Abstract
The aim of this study was to explore the effects of Garcinia mangostana (mangosteen) and Curcuma longa independently and synergistically in modulating oxidative stress, dyslipidemia, and hyperglycemia commonly observed in high-fat diet-induced obesity in rodent models. Male albino Wistar rats were divided into eight experimental groups, fed on a normal diet or high-fat diet (HFD), then given mangosteen extract (400 mg /kg /day) and/or curcumin (80 mg/kg /day) for 6 weeks. Oxidative stress markers, glucose, and lipid fractions were measured in the sera. Mangosteen pericarp extract (MPE) induced a remarkable decrease in BMI (from 0.86 to 0.81 gm/cm2), while curcuma either alone or in combination was more effective, as treated rats recorded BMIs of 0.78 and 0.79 gm/cm2, respectively. Regarding the antioxidant effects, MPE induced a significant increase of GSH in obese rats (123.86 ± 15.53 μg/ml vs 288.72 ± 121.37 μg/ml). As anti-atherogenic agents MPE demonstrate significant effect recorded higher level of HDL-C in treated animals, but ineefective as anti-dyslipidemic agent. Curcumin was more effective in reducing LDL-C levels in obese rats. Both extracts effectively reduced blood glucose. The present study demonstrated that MPE and curcumin were independently and synergistically effective in treating obesity-induced atherogenesis.
Subject terms: Biochemistry, Drug discovery
Introduction
Obesity is a complex metabolic disorder with multifactorial etiology. Obesity can lead to reduced quality of life due to numerous associated complications, including cardiovascular disease, diabetes, cancer, asthma, sleep disorders, liver and renal dysfunction, and sterility. Increasing evidence suggests that oxidative stress plays a critical role in linking obesity to these associated complications1–3.
Recent studies have proposed that the causes of oxidative stress in obesity include hyperleptinemia, hyperglycemia, insufficient antioxidant capacity, increased free radical generation, mitochondrial dysfunction, and chronic inflammation4,5. Accumulating evidence also shows that the quality of diet has demonstrated a negative shift toward consumption of high-energy foods, while intake of nutrient-rich foods such as fruits and vegetables has decreased6,7. Such low-quality diets have been linked to a higher risk of obesity8 consequently, dietary modification is frequently recommended as the main approach in prevention and treatment of obesity9,10. Many studies have also indicated that natural products such as phytochemicals in fruits and vegetables can be vital modulators of the risks accompanying obesity.
Mangosteen (Garcinia mangostana L.) fruit has a unique sweet–sour taste, and is rich in valuable compounds, such as xanthones. Mangosteen has been used in various traditional remedies to treat fever, diarrhea, and to promote wound healing. Recently, it has been used as a chief constituent in health supplements for weight loss and for supporting overall health11, due to its well-known anti-oxidative and anti-inglammatory medicinal properties12–14. Mangosteen pericarp contains mostly sugars (nearly 50% of total metabolites), followed by additional metabolites such as organic acids, alcohols, sugar acids, and aromatic compounds. Additionally, mangosteen pericarp contains several phenolic compounds, such as benzoic acid, tyrosol, and protocatechuic acid, which are known to have anti-oxidative and anti-inflammatory effects.
Interestingly, various studies have revealed that spices such as curcumin may show efficacy in controlling weight gain or reducing obesity. Curcumin is a bioactive polyphenol component found in turmeric rhizomes15. Clinical effects of curcumin in reducing weight and body mass index (BMI) have not been thoroughly assessed, and the results of many studies are unreliable and contradictory. Multiple studies have reported that a bioavailable form of curcumin resulted in improved weight management in obese humans and experimental animals16,17.
High-energy diets have been utilized to induce obesity and related metabolic disorders in rodent models; however, dietary mediation has not been absolutely standardized18. Usually, these diets comprise a simple exchange of carbohydrate-derived calories with fat-derived calories and are compared with a standard chow diet (SCD) as a control.
In this study, we sought to determine the potency of Garcinia mangostana pericarp extract and curcumin either independently or synergistically in modulating biochemical markers of oxidative stress, hyperlipidemia, and hyperglycemia in a high-fat diet-induced rodent model of obesity.
