Abstract
Drought leads to reductions in plant growth and crop yields. Arbuscular mycorrhizal fungi (AMF), which form symbioses with the roots of the most important crop species, alleviate drought stress in plants. In the present work, we identified 14 GH3 genes in apple (Malus domestica) and provided evidence that MdGH3-2 and MdGH3-12 play important roles during AM symbiosis. The expression of both MdGH3-2 and MdGH3-12 was upregulated during mycorrhization, and the silencing of MdGH3-2/12 had a negative impact on AM colonization. MdGH3-2/12 silencing resulted in the downregulation of five genes involved in strigolactone synthesis, and there was a corresponding change in root strigolactone content. Furthermore, we observed lower root dry weights in RNAi lines under AM inoculation conditions. Mycorrhizal transgenic plants showed greater sensitivity to drought stress than WT, as indicated by their higher relative electrolytic leakage and lower relative water contents, osmotic adjustment ability, ROS scavenging ability, photosynthetic capacity, chlorophyll fluorescence values, and abscisic acid contents. Taken together, these data demonstrate that MdGH3-2/12 plays an important role in AM symbiosis and drought stress tolerance in apple.
Subject terms: Drought, Arbuscular mycorrhiza
Introduction
To cope with a variety of environmental challenges, plants have evolved many strategies, including interactions with other microorganisms1. Arbuscular mycorrhizal (AM) symbiosis is one of the most complex and beneficial interrelationships and is formed between the roots of nearly 80% of terrestrial plants and fungi from the phylum Glomeromycota2–4. This symbiosis evolved more than 450 million years ago, making it one of the most ancient symbioses, and it provides a significant selective advantage to both symbiotic partners5. After a molecular dialog between the two partners, the AM fungus penetrates the host roots and forms branched structures in cortical cells in which nutrient exchange occurs6. As obligate symbionts, AM fungi (AMF) complete their life cycle using the carbon sources provided by their host plants. In exchange, fungi not only improve the availability of mineral nutrients (especially phosphate) for the partner but also enhance its resistance to various environmental stresses2,7,8.
The establishment of AM symbiosis is a complex process that requires molecular communication between the two partner organisms9,10. Over the past decade, great progress has been made in understanding how AM symbiosis is established11–13. The process of symbiosis formation is relatively consistent between different plant and AMF species and can be divided into several stages. First, before coming into contact with the AMF, the plant roots secrete strigolactone (SL) “branching factors”. These branching factors stimulate the spores of AM fungi germination and branching, which continue to approach the roots14. At the same time, AMF also secrete signaling substances called “mycorrhizal factors”, which include sulfated and nonsulfated lipochitooligosaccharides15,16. After the plant roots recognize these substances, the host plants activate common symbiotic signaling pathways17. When the fungal mycelium comes into contact with the plant root epidermal cells, it differentiates into an attachment structure called a hyphopodium, and the root epidermal cells also undergo cytoskeletal changes. The fungal hyphae penetrate the epidermal cells and continue to elongate toward the cortical cells, continuously growing through the root tissue18. When the mycelium extends to the endothelial layer of cells and penetrates the cell wall, it continues to branch in the cells, forming a highly branched, tree-like structure that becomes an arbuscule. Arbuscules are the main site of the exchange of mineral nutrients between the host cell and the fungi12,13.
Plant hormones are multifunctional molecules that act as central regulators of all plant developmental processes, including the development of the plant-AMF interaction12,13,19–22. Several studies have reported that different hormones are involved in AM formation12,20,23,24, and auxin and its derivatives are thought to play a particularly important role. Hanlon and Coenen25 showed that the roots of two auxin-related mutants were poorly colonized. Similarly, a low-auxin bsh mutant of pea (Pisum sativum) exhibited a reduction in mycorrhizal colonization and strigolactone exudation26. Guillotin et al.9 showed that Sl-IAA27 may control SL biosynthesis via the regulation of NSP1. Although all plant hormones can perform their biological functions independently, the regulation of a plant’s development throughout its lifespan requires the synergistic action of multiple hormones24.
In agricultural ecosystems, drought is one of the most serious environmental factors that limit plant growth and crop productivity27,28. AM symbiosis can improve the performance of plants under various environmental stresses, such as drought, and can alter plant water relations under conditions of both water sufficiency and water stress3,29–31. The regulation of plant drought resistance by AMF is a very complex process involving many metabolites and metabolic pathways32,33. AMF symbiosis has been shown to improve plant drought adaptability by up- and downregulating many physiological and biochemical processes. Specifically, AMF can directly promote the absorption and transport of plant nutrients and water, increase plant osmotic regulation, improve plant gas exchange capacity and water use efficiency, and increase plant antioxidant capacity4,30,34.
In this study, the expression of two apple early auxin-responsive genes, MdGH3-2 (MD02G1180300) and MdGH3-12 (MD15G1290900), was shown to be significantly induced by AM fungi. To further understand their potential roles in the mycorrhizal process, an RNA interference (RNAi) approach was used to repress their expression. We hypothesized that their repression would not only affect mycorrhization but would also affect drought resistance in apple under drought conditions. Physiological parameters such as photosynthetic parameters, antioxidant system activity, osmotic adjustment, and ABA levels under AM inoculation were assessed. Overall, the data show that MdGH3-2/12 are involved in AM symbiosis and drought stress tolerance in apple.
Results
Identification of MdGH3 genes
In total, fifteen MdGH3 family genes were identified. However, we found that there was one gene (MD09G1091000) with 280 amino acids, and the predicted conserved domain indicated that it was incomplete. Therefore, this gene was not analyzed further. The basic information for the MdGH3 genes, including gene names, gene IDs, chromosome locations, protein lengths, theoretical isoelectric points (pIs), and molecular weights (MWs), is listed in Table 1. The sizes of the MdGH3 proteins ranged from 562–709 amino acids. The MW and pI of the MdGH3 proteins ranged from 62531.86 Da (MdGH3-7) to 80113.10 Da (MdGH3-3) and from 5.41 (MdGH3-11) to 7.54 (MdGH3-3), respectively.
Table 1.
