), 21 patients with precursor T-ALL (Pre-T-ALL), 10 patients with cortical T-ALL, and 3 patients with medullary T-ALL. Four patients had confirmed T cell lymphoma, with a median age of 33 years (range 8–60 years), including one patient with peripheral T cell lymphoma (PTCL), two patients with angioimmunoblastic T cell lymphoma (AITL), one patient with unclassified CD4+ T cell lymphoma, and five patients with suspected abnormal proliferation of T cell lymphoma. The clinical, pathological, and morphological information of the patients were reviewed to determine the reliability of patient diagnosis. This study was approved by the hospital ethics committee of the Hebei Yanda LU Dao-Pei Hospital. Consent forms were obtained from all patients for study participation. Sample source details are shown in Table 1.
Table 1.
Clinical information of patients
| Characteristics | Healthy donor (n = 25) | T-ALL patient (n = 39) | T-NHL patients (n = 4) | Patient with suspected abnormal hyperplasia of T-cell lymphoma (n = 5) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pro-T-ALL patient (n = 5) | Pre-T-ALL patient (n = 21) | Cortical T-ALL patient (n = 10) | Medullary T-ALL patient (n = 3) | PTCL patient (n = 1) | AITL patient (n = 2) | Patient with unclassified CD4 + T-cell lymphoma (n = 1) | |||
| Male | 15 | 29 | 3 | 2 | |||||
| Female | 10 | 10 | 1 | 3 | |||||
| Age range | 16–53 | 1–69 | 8–60 | 2–69 | |||||
| Median age | 37 | 16 | 33 | 28 | |||||
T-ALL T lineage acute lymphocytic leukemia; Pro-T-ALL early precursor T lineage acute lymphocytic leukemia; Pre-T-ALL precursor T lineage acute lymphocytic leukemia; PTCL Peripheral T-cell lymphomas; AITL angioimmunoblastic T-cell lymphoma; T-NHL T-cell Non-Hodgkin's lymphomas
Flow Cytometry
Bone marrow samples were collected in dipotassium ethylenediamine tetraacetic acid (EDTA-K2) anticoagulant. These samples were stored at 25 °C for 72 h after collection. The bone marrow samples were then diluted with 0.1 M hydrochloric acid solution. A counter star cell counter was used to count the number of nucleated cells in each sample, and the specimens were diluted to a nucleated cell count of a total of 1 × 106/ml. R-phycoerythrin (PE) mouse anti-human-TRBC1 antibody (clone JOVI-1, BD Phamingen) and CD45/SSC and CD3/SSC gate antibodies (APC, mouse anti-human, CD7, Clone: M-T701; V500, mouse anti-human, CD45, Clone: HI30; FITC, mouse anti-human-CD4, clone: RPA-T4; APC, mouse Anti-CD3, clone: SP34-2) purchased from BD Phamingen, were used and incubated in the dark at 25 °C. Then, 2 ml of diluted hemolysin (BD Bioscience) was added, incubated at 25 °C for 10 min in the dark, subsequently adding 2 ml of phosphate buffered saline (PBS) to stop hemolysis, and washed twice by centrifugation at 1500 rpm. The cells were then resuspended in PBS, the antibodies were added, and the setups were incubated for 30 min at 4 °C. The setups were then subject to flow cytometry (BD FacsCanto II, BD Biosciences), until 100,000 events were acquired. Cells were analyzed using BD Diva and BD Cellquest software.
TCR Gene Rearrangement Detection
An Identification gene clonality assay kit (Invivoscribe Technologies, USA) was used to detect and analyze the TCRB, TCR delta chain (TCRD), and TCR gamma chain (TCRG) gene resequencing of the samples positive for TRBC1 as detected by flow cytometry. Genomic DNA was extracted from bone marrow samples. The DNA was resuspended in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0) to a final concentration of 100–400 μg/ml. After passing quality inspection, polymerase chain reaction (PCR) amplification of TCRB, TCRD, and TCRG recombination was performed using the relevant primers, and detected by AB 9700 (Applied Biosystems, USA), according to the manufacturer instructions. PCR amplification was performed using an ABI 7500 RT-PCR system (Applied Biosystems, Foster City) with the following parameters: pre-denaturation at 95 °C for 7 min, 34 cycles of 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 90 s, a solution curve stage at 72 °C for 10 min, then indefinitely held at 15 °C until collection. Capillary gel electrophoresis was used to separate amplification products on an AB 3500XL gene sequencing instrument (Applied Biosystems, USA), and the results were analyzed using GeneMapper ID-X software.