Results
Effect of treatments on weight gain and BMI
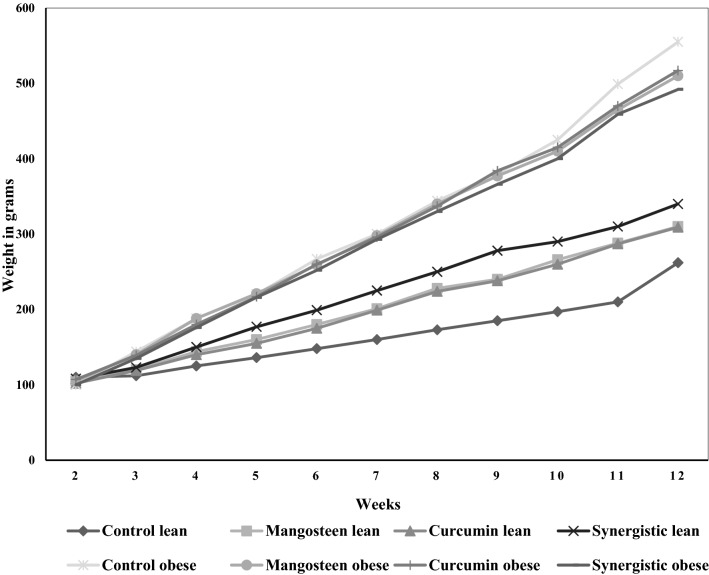
Body weight, weight gain, and BMI in normal and HFD-induced obese rats in response to mangosteen extract and curcumin are shown in Table 1 and Fig. 1. There was a significant difference between normal and obese experimental groups in weight (p ˂ 0.001). While the BMI in obese controls (Group 5) reached 0.86 ± 0.09 gm/cm2, the normal diet controls (Group 1) recorded a significantly lower BMI of 0.55 ± 0.04 gm/cm2 (p ˂ 0.001). Both mangosteen extract and curcumin ameliorated the increase in BMI as measure of obesity; while MPE induced a remarkable decrease in BMI (from 0.86 to 0.81 gm/cm2), curcuma either alone or in combination was more effective, as BMIs were reduced to 0.78 and 0.79 gm/cm2, respectively.
Table 1.
Independent and synergistic effects of curcumin and mangosteen extracts on BMI of lean and HFD-induced obese rats.
| Parameters | Groups | Mean ± SD | p-valuea | p-valueb |
|---|---|---|---|---|
| BW (g) | Control lean | 306.80 ± 32.38 | ||
| Mangosteen lean | 341.00 ± 70.84 | 0.355 | ||
| Curcumin lean | 360.00 ± 52.56 | 0.090 | ||
| Synergistic lean | 317.00 ± 15.67 | 0.544 | ||
| Control obese | 528.40 ± 33.92 | 0.001 | ||
| Mangosteen obese | 504.00 ± 14.85 | 0.006 | 0.179 | |
| Curcumin obese | 524.00 ± 21.63 | 0.001 | 0.813 | |
| Synergistic obese | 464.00 ± 38.19 | 0.001 | 0.023 | |
| Weight gain (g) | Control lean | 152.00 ± 30.83 | ||
| Mangosteen lean | 210.80 ± 72.81 | 0.153 | ||
| Curcumin lean | 207.20 ± 46.40 | 0.058 | ||
| Synergistic lean | 207.00 ± 13.25 | 0.006 | ||
| Control obese | 458.60 ± 35.66 | 0.001 | ||
| Mangosteen obese | 405.00 ± 16.67 | 0.003 | 0.016 | |
| Curcumin obese | 411.20 ± 21.05 | 0.001 | 0.034 | |
| Synergistic obese | 392.00 ± 38.63 | 0.001 | 0.022 | |
| BMI (g/cm2) | Control lean | 0.55 ± 0.04 | ||
| Mangosteen lean | 0.61 ± 0.06 | 0.102 | ||
| Curcumin lean | 0.65 ± 0.09 | 0.065 | ||
| Synergistic lean | 0.57 ± 0.02 | 0.423 | ||
| Control obese | 0.86 ± 0.09 | 0.001 | ||
| Mangosteen obese | 0.81 ± 0.06 | 0.001 | 0.360 | |
| Curcumin obese | 0.78 ± 0.03 | 0.030 | 0.105 | |
| Synergistic obese | 0.79 ± 0.07 | 0.001 | 0.259 |
aSignificant difference between normal weight and obese rats in each group (control, mangosteen, curcumin, and synergistic).
bSignificant difference between each group and respective normal weight or obese control groups.