The GH3 family in apple
| Gene name | Gene ID (GDDH13) | Gene ID (V1.0) | Chromosome location | Length (aa) | MW (Da) | pI |
|---|---|---|---|---|---|---|
| MdGH3-1 | MD01G1076500 | MDP0000233483 | Chr01:18316873..18322302 | 562 | 62595.79 | 6.01 |
| MdGH3-2 | MD02G1180300 | MDP0000612660 | Chr02:16058335..16061924 | 599 | 67466.49 | 6.23 |
| MdGH3-3 | MD03G1215800 | MDP0000132162 | Chr03:29660247..29662938 | 709 | 80113.10 | 7.54 |
| MdGH3-4 | MD03G1284700 | MDP0000568498 | Chr03:36483028..36485639 | 596 | 67617.14 | 6.43 |
| MdGH3-5 | MD05G1092900 | MDP0000209432 | Chr05:19753185..19755519 | 601 | 67564.48 | 5.92 |
| MdGH3-6 | MD05G1092300 | MDP0000873893 | Chr05:19618797..19621192 | 577 | 64773.60 | 6.30 |
| MdGH3-7 | MD07G1145900 | MDP0000786650 | Chr07:21291062..21294030 | 562 | 62531.86 | 6.22 |
| MdGH3-8 | MD11G1293000 | MDP0000231245 | Chr11:41253547..41255976 | 575 | 64085.78 | 5.49 |
| MdGH3-9 | MD11G1304200 | MDP0000204381 | Chr11:41930461..41932991 | 596 | 67407.79 | 5.87 |
| MdGH3-10 | MD11G1230400 | MDP0000402444 | Chr11:33501665..33504059 | 614 | 69316.51 | 7.03 |
| MdGH3-11 | MD13G1132300 | MDP0000226842 | Chr13:10035347..10038041 | 611 | 69630.77 | 5.41 |
| MdGH3-12 | MD15G1290900 | MDP0000872868 | Chr15:26870302..26873401 | 599 | 67351.40 | 5.45 |
| MdGH3-13 | MD16G1142800 | MDP0000834656 | Chr16:10976308..10979216 | 611 | 69665.62 | 5.53 |
| MdGH3-14 | MD17G1081000 | MDP0000214081 | Chr17:6765282..6769196 | 613 | 69505.96 | 6.85 |
Phylogenetic and gene structure analysis
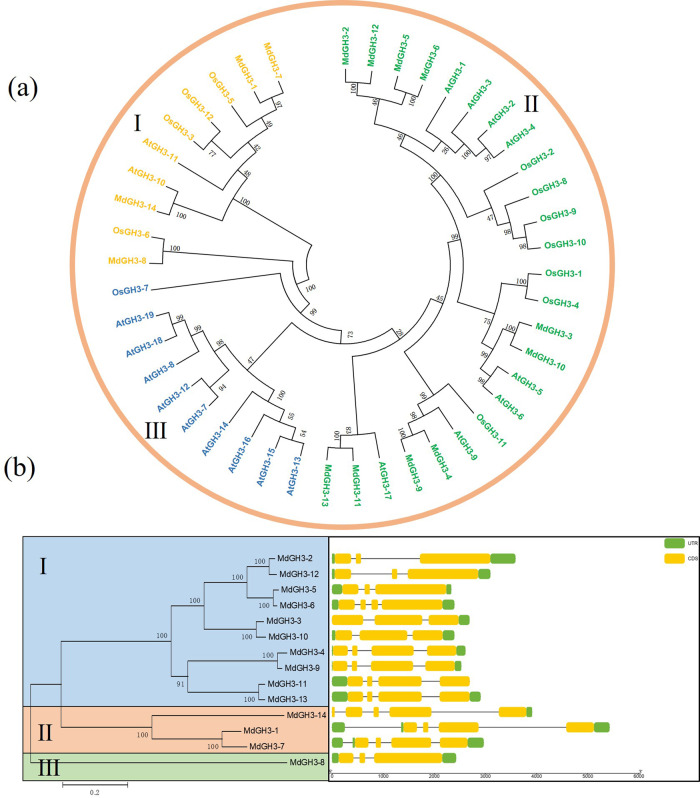
To investigate the evolutionary relationships among apple, Arabidopsis (Arabidopsis thaliana), and rice (Oryza sativa L.) GH3s proteins, we constructed a phylogenetic tree (Fig. 1a). The GH3 members could be classified into three subgroups, numbered I, II, and III. There were 10, 25, and 10 GH3s in the three subgroups, respectively. Fourteen MdGH3s were unevenly distributed among three different subgroups. The gene structure analysis showed that in group I, MdGH3-2, MdGH3-3, MdGH3-5, MdGH3-10, and MdGH3-12 contained three exons, and the other genes contained four exons. In group II, MdGH3-14 contained five exons, and MdGH3-1 and MdGH3-7 contained four exons. In group III, MdGH3-8 had three exons. Even within the same phylogenetic subgroup, the MdGH3 members exhibited different structural patterns of exon–intron junctions (Fig. 1b).
Fig. 1. Phylogenetic analysis of GH3 proteins from apple, Arabidopsis, and rice and gene structures of MdGH3 genes.
a The phylogenetic tree was constructed with the MEGA5 program using the neighbor-joining method. b Gene structures were constructed using TBtools
Expression and subcellular localization of MdGH3-2 and MdGH3-12
The expression of MdGH3-2 and MdGH3-12 differed among plant organs. In ‘Royal Gala’, MdGH3-2, and MdGH3-12 were highly expressed in mature flowers, but their expression levels were lower in branches and bark (Fig. 2a, b). In ‘Winter Red’, MdGH3-2 and MdGH3-12 were highly expressed in roots (Fig. 2c, d). When we investigated the expression of MdGH3s after inoculation with Rhizophagus irregularis, we found that MdGH3-2 and MdGH3-12 were significantly induced in mycorrhizal plants compared with their expression in nonmycorrhizal (NM) plants (Fig. 2e, Fig. S1). Furthermore, subcellular localization analysis of MdGH3-2 and MdGH3-12 showed that their encoded proteins were localized to membranes and nuclei (Fig. 2f). The nucleotide sequences of MdGH3-2 and MdGH3-12 were highly (up to 90%) similar (Fig. S2), and it was therefore not possible to design primers that specifically knocked down MdGH3-2 or MdGH3-12. The two genes could only be silenced simultaneously.