Statistical Analysis
All data statistics and scatter plots were analyzed using SPSS 13.0 software. Statistical results are reported as mean values ± standard deviation (SD). The percentage of TRBC1 positive events were calculated by Student’s t-test and one-way ANOVA for comparisons between multiple groups. All differences were adjusted and p values < 0.05 were considered to be significant.
Results
Different Expression Patterns of TRBC1 were Found in Bone Marrow Samples
In the bone marrow samples, TRBC1 showed a partial expression in CD3+ T-lymphocyte cells in all healthy donors, and the percentages of TRBC1-positive cells ranged from 21.7 to 44.0% (mean = 30.15%). The mean ± 2 times SD of all results ranged from 30.15 ± 12.06%. The range of the 95% confidence interval (CI) was 23.83% ± 2.74% (Fig. 1a). The detailed statistical results are shown in Tab 2. The positive expression rate of TRBC1 in T-ALL samples was 12.8% (5/39 cases). Among them, the expression rate of TRBC1 with all-positive values was 10.3% (4/39 cases). Moreover, the positive expression rate of TRBC1 in T-NHL patients was 25% (1/4 cases), and the positive expression rate of TRBC1 collectively comprise about 20% (1/5 cases) of the suspected T cell lymphoma patients. T-lymphocyte cells exhibited a partial expression of TRBC1 in all healthy donors, while the neoplastic cells showed a polytypic expression of TRBC1 (Fig. 1a).
Fig. 1.
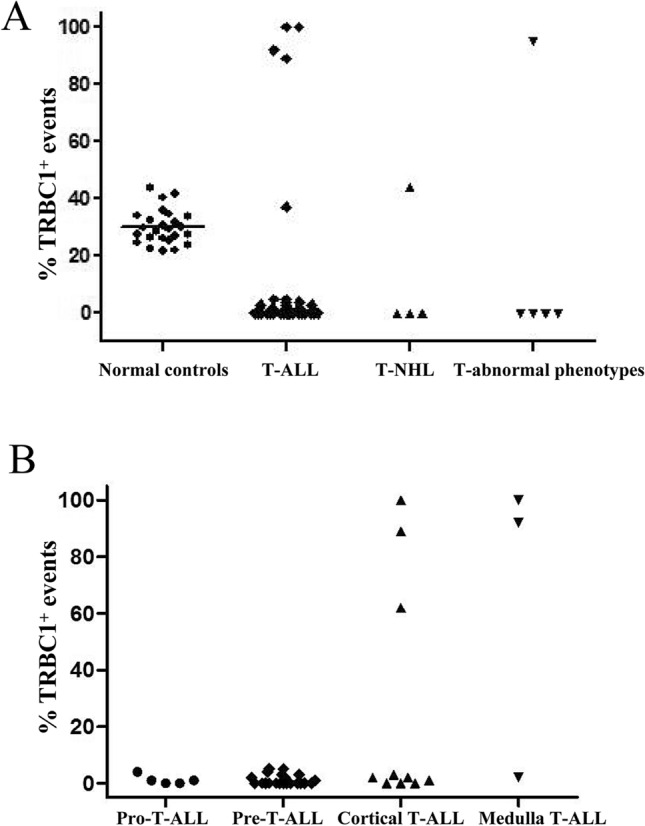
The expression patterns of TRBC1 on malignant CD3-positive T cell subsets from patients. a T cell receptor (TCR) αβ CD3-positive T cell subsets was identified in representative bone marrow sample from healthy donors and patients. b Percentages of TRBC1-positive events on benign CD3-positive subsets identified. The graph shows data from 39 T-ALL patients with no evidence of T cell malignancy. Each dot represents a sample. Lines depict the mean percentages for each group
Table 2.
The expression of TRBC1 in patients
| Characteristics | Number of patient | Positive expression | Negative | Positive rate (%) | Positive rate (%) | Total positive rate (%) | |
|---|---|---|---|---|---|---|---|
| All positive (case) | Partially positive (case) | ||||||
| T-ALL(39) | |||||||
| Pro-T-ALL | 5 | 0 | 0 | 5 | 0 | 12.8 | 10.3 |
| Pre-T-ALL | 21 | 0 | 0 | 20 | 0 | ||
| Cortical T-ALL | 10 | 2 | 1 | 7 | 30% | ||
| Medullary T-ALL | 3 | 2 | 0 | 1 | 66.7% | ||
| T-NHL (4) | |||||||
| AITL | 2 | 0 | 0 | 2 | 0 | 25 | 25 |
| PTCL | 1 | 0 | 0 | 1 | 0 | ||
| Unclassified CD4+ T-NHL | 1 | 0 | 1 | 0 | 100% | ||
| Suspected T-cell lymphoma (5) | 5 | 1 | 0 | 4 | 20% | 20 | 20 |
T-ALL T lineage acute lymphocytic leukemia; Pro-T-ALL early precursor T lineage acute lymphocytic leukemia; Pre-T-ALL precursor T lineage acute lymphocytic leukemia; T-NHL T-cell Non-Hodgkin's lymphomas; AITL angioimmunoblastic T-cell lymphoma; PTCL Peripheral T-cell lymphomas
In addition, we also detected the expression of TRBC1 in the T-ALL and T-NHL subsets. One case of unclassified CD4+ T-NHL showed positive expression (100%). The TRBC1 expression rate of medullary T-ALL was 66.7% (2/3) of the patients with T-ALL. These results suggested that all CD3+ T cell subsets identified on T-ALL showed a polytypic TRBC1 expression pattern (Fig. 1b), with percentages of TRBC1-positive events ranging from 0 (Pro-T-ALL and Pre-T-ALL) to 66.7% (medullary T-ALL). Importantly, the positive expression rate of TRBC1 increased with mature development in T-ALL patients. In our practice, patients with T-lymphoma have a negative TRBC1 expression rate of 83.33% (40/48 cases), a positive expression rate of 10.42% (5/48 cases), and a partial expression rate of 4.17% (2/48 cases). Surprisingly, healthy patients showed a partial expression rate of 100% (Table 2). The above results indicate that TRBC1 negative expression or positive expression patterns are present in patients with T-lymphoid tumors, with only two cases of partial expression patterns. However, healthy patients predominantly showed a partial expression pattern.