Fig. 2. MdGH3-2 and MdGH3-12 expression in various tissues, response to AM colonization, and protein localization.
a Expression profiles of MdGH3-2 in ‘Royal Gala’ (Malus domestica). b Expression profiles of MdGH3-12 in ‘Royal Gala’ (Malus domestica). c Expression profiles of MdGH3-2 in ‘Winter Red’. d Expression profiles of MdGH3-12 in ‘Winter Red’. e Expression of MdGH3-2 and MdGH3-12 in M and NM apple roots inoculated with Rhizophagus irregularis over 8 weeks. f MdGH3-2 or MdGH3-12 localized in the nucleus and membranes of tobacco leaf epidermal cells. AtCBL1n-mCherry served as a marker for plasma membrane protein localization. M=mycorrhizal, NM=nonmycorrhizal. Fruit1=4 months after anthesis, Fruit2 = 7 months after anthesis. Bars = 50 μm. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by the independent-samples t-test
MdGH3-2 and MdGH3-12 are positive regulators of mycorrhization
We next investigated whether MdGH3-2/12 plays a role during mycorrhization. We obtained two individual RNAi lines and used genome walking to identify the T-DNA integration site. The results showed that their insertion sites were located on two different chromosomes (Table S1). The expression levels of MdGH3-2 in lines Ri-1 and Ri-9 were reduced by 36% and 25%, respectively. The expression levels of MdGH3-12 in lines Ri-1 and Ri-9 were reduced by 30% and 28%, respectively (Fig. S3). We also detected the expression levels of other GH3 family genes in the two transgenic lines. The results showed that other genes were not affected by the silencing of MdGH3-2/12 (Fig. S4). The two RNAi lines and wild-type (WT) plants were inoculated with R. irregularis. After 8 weeks, symbiotic relationships had formed in both the WT and RNAi lines (Fig. 3b–d). However, the frequency of colonization (F) and arbuscule abundance (a) in the two RNAi lines were significantly lower than those in WT (Fig. 3e). Taken together, these data indicated that MdGH3-2/12 were involved in AM symbiosis.
Fig. 3. Mycorrhizal phenotype of RNAi lines and WT 8 weeks after inoculation with Rhizophagus irregularis.
a–d Root segments stained with trypan blue. a Roots of WT under the NM condition. b–d Arbuscules of WT, Ri-1 and Ri-9. e Colonization percentages in the RNAi lines and WT. ‘F’, frequency of colonization in the root system; ‘a’, arbuscule abundance (percentage) in the colonized root sections. Bars = 100 μm. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
Expression of strigolactone biosynthesis genes and strigolactone production
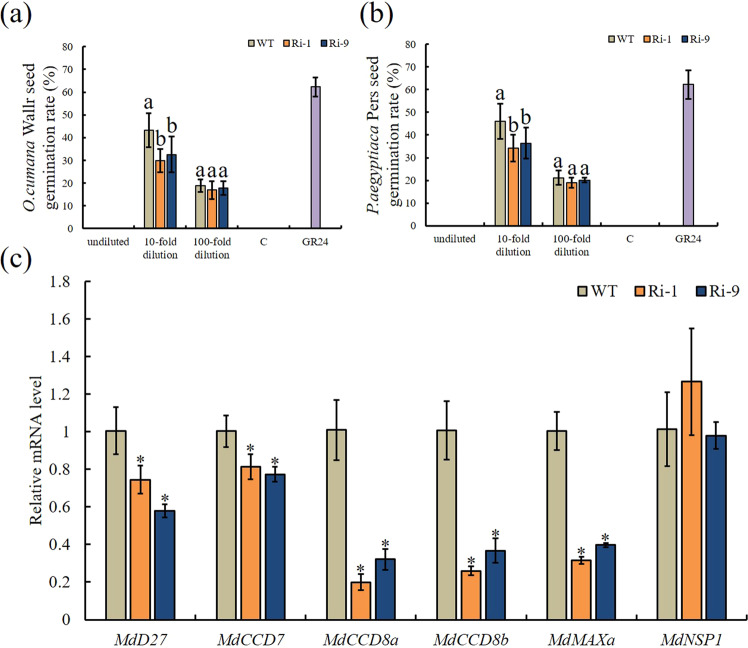
Because SL plays important role during AM symbiosis, we also analyzed SL production. To quantify SL production in the WT and RNAi lines, we performed a germination bioassay with seeds of Orobanche cumana Wallr. and Orobanche aegyptiaca Pers. using root extracts from the NM WT and RNAi lines (Fig. 4a, b). As expected, the seed germination rates were null when the seeds were treated with sterile distilled water. However, when treated with GR24, the germination rates of the O. cumana and O. aegyptiaca seeds were 62.30% and 62.22%, respectively. Furthermore, significantly reduced germination rates were obtained when both seed species were treated with RNAi-line root extracts at a 10-fold dilution. The germination rates of the seeds after treatment with the WT and RNAi extracts at a 100-fold dilution did not differ significantly, but the germination rates of the seeds treated with the RNAi line extracts were slightly lower than those of the seeds treated with the WT extracts. As a second approach to analyzing SL production, the expression patterns of SL biosynthetic pathway genes were analyzed (Fig. 4c). The results showed that the expression of those genes in NM RNAi lines was significantly lower than that in NM WT plants. The expression of MdNSP1, an important GRAS transcription factor in the nodulation process that is involved in AM symbiosis and regulates the expression of D27 and MAXa, did not differ significantly among genotypes.
Fig. 4. Quantification of strigolactones and expression of strigolactone biosynthesis genes in roots of WT and RNAi lines without mycorrhization.