Representative Expression of TRBC1
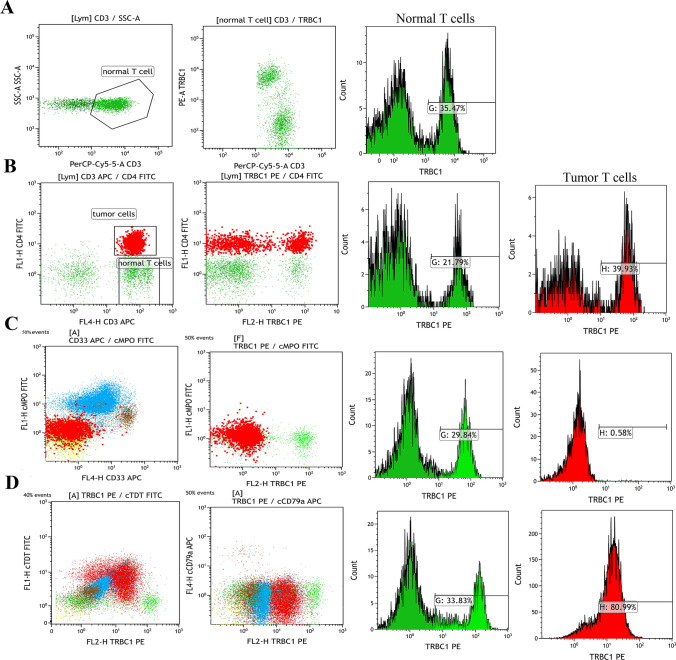
We also evaluated the percentages of TRBC1-positive events according to the fluorescence threshold at the approximate midpoint between TRBC1-positive and TRBC1-negative background benign T cells. Representative flow cytometry plots from 25 bone marrow samples of healthy donors showed a 35.47% expression rate of TRBC1 in CD3+ T cells (Fig. 2a). An unclassified CD4+ T-NHL patient showed a partial positive expression rate of TRBC1 (39.93%) in CD3+ CD4+ T malignant cells. TRBC1 expression rate was 21.79% in normal CD3+ CD4− T lymphocytes (Fig. 2b). A representative pre-T-ALL patient with negative TRBC1 expression showed 0.58% TRBC1 expression rate in CD3+ CD4+ T malignant cells, and a 29.84% expression rate of TRBC1 in normal T lymphocytes (CD3+ CD4− T cells) (Fig. 2c). A representative cortical T-ALL patient with positive TRBC1 expression showed a expression rate of TRBC1 in CD3+ CD4+ T malignant cells was 80.99%, and the TRBC1 expression rate in normal T lymphocytes (CD3+ CD4− T cells) was 33.83% (Fig. 2d).
Fig. 2.
The expression patterns of TRBC1 on tumor cells and normal T cells. a Representative flow cytometry plots from 25 healthy donor bone marrow samples showed expression patterns of TRBC1 on normal CD3-positive T cell populations. b Gates were set in selecting an unclassified CD4+ T-NHL patient with partially positive TRBC1, and dot plots of subclass CD4-FITC/TRBC1 PE with quadrant markers were created. The histogram shows CD4+ T-NHL tumor cells positive for TRBC1 in the R7 gates and CD3+CD4− normal T cells positive for TRBC1 in the R3 gates. c Bone marrow involvement by a representative Pre-T-ALL patient with non-TRBC1-expressing T cell malignancy, showing an expanded cytoplasmic MPO (cMPO)-negative T cell population with an aberrant negative expression of TRBC1. Gated neoplastic cells that are TRBC1 expression-negative (monotypic) are red, and the normal T cells with partial expression of TRBC1 are green. d Bone marrow sample involvement by a representative cortical T-ALL patient with a positive-TRBC1-expressing T cell malignancy (red), showing an expanded cTDT-positive T cell population with aberrant positive expression of TRBC1; green is normal and is negatively expressed, red is abnormal and positively expressed
TCR Rearrangement Presented in Positive TRBC1 Expression Patients
To verify the flow cytometry results, we analyzed TCR gene diversity through the detection of TCRB, TCRD, and TCRG rearrangements in five patients with TRBC1-positive expression and three TRBC1-negative patients (Table 3). T cell rearrangement tests found that the TCRB-positive rearrangement rate was 60% (3/5 cases), TCRD-positive rearrangement rate was 40% (2/5 cases), and TCRG-positive rearrangement rate 80% (4/5 cases) in five TRBC1-positive patients. T cell gene rearrangement examinations on bone marrow samples of patients with positive TRBC1 indicated tracings consistent with small monoclonal T cell subsets. The TCRG gene rearrangement results of a representative cortical T-ALL patient showed a non-Gaussian distribution, but is monoclonal-positive (Fig. 3). These results showed that at least one TCR was rearranged in five patients with positive TRBC1 expression, which was consistent with TRBC1 monoclonal expression. We also analyzed the bone marrow samples of patients with negative TRBC1 by T cell gene rearrangement examinations, indicated that the TCRB, TCRD and TCRG-negative rearrangement rate were 100% (3/3 cases) in three TRBC1-negative patients (Fig. 4).