Germination of Orobanche cumana Wallr. a and Orobanche aegyptiaca Pers. b seeds in response to a range of concentrations of root exudates of WT and RNAi lines. Sterile water (C) and 1 mg L-1 synthetic strigolactone (GR24) were used as negative and positive controls. c Expression of strigolactone biosynthetic genes and MdNSP1 in WT and RNAi lines under NM conditions. NM=nonmycorrhizal. Data are expressed as means ± SDs (n = 4 for c, n = 12 for a and b). Data with different letters and asterisks differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
Morphometric data on plant biomass
Growth promotion in host plants is the most direct effect of AMF inoculation. We, therefore, measured plant growth parameters and biomass production prior to the DS treatment. As expected, the plants inoculated with R. irregularis consistently exhibited greater plant growth and biomass production than the NM plants (Fig. 5a–d). Under WW+NM conditions, the plant height, stem diameter, and shoot and root dry weight did not differ significantly among genotypes. This trend was maintained under WW+M conditions, with the exception of root dry weight, which was significantly lower in Ri-1 and Ri-9 than in WT.
Fig. 5. Influence of AM symbiosis on plant growth parameters and biomass production in WT and RNAi lines.
a Plant height. b Stem diameter. c, d Shoot and root dry weight. M = mycorrhizal, NM = nonmycorrhizal. Data are expressed as means ± SDs (n = 5 for c and d, n = 10 for a, b). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
RNAi-MdGH3-2/12 plants are more sensitive to drought stress
RNAi-MdGH3-2/12 transgenic lines with reduced MdGH3-2 and MdGH3-12 expression had lower root SL content and lower levels of AMF colonization. We have previously shown that AM fungi enhance drought resistance in apple. Therefore, we wanted to assess whether repression of both MdGH3-2 and MdGH3-12 would affect the performance of apple under drought conditions because of lower AM colonization levels. Under NM and WW+M conditions, the phenotypes did not differ significantly among genotypes. However, under DS+M conditions, the RNAi lines showed more wilting and necrosis than the WT (Fig. 6a). This phenotype was also reflected in the REL and RWC values. Under the NM and WW+M treatments, there were no significant differences in RWC and REL among genotypes. However, under the DS+M treatment, the RWC was significantly lower in Ri-1 and Ri-9, at 77.50% and 82.13% that of WT, respectively (Fig. 6b). The REL was significantly higher in Ri-1 and Ri-9, at 1.31 and 1.30 times that of WT (Fig. 6c).
Fig. 6. Drought tolerance in RNAi lines and WT.
a Drought tolerance in RNAi lines and WT under M and NM conditions. b Relative water content (RWC). c Relative electrolyte leakage (REL). WW = well-watered, DS = drought stress, M = mycorrhizal, NM = nonmycorrhizal. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
Gas exchange and Fv/Fm
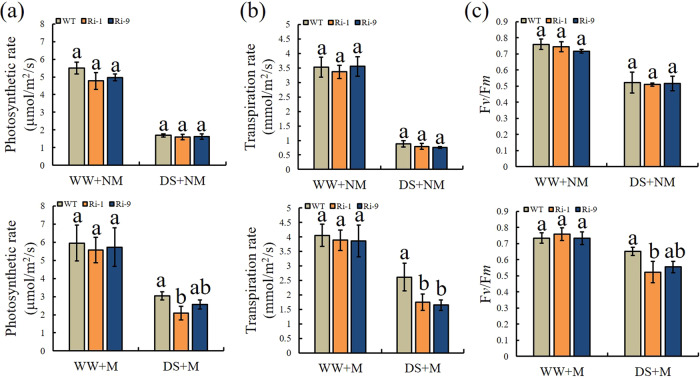
Under the NM treatments, the values of Pn, Tr, and Fv/Fm were similar in the WT and RNAi lines, and the differences among them were not significant (Fig. 7). The results were similar for Pn, Tr, and Fv/Fm under the WW+M treatment. However, under the DS+M treatment, the values of Pn, Tr, and Fv/Fm were higher in the WT than in the RNAi lines. The Pn of WT was 1.46 and 1.18 times those of Ri-1 and Ri-9, respectively. Tr was significantly lower in Ri-1 and Ri-9 than in WT, at 66.79% and 63.03% that of WT, respectively. The same pattern was observed for Fv/Fm, and the Ri-1 and Ri-9 values were 80.27% and 85.12% that of WT, respectively.
Fig. 7. Effect of drought and AM symbiosis on Pn, Tr, and Fv/Fm in the RNAi lines and WT.
a Photosynthetic rate (Pn). b Transpiration rate (Tr). c Maximum photochemical efficiency of PSII (Fv/Fm). WW=well-watered, DS=drought stress, M=mycorrhizal, NM=nonmycorrhizal. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
H2O2 and O2- generation, antioxidant enzyme activity, and proline contents
The drought treatment increased the O2- and H2O2 concentrations in all plant types. Lower amounts of O2- and H2O2 were detected in the WT than in the RNAi lines under DS+M (Fig. 8a, b). The O2- concentration in Ri-1 was significantly increased, by 1.12 times, relative to that in WT, and the H2O2 concentrations in Ri-1 and Ri-9 were significantly increased, by 1.23 and 1.20 times, relative to that in WT, respectively. POD and SOD activities did not differ among genotypes under the NM and WW+M treatments (Fig. 8c, d). Under DS+M, the POD activities of Ri-1 and Ri-9 were significantly decreased, to 88.02% and 88.82% of the WT level, respectively. The SOD activities of Ri-1 and Ri-9 were significantly decreased, to 80.53% and 82.05% of WT levels, respectively. A similar pattern was observed for the proline content (Fig. 8e). Under DS+M conditions, the proline contents of Ri-1 and Ri-9 were significantly decreased, to 84.07% and 84.48% of WT levels, respectively. Together, these findings suggest that the RNAi lines exhibited reduced antioxidant enzyme activity and lower osmoprotectant contents under DS+M conditions than WT.
Fig. 8. Effect of drought and AM symbiosis on the levels of ROS, antioxidant enzyme activity, and proline in WT and RNAi lines.