Table 3.
Rearrangement of five TRBC1-positive and three TRBC1-negative patients
| Patients | All (case) | TCR rearrangement result |
|---|---|---|
| TRBC1-positive patients | ||
| Cortical T-ALL | 2 | TCRB and TCRD positive rearrangement (1 case) |
| TCRB, TCRD and TCRG positive rearrangement (1 case) | ||
| Medullary T-ALL | 2 | TCRB and TCRG positive rearrangement (1 case) |
| TCRG positive rearrangement (1 case) | ||
| Suspected T-cell lymphoma | 1 | TCRG positive rearrangement (1 case) |
| TRBC1-negative patients | ||
| AITL | 2 | TCRB, TCRD and TCRG negative rearrangement (1 case) |
| TCRB, TCRD and TCRG negative rearrangement (1 case) | ||
| PTCL | 1 | TCRB, TCRD and TCRG negative rearrangement (1 case) |
T-ALL T lineage acute lymphocytic leukemia; AITL angioimmunoblastic T-cell lymphoma; PTCL Peripheral T-cell lymphomas; TCRB T cell receptor beta chain; TCRD T cell receptor delta chain; TCRG T cell receptor gamma chain
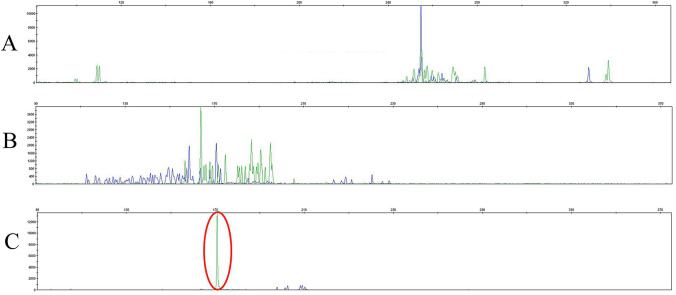
Fig. 3.
TCRG gene was monoclonal-positive in a suspected T cell lymphoma patient with TCRB1-positive. a The TCRB gene showed negative rearrangement in a suspected T cell lymphoma patient who was TCRB1-positive. b The TCR delta chain (TCRD) gene showed negative rearrangement in a suspected T cell lymphoma patient who was TCRB1-positive. c TCR gamma chain (TCRG) gene rearrangement in the bone marrow cells of patients with TRBC1-positive expression and of a suspected T cell lymphoma patient who is TCRB1-positive
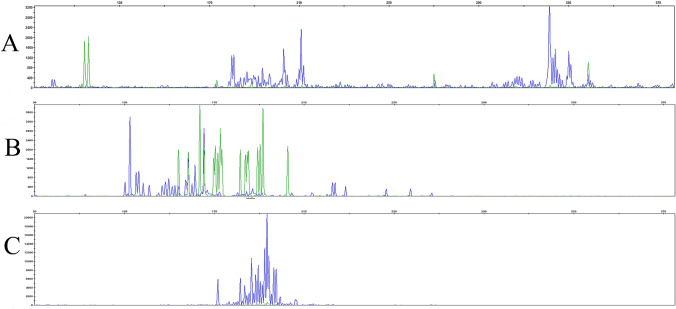
Fig. 4.