Concentrations of a O2- and b H2O2, c, d peroxidase (POD) and superoxide dismutase (SOD) activity, and e proline content. WW=well-watered, DS=drought stress, M=mycorrhizal, NM=nonmycorrhizal. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
ABA accumulation
Abscisic acid is a plant hormone that is very important to plant growth and development. It is usually related to plant responses to abiotic stresses such as drought. ABA is also related to AM symbiosis. As expected, drought enhanced the accumulation of ABA, especially under M conditions (Fig. 9). The data also showed that under the DS+M treatment, ABA accumulation in the WT was 1.18 and 1.53 times those in Ri-1 and Ri-9, respectively, and was significantly higher than that in Ri-9, reaching 113.94 ng g-1 FW.
Fig. 9. Effect of drought and AM symbiosis on root abscisic acid (ABA) content in WT and RNAi lines.
WW = well-watered, DS = drought stress, M = mycorrhizal, NM = nonmycorrhizal. Data are expressed as means ± SDs (n = 3). Data with different letters differ significantly (P < 0.05), as determined by one-way ANOVA and Tukey’s test
Discussion
As global temperatures increase, soil water shortages often occur35, and the resulting drought is one of the main factors limiting crop production worldwide28. In the process of evolution, plants have evolved mechanisms to flexibly adapt to adverse conditions36. One of these strategies is the establishment of AM symbiosis4,37. It is generally believed that this symbiotic relationship is a key component of plants’ ability to cope with drought38. Our previous research showed that AMF can directly increase plant osmotic regulation, improve plant gas exchange capacity and water use efficiency, and improve plant antioxidant capacity under drought stress39. We hypothesized that changes in the AMF colonization level would affect plant growth and alter plant performance under drought.
Many studies have shown that plant hormones, as signaling molecules, are involved in the regulation of AM symbiosis19. Moreover, interactions among different hormones regulate various plant physiological processes, as well as interactions between plants and microorganisms. Many auxin-responsive genes play roles in the regulation of mycorrhization. One example of this is the tomato GH3 family member GH3.4, whose expression is highly correlated with mycorrhizal colonization levels40. The auxin amidohydrolases MtIAR33 and MtIAR34 are significantly upregulated by inoculation with AM fungi41. Another study showed that the positive effect of ABA on AM colonization was mediated mainly through PP2AB'112. In our study, we identified 14 GH3 genes in apple, and MdGH3-2 and MdGH3-12 were significantly induced by mycorrhizal inoculation. We used an RNAi approach to repress the expression of both MdGH3-2 and MdGH3-12 to gain insight into their functions during AM symbiosis and ultimately determine how they affect apple performance under drought stress. Interestingly, the expression levels of five SL synthesis genes were downregulated in the roots of the two transgenic lines relative to those in the WT. We also measured MdNSP1 expression, and no significant differences were observed between RNAi lines and WT. These results suggest that MdGH3-2/12 do not affect MdD27 and MdMAXa expression through the regulation of NSP1. Moreover, a germination assay using two types of Orobanche seeds demonstrated that the root SL contents of the RNAi lines were lower than that of WT. We also observed a lower root AMF colonization frequency and arbuscule abundance in the two transgenic lines. These data suggest that MdGH3-2 and MdGH3-12 regulate SL biosynthesis and mycorrhization in apple.
A large body of research has documented the beneficial effects of different AM fungi on plant growth30,42. Here, we also showed that mycorrhizal symbiosis improved plant growth performance and increased plant biomass. In addition to the lower AMF colonization levels in the two RNAi lines, we also observed lower root dry weights in the Ri-1 and Ri-9 lines under WW+M conditions relative to that in WT. These results indicate that higher levels of AMF colonization improved the growth of mycorrhizal plants. Drought stress negatively affects plant growth and causes a series of physiological, biochemical, and molecular changes32. A large number of studies have reported that AM fungi enhance the ability of plants to resist drought stress through multiple mechanisms: improving the soil structure, promoting the formation of soil aggregates, and increasing soil nutrient and moisture retention; accelerating plant nutrient and water absorption through the activity of extraradical hyphae; improving plant photosynthetic function; increasing the efficiency and capacity of the antioxidant system to alleviate harmful ROS effects, and increasing the expression of drought resistance genes29,30. Here, we were interested in determining whether lower AMF colonization levels in transgenic apples would increase their sensitivity to drought stress.
Drought stress can lead to a decrease in plant RWC, which is an important index for the evaluation of plant water status43. We found that under DS+M, RWC was significantly lower in the two RNAi lines than in WT. REL is an important indicator for evaluating membrane integrity and the extent of tolerance to abiotic stress44. Here, REL was significantly lower in WT than in the two RNAi lines under DS+M conditions. These data indicated that silencing MdGH3-2/12 led to greater cell damage under DS+M conditions. Increased ROS production is a typical physiological response induced by drought stress44. High levels of ROS accumulation are harmful to cells and eventually lead to oxidative stress injury. Here, the RNAi lines accumulated more H2O2 and O2- than the WT under DS+M. To maintain the normal physiological and metabolic functions of cells under stress, plants have developed a complete set of ROS scavenging defenses over the course of their long-term evolution, including enzymatic defenses such as SOD and POD. Multiple studies have shown that AM symbiosis increases the activity of antioxidant enzymes under drought stress29,30. Likewise, we found that POD and SOD activities were significantly lower in transgenic plants under the DS+M treatment than in WT. This result was also consistent with the measured contents of H2O2 and O2- in the plants.
Mycorrhizal seedlings usually have higher photosynthetic rates than nonmycorrhizal seedlings under drought stress39. ROS produced by abiotic stress can damage components of the photosynthetic system and inhibit the repair of PSII. Plants can reduce ROS accumulation and damage to the photosynthetic system by inducing the production of antioxidant enzymes. The greater decrease in Pn and Tr in RNAi lines under drought stress may reflect the reduced capacity of the antioxidant system. The same trend was observed in Fv/Fm. Under drought stress, plant drought resistance is related to the accumulation of osmoregulatory substances such as proline. Here, we observed lower proline concentrations in RNAi lines under DS+M. This may also have contributed to the greater drought sensitivity observed in the transgenic lines.
The increased tolerance of mycorrhizal plants to drought stress is related to changes in plant hormone homeostasis. Of these changes in hormonal activity, changes in ABA signaling have been studied the most in-depth34. As expected, drought caused an increase in ABA, and the ABA content of mycorrhizal plants increased more than that of nonmycorrhizal plants. In addition to its importance to drought tolerance, the production of ABA is essential for the establishment and functioning of AM symbiosis20,45. Hence, the increased ABA levels in AM symbiosis-stressed plants served not only to improve drought tolerance but also to enhance and maintain AM symbiosis. In our study, lower ABA levels were observed in the RNAi lines due to the lower AMF colonization rate in the RNAi lines.
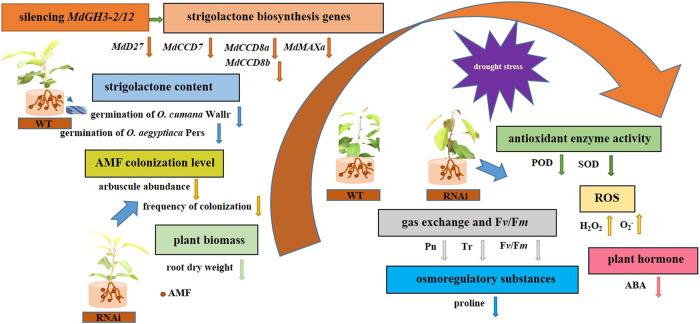
In brief, we functionally characterized MdGH3-2/12 by downregulating them in apple and addressed their role in AM symbiosis (Fig. 10). Although further studies are necessary to fully understand how MdGH3-2/12 regulates AM symbiosis, our data demonstrate their importance for the establishment of AM symbiosis and for drought stress resistance.
Fig. 10. A working model for silencing MdGH3-2/12 in response to AMF inoculation and drought in apple.
MdGH3-2/12 silencing resulted in the downregulation of MdD27, MdCCD7, MdCCD8a, MdCCD8b, and MdMAXa, and there was a corresponding change in root strigolactone content. Furthermore, we observed lower root dry weights in RNAi lines under AM inoculation conditions. Under drought stress, mycorrhizal transgenic plants showed higher sensitivity than WT, as indicated by higher relative electrolytic leakage and lower relative water contents, osmotic adjustment ability, ROS scavenging ability, gas exchange capacity, chlorophyll fluorescence values, and abscisic acid contents
Materials and methods
Plant material, growth conditions, and treatments
The leaves, branches, bark, flower buds, and mature flowers of ‘Royal Gala’ (Malus domestica) apple trees and the fruits, leaves, and roots of ‘Winter Red’, a cultivar of crabapple (Malus spp.), grown at the horticultural experimental station of Northwest A&F University (Yangling, Shaanxi, China) were sampled. The mature leaves of ‘Qinguan’ apple were used for gene cloning. A line with high regeneration capacity isolated from ‘Royal Gala’ (M. domestica) named GL-3 was used for genetic transformation46. After rooting on MS agar medium, the plants were transferred to small plastic pots (8 × 8 cm) that contained a substrate/perlite/vermiculite mixture (4:1:1, v/v/v) that had been sterilized by autoclaving at 121 °C for 2 h. After one month, the mycorrhization assay plants were moved to larger plastic pots (9 × 10 cm) that contained the sterilized mixture described above. Ten grams of fungal inoculum was added, and noninoculated control plants received the same amount of sterilized mycorrhizal inoculum. The AM fungus was R. irregularis. Two months after transplantation, AMF seedlings and non-AMF seedlings were exposed to well-watered or drought conditions, with 40 pots in each treatment. Water was withheld from the drought-stressed seedlings for 9 days.
Genetic transformation of apple
A 185-bp fragment of MdGH3-2 was cloned into the Gateway transfer vector pDONR222 and then into the binary vector pK7WIWG2D to create the two RNAi silencing constructs. A schematic drawing of the silencing vector is shown in Fig. S5. The Agrobacterium-mediated transformation of apple was performed as previously described, using GL-3 as the genetic background44,46,47. The primers used for constructing all vectors are shown in Table S2. A Genome Walking Kit (Takara, Dalian, China) was used to identify the transgene insertion site.
Biomass production and observation of AM development
After 8 weeks of the NM and M treatments, the NM and M seedlings were harvested and separated into shoots and roots to measure their biomass. Growth parameters, including stem diameter and plant height, were also recorded. The root staining procedure followed the methods of Koske and Gemma48 and Huang et al39. The degree of root mycorrhizal colonization was calculated according to the method of Trouvelot et al.49 under an Olympus microscope.
Subcellular localization assays in Nicotiana benthamiana leaves
The coding regions without stop codons of MdGH3-2 and MdGH3-12 were recombined into the pcambia2300-GFP vector (Fig. S5). The AtCBL1n:mCherry construct was used as a marker for plasma membrane protein localization50. Then, 100 mg ml−1 4′,6-diamidino-2-phenylindole (DAPI, MP Bio, Santa Ana, CA) was used to locate the fluorescent proteins in the nucleus. Instantaneous transformation and fluorescence signal observation were performed as described by Liu et al.51.
Germination assay
After 8 weeks of the NM treatment, the roots were placed in envelopes in a 105 °C oven for 1 h. The oven temperature was then adjusted to 80 °C, and the roots were fully dried. The supernatant obtained by centrifugation was diluted with methanol to final concentrations of 10 and 1 mg/L. Germination bioassays were performed according to Ma et al.52.
Measurements of ABA content
Frozen root tissue (0.1 g) was ground into a 1 mL extraction solution containing methanol:isopropanol, 20:80 (v/v), with 1% glacial acetic acid. After standing at 4 °C for 12 h, the mixture was centrifuged at 12000 g for 5 min at 4 °C. Afterward, the supernatants were combined and passed through a 0.22-μm PTFE filter. The concentrations of ABA were determined as described by Guo et al.53.
Photosynthetic characteristics and Fv/Fm
Gas exchange parameters, including the net photosynthetic rate (Pn) and transpiration rate (Tr), were monitored using a portable photosynthesis system (LI-6800, Li-Cor Biosciences, Lincoln, NE, USA). For each treatment, the 4th–6th leaves from the stem base were selected for measurement. Fv/Fm was measured using a MINI-PAM-II fluorometer (Imaging PAM, Walz, Effeltrich, Germany).