TCR gene were monoclonal-negative in a representative AITL patient with TCRB1-negative. a The TCRB gene showed negative rearrangement in a AITL patient who was TCRB1-negative. b The TCRD gene showed negative rearrangement in a AITL patient who was TCRB1-negative. c TCRG gene rearrangement in the bone marrow cells of patients with TRBC1-negative expression and of a AITL patient who is TCRB1-negative
Discussion
At present, T cell lymphoma has a high mortality rate and a low cure rate. The five-year disease-free survival rate for T-ALL is less than 50% in adults and less than 70% in children [5–7]. It was reported that the TRBC1 expression rate is 25% to 47% in normal T cells [12], which is in alignment with the results of CD3+ T cells (30.15%) from normal donors. The expression of TRBC1 and TRBC2 on TCR is similar to the kappa (κ) light chain and lambda on B lymphocytes. Monoclonal expression occurs when T cells are activated or abnormally proliferating, where TRBC1 expression can be positive or negative [13]. In this study, there were 5 positive results and 41 negative results. Two partial expression results were greater than the highest level of normal results in the 48 patients tested. As a result, the expression rate of TRBC1 in the two patients with partially abnormal expression patterns was greater than the highest of the normal donors. Therefore, the abnormal rate of TRBC1 expression in tumors is 100%. This result is consistent with the studies conducted by Shimin et al. [21] and Novikov ND et al. [22], where they reported that the sensitivity of TRBC1 detection in malignant T cell tumors is 100% and 97%, respectively. Maciocia and colleagues reported that TRBC1 CAR-T cells specifically eliminated malignant T cell lines expressing TRBC1 while sparing TRBC2-expressing T cells [13]. However, the expression of TRBC2 in the bone marrow of patients with negative TRBC1 is unknown.
Furthermore, we have also confirmed that the expression rate of TRBC1 gradually increases for T-ALL patients, ranging from 0 in patients with pro-T-ALL and pre-T-ALL, to 66.7% in patients with medullary T-ALL with T cell development. Combined with the studies in TCR rearrangement, the expression rate of TRBC1 gradually increased with the progress of T cell development. However, this approach is beleaguered by several limitations, such as the unknown expression levels of TRBC2 and a small sample size of the patients with medulla T-ALL. Collection and observation of the expression of TRBC1 expression through more samples of T-ALL should be done. Went et al. [10] and Pui et al. [11] indicated that the expression rate of TRBC1 is greater than 95% in PTCL-NOS, and it is also expressed in almost all vascular immunoblast AITL. The expression of TRBC1 was negative in the AITL patient samples (two cases) and PTCL patient samples (one case) that we collected, which was inconsistent with the results reported by Went et al. and Pui et al. We believe that the expression of TRBC1 might be not detected for two reasons: the TRBC1 expression experiment was of small sample size and detected by flow cytometry, resulting to a small statistical significance, and the difference in gene expression patterns of patients from different races. Therefore, further evaluation and detection on patients with T-NHL needs to be done. The PCR technique was the most specific, sensitive, and rapid detection method for clonal gene rearrangement [23]. We used the TCR rearrangement test to further verify the positive result of TRBC1 expression, which was consistent with the results of flow cytometry. Thus, we expected that TRBC1 can serve as an indicator and target for T lymphoid tumors.
CAR-T cell immunotherapy, with TRBC1 as a clinical target, has been gradually developed in some foreign laboratories. Maciocia et al. [13] established the TRBC1-CAR-T model for the treatment of patients with PTCL, which was achieved in vitro culture and vivo animal models. The effective killing effect of TRBC1-CAR-T cells in TRBC2+ T cells can maintain the body's immune function in animal models. Onuoha et al. [24] conducted a phase I/II clinical trial to evaluate the safety and effectiveness of TRBC1-specific CAR-T cells in TRBC1-positive malignant T cell tumors. In the present study, the results suggested that the expression pattern of TRBC1 in patients with T lymphocyte tumors was significantly different from the patients with healthy bone marrow tissues.
Conclusion
In conclusion, the present study identified that multiple TRBC1 expression patterns were present in samples from a variety patients with T-lymphoid tumors, as shown by flow cytometry, while healthy patients showed a pattern of partial expression. The expression of TRBC1 was verified through TCR gene rearrangement detection in TRBC1-positive patients. Therefore, TRBC1 may be a diagnostic and therapeutic target for T lymphocyte tumors, providing a basis for CAR-T immune therapy.
Author contributions
Conceptualization and funding acquisition: HW. Data curation: MC, AW and MF. Formal analysis: MC, SL and XW. Investigation: MC, AW, SL, XW, MG, JZ and MF. Methodology: AW and JZ. Writing—original draft: MC, AW, SL, and JZ. Writing—review and editing: MC, XW, MG and MF. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Conflict of interest
The authors declared that they have no conflicts of interest.