Relative water content and relative electrolytic leakage
The RWC and REL were determined and calculated according to the method described by Sun et al.44 and Dionisio-Sese and Tobita54.
Determination of ROS accumulation, proline, and antioxidant system activity
The levels of H2O2, superoxide radical (O2-), and proline and the activities of superoxide dismutase (SOD) and peroxidase (POD) were detected via Suzhou Comin Biotechnology test kits (Suzhou Comin Biotechnology Co., Ltd, Suzhou, China).
RNA extraction, DNA isolation, and qRT-PCR analysis
Total RNA was extracted from apple plants via a Wolact plant RNA isolation Kit (Wolact, Hong Kong, China). Apple genomic DNA was isolated with a Wolact Plant Genomic DNA Purification Kit (Wolact, Hong Kong, China). qRT-PCR analysis was carried out as previously described by Huo et al.55. Malate dehydrogenase (MDH) transcription was used to normalize the levels of different genes. Table S2 lists the primers used in this study.
Identification and phylogenetic and gene structure analyses of GH3 genes
The protein sequences of 19 GH3 genes from Arabidopsis were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov) according to Xu et al.56. All AtGH3 proteins were used as queries in a BLASTP search against the apple genome database (https://www.rosaceae.org/species/malus/malus_x_domestica). Candidate sequences were confirmed using the Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and SMART Database (http://smart.emblheidelberg.de/) and compared with Yuan et al.57. The OsGH3 protein sequences were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov) according to Jain et al.58. The phylogenetic trees were estimated with the MEGA5 program using the neighbor-joining method. Gene structures were constructed using TBtools (version 1.046). Sequence alignment of MdGH3-2 and MdGH3-12 was performed with DNAMAN (version 6.0.3.99).
Statistical analysis
SPSS 16.0 was used for the statistical analysis. The data were subjected to independent-samples t-tests and one-way ANOVA and expressed as means ± SDs.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFD1000303) and by the earmarked fund for the China Agriculture Research System (CARS-27). The authors are grateful to Professor Yongqing Ma (Institute of Soil and Water Conservation, Northwest A & F University) for providing O. cumana Wallr. and O. aegyptiaca Pers. seeds and Dr. Zhihong Zhang (Shenyang Agricultural University) for providing tissue-cultured GL-3 plants.
Author contributions
D.H. designed and wrote the manuscript. Q.W. analyzed the data. D.H., Q.W., M.M., Z.Z., and G.J. performed the experiments. F.M. and C.L. revised the manuscript. All the authors read and approved the final manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
These authors contributed equally: Dong Huang, Qian Wang.
Contributor Information
Fengwang Ma, Email: fwm64@nwsuaf.edu.cn.
Chao Li, Email: lc453@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41438-021-00524-z.
References
- 1.Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 2.Bucher M, Hause B, Krajinski F, Kuster H. Through the doors of perception to function in arbuscular mycorrhizal symbioses. N. Phytol. 2014;204:833–840. doi: 10.1111/nph.12862. [DOI] [PubMed] [Google Scholar]
- 3.Chitarra W, et al. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016;171:1009–1023. doi: 10.1104/pp.16.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Lozano JM, et al. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016;39:441–452. doi: 10.1111/pce.12631. [DOI] [PubMed] [Google Scholar]
- 5.Remy W, Taylor T, Hass H, Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Nat. Acad. Sci. USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doidy J, et al. The Medicago truncatula sucrose transporter family: characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Mol. Plant. 2012;5:1346–1358. doi: 10.1093/mp/sss079. [DOI] [PubMed] [Google Scholar]
- 7.Baier MC, et al. Knockdown of the symbiotic sucrose synthase MtSucS1 affects arbuscule maturation and maintenance in mycorrhizal roots of Medicago truncatula. Plant Physiol. 2010;152:1000–1014. doi: 10.1104/pp.109.149898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant. Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 9.Guillotin B, et al. Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom) N. Phytol. 2017;213:1124–1132. doi: 10.1111/nph.14246. [DOI] [PubMed] [Google Scholar]
- 10.MacLean AM, Bravo A, Harrison M. J. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell. 2017;29:2319–2335. doi: 10.1105/tpc.17.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 12.Charpentier M, Sun J, Wen J, Mysore KS, Oldroyd GE. Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the PROTEIN PHOSPHATASE 2A complex. Plant Physiol. 2014;166:2077–2090. doi: 10.1104/pp.114.246371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemadi M, et al. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014;166:281–292. doi: 10.1104/pp.114.246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chory J, et al. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLOS Biol. 2006;4:e226. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genre A, et al. Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. N. Phytol. 2013;198:190–202. doi: 10.1111/nph.12146. [DOI] [PubMed] [Google Scholar]
- 16.Maillet F, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63. doi: 10.1038/nature09622. [DOI] [PubMed] [Google Scholar]
- 17.Harrison MJ. Cellular programs for arbuscular mycorrhizal symbiosis. Curr. Opin. Plant. Biol. 2012;15:691–698. doi: 10.1016/j.pbi.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Martin P. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1991. [DOI] [PubMed] [Google Scholar]
- 19.Foo E, Ross JJ, Jones WT, Reid JB. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Ann. Bot. 2013;111:769–779. doi: 10.1093/aob/mct041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera-Medina MJ, Steinkellner S, Vierheilig H, Bote JAO, Garrido JMG. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. N. Phytol. 2007;175:554–564. doi: 10.1111/j.1469-8137.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 21.Santner A, Calderon-Villalobos LI, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, et al. Overexpression of MdATG18a in apple improves resistance to Diplocarpon mali infection by enhancing antioxidant activity and salicylic acid levels. Hortic. Res. 2018;5:57. doi: 10.1038/s41438-018-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Y, et al. DELLA proteins are common components of symbiotic rhizobial and mycorrhizal signalling pathways. Nat. Commun. 2016;7:12433. doi: 10.1038/ncomms12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda N, et al. Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol. 2015;167:545–557. doi: 10.1104/pp.114.247700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanlon MT, Coenen C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. N. Phytol. 2011;189:701–709. doi: 10.1111/j.1469-8137.2010.03567.x. [DOI] [PubMed] [Google Scholar]
- 26.Foo E. Auxin influences strigolactones in pea mycorrhizal symbiosis. J. Plant Physiol. 2013;170:523–528. doi: 10.1016/j.jplph.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Sheffield J, Wood EF, Roderick ML. Little change in global drought over the past 60 years. Nature. 2012;491:435–438. doi: 10.1038/nature11575. [DOI] [PubMed] [Google Scholar]
- 28.Trenberth KE, et al. Global warming and changes in drought. Nat. Clim. Chang. 2013;4:17–22. doi: 10.1038/nclimate2067. [DOI] [Google Scholar]
- 29.Fan QJ, Liu JH. Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol. Plant. 2011;33:1533–1542. doi: 10.1007/s11738-011-0789-6. [DOI] [Google Scholar]
- 30.Mo YL, et al. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016;7:644. doi: 10.3389/fpls.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu QS, He JD, Srivastava AK, Zou YN, Kuca K. Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol. 2019;39:1149–1158. doi: 10.1093/treephys/tpz039. [DOI] [PubMed] [Google Scholar]
- 32.Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 33.Ruiz-Lozano JM, Aroca R. Modulation of aquaporin genes by the arbuscular mycorrhizal symbiosis in relation to osmotic stress tolerance. Symbioses Stress, Springe. Neth. 2010;17:359–374. [Google Scholar]
- 34.Osakabe Y, Osakabe K, Shinozaki K, Tran LS. Response of plants to water stress. Front. Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talbi S, et al. Drought tolerance in a saharian plant Oudneya africana: role of antioxidant defences. Environ. Exp. Bot. 2015;111:114–126. doi: 10.1016/j.envexpbot.2014.11.004. [DOI] [Google Scholar]
- 36.Pierik R, Testerink C. The art of being flexible: how to escape from shade, salt, and drought. Plant Physiol. 2014;166:5–22. doi: 10.1104/pp.114.239160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miransari M, Abrishamchi A, Khoshbakht K, Niknam V. Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit. Rev. Biotechnol. 2014;34:123–133. doi: 10.3109/07388551.2012.731684. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Sanchez M, Aroca R, Munoz Y, Polon R, Ruiz-Lozano JM. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010;167:862–869. doi: 10.1016/j.jplph.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Huang D, et al. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020;149:245–255. doi: 10.1016/j.plaphy.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Liao DH, et al. The characterization of six auxin-induced tomato GH3 genes uncovers a member, SlGH3.4, strongly responsive to arbuscular mycorrhizal symbiosis. Plant Cell Physiol. 2015;56:674–687. doi: 10.1093/pcp/pcu212. [DOI] [PubMed] [Google Scholar]
- 41.Campanella JJ, Smith SM, Leibu D, Wexler S, Ludwig-Müller J. The auxin conjugate hydrolase family of Medicago truncatula and their expression during the interaction with two symbionts. J. Plant Growth Regul. 2008;27:26–38. doi: 10.1007/s00344-007-9027-2. [DOI] [Google Scholar]
- 42.Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012;169:704–709. doi: 10.1016/j.jplph.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Wang SC, et al. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012;51:81–89. doi: 10.1016/j.plaphy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Sun X, et al. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018;16:545–557. doi: 10.1111/pbi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahadur A, et al. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019;20:4199. doi: 10.3390/ijms20174199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai HY, et al. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Horticulturae. 2013;164:202–208. doi: 10.1016/j.scienta.2013.09.033. [DOI] [Google Scholar]
- 47.Xie YP, et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. N. Phytol. 2018;218:201–218. doi: 10.1111/nph.14952. [DOI] [PubMed] [Google Scholar]
- 48.Koske RE, Gemma JN. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989;92:486–505. doi: 10.1016/S0953-7562(89)80195-9. [DOI] [Google Scholar]
- 49.Trouvelot, A., Kough, J. L. & Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche demethodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi- Pearson V., Gianinazzi S., eds Physiological and genetical aspects of mycorrhizae. INRA Press, Paris, France: Proceedings of the 1st European Symposium on mycorrhizae Institut National de la Recherche Agronomique, 217–221 (1986).
- 50.Batistic O, Waadt R, Steinhorst L, Held K, Kudla J. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J. 2010;61:211–222. doi: 10.1111/j.1365-313X.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu YF, et al. Biochemical and functional characterization of AcUFGT3a, a galactosyltransferase involved in anthocyanin biosynthesis in the red-fleshed kiwifruit (Actinidia chinensis) Physiol. Plant. 2018;162:409–426. doi: 10.1111/ppl.12655. [DOI] [PubMed] [Google Scholar]
- 52.Ma YQ, et al. Induction of seed germination in Orobanche spp. by extracts of traditional Chinese medicinal herbs. Sci. China Life Sci. 2012;55:250–260. doi: 10.1007/s11427-012-4302-2. [DOI] [PubMed] [Google Scholar]
- 53.Guo TL, et al. Overexpression of the RNA binding protein MhYTP1 in transgenic apple enhances drought tolerance and WUE by improving ABA level under drought condition. Plant Sci. 2019;280:397–407. doi: 10.1016/j.plantsci.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- 55.Huo LQ, et al. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020;7:21. doi: 10.1038/s41438-020-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu DB, et al. Identifcation and expression analysis of auxin-responsive GH3 family genes in Chinese hickory (Carya cathayensis) during grafting. Mol. Biol. Rep. 2020;47:4495–4506. doi: 10.1007/s11033-020-05529-w. [DOI] [PubMed] [Google Scholar]
- 57.Yuan, H. Z. et al. Genome-wide analysis of the GH3 family in apple (Malus × domestica). BMC Genomics. 14, 297. [DOI] [PMC free article] [PubMed]
- 58.Jain, M., Kaur, N., Tyagi, A. K. & Khurana, J. P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genomic. 6, 36–46. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.