Consent to Participate
Consent forms were obtained from all patients before participation in the study.
Ethics Approval
This study was approved by the hospital ethics committee of the Hebei Yanda LU Dao-Pei Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2.Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. 2008;8(5):380–390. doi: 10.1038/nri2304. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 5.Iacobucci I, Mullighan CG. Genetic basis of acute lymphoblastic leukemia. J Clin Oncol. 2017;35(9):975–983. doi: 10.1200/JCO.2016.70.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993) Blood. 2009;114(25):5136–5145. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21(19):3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 8.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24(16):2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- 11.Pui C-H, Behm FG, Singh B, Schell MJ, Williams D, Rivera G, et al. Heterogeneity of presenting features and their relation to treatment outcome in 120 children with T-cell acute lymphoblastic leukemia. Blood. 1990;75(1):174–179. doi: 10.1182/blood.V75.1.174.174. [DOI] [PubMed] [Google Scholar]
- 12.Tunnacliffe A, Kefford R, Milstein C, Forster A, Rabbitts TH. Sequence and evolution of the human T-cell antigen receptor beta-chain genes. Proc Natl Acad Sci U S A. 1985;82(15):5068–5072. doi: 10.1073/pnas.82.15.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciocia PM, Wawrzyniecka PA, Philip B, Ricciardelli I, Akarca AU, Onuoha SC, et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23(12):1416–1423. doi: 10.1038/nm.4444. [DOI] [PubMed] [Google Scholar]
- 14.Poggio T, Duyster J, Illert AL. Current Immunotherapeutic approaches in T cell Non-hodgkin lymphomas. Cancers (Basel). 2018;10(9):339. doi: 10.3390/cancers10090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164(4):536–545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huppa JB, Axmann M, Mörtelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, et al. TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463(7283):963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide–MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;43(7030):238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 19.Jamal S, Picker LJ, Aquino DB, McKenna RW, Dawson DB, Kroft SH. Immunophenotypic analysis of peripheral T-cell neoplasms. A multiparameter flow cytometric approach. Am J Clin Pathol. 2001;116(4):512–526. doi: 10.1309/QF6N-VAQW-N74H-4JE2. [DOI] [PubMed] [Google Scholar]
- 20.Scherer LD, Brenner MK, Mamonkin M. Chimeric Antigen Receptors for T-Cell Malignancies. Front Oncol. 2019;9:126. doi: 10.3389/fonc.2019.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi M, Jevremovic D, Otteson GE, Timm MM, Olteanu H, Horna P. Single antibody detection of T-cell receptor αβ clonality by flow cytometry rapidly identifies mature T-cell neoplasms and monotypic small CD8-positive subsets of uncertain significance. Cytometry B Clin Cytom. 2020;98(1):99–107. doi: 10.1002/cyto.b.21782. [DOI] [PubMed] [Google Scholar]
- 22.Novikov ND, Griffin GK, Dudley G, Drew M, Rojas-Rudilla V, Lindeman NI, et al. Utility of a simple and robust flow cytometry assay for rapid clonality testing in mature peripheral T-cell lymphomas. Am J Clin Pathol. 2019;151(5):494–503. doi: 10.1093/ajcp/aqy173. [DOI] [PubMed] [Google Scholar]
- 23.Ji H, Zhu M, Zhao T. The application of PCR technique in genetic diagnosis of non-Hodgkin's lymphoma. Zhonghua Bing Li Xue Za Zhi. 1994;23(4):207–210. [PubMed] [Google Scholar]
- 24.Onuoha S, Ferrari M, Bulek A, Bughda R, Manzoor S, Srivastava S, et al. Structure guided engineering of highly specific chimeric antigen receptors for the treatment of T cell lymphomas. Blood. 2018;132(Supplement 1):1661. doi: 10.1182/blood-2018-99-119564. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.