Figure 1.
Body weight gain in different groups.
Effect of treatments on oxidative stress and antioxidant related variables
Levels of malondialdehyde (MDA) serve as a marker of lipid peroxidation and oxidative stress. Glutathione (GSH) levels, GST activity, and vitamin C levels also serve as markers of anti-oxidative capacity. Unexpectedly, obese controls did not show any significant increase in MDA levels or decrease in GSH levels, GST activity, or vitamin C levels (Table 2). While MPE did not affect these antioxidant markers in normal weight rats, it induced a significant increase of GSH in obese rats (123.86 ± 15.53 μg/ml vs 288.72 ± 121.37 μg/ml). There was also a significant increase in GST activity in rats treated with both mangosteen extract and curcumin (p ˂ 0.016), although independently these compounds did not achieve a significant effect. On the other hand, vita min C levels did not demonstrate any change either in HFD or in treated animals (Table 2).
Table 2.
Independent and synergistic effects of curcumin and mangosteen extract on oxidative stress- and antioxidant-related markers in lean and HFD-induced obese rats.
| Parameters | Groups | Mean ± SD | p-valuea | p-valueb |
|---|---|---|---|---|
| MDA (µg/ml) | Control lean | 108.95 ± 12.54 | ||
| Mangosteen lean | 100.18 ± 0.98 | 0.193 | ||
| Curcumin lean | 94.77 ± 8.12 | 0.067 | ||
| Synergistic lean | 93.17 ± 11.36 | 0.071 | ||
| Control obese | 94.97 ± 13.70 | 0.131 | ||
| Mangosteen obese | 81.21 ± 23.90 | 0.114 | 0.296 | |
| Curcumin obese | 94.73 ± 24.01 | 0.997 | 0.984 | |
| Synergistic obese | 101.98 ± 9.61 | 0.222 | 0.377 | |
| GSH (µg/ml) | Control lean | 148.31 ± 36.42 | ||
| Mangosteen lean | 123.86 ± 15.53 | 0.205 | ||
| Curcumin lean | 125.11 ± 16.23 | 0.230 | ||
| Synergistic lean | 162.51 ± 47.20 | 0.609 | ||
| Control obese | 168.50 ± 20.49 | 0.312 | ||
| Mangosteen obese | 288.72 ± 121.37 | 0.017 | 0.047 | |
| Curcumin obese | 140.94 ± 8.25 | 0.088 | 0.024 | |
| Synergistic obese | 152.84 ± 36.07 | 0.726 | 0.423 | |
| GST activity (µ/ml) | Control lean | 1.35 ± 0.42 | ||
| Mangosteen lean | 1.73 ± 0.39 | 0.115 | ||
| Curcumin lean | 1.23 ± 0.27 | 0.599 | ||
| Synergistic lean | 0.68 ± 0.86 | 0.249 | ||
| Control obese | 1.64 ± 1.46 | 0.600 | ||
| Mangosteen obese | 2.77 ± 2.33 | 0.599 | 0.346 | |
| Curcumin obese | 1.37 ± 0.23 | 0.293 | 0.465 | |
| Synergistic obese | 2.39 ± 0.53 | 0.016 | 0.117 | |
| Vit. C (µg/ml) | Control lean | 0.04 ± 0.01 | ||
| Mangosteen lean | 0.05 ± 0.03 | 0.602 | ||
| Curcumin lean | 0.07 ± 0.09 | 0.675 | ||
| Synergistic lean | 0.03 ± 0.01 | 0.094 | ||
| Control obese | 0.03 ± 0.00 | 0.075 | ||
| Mangosteen obese | 0.02 ± 0.01 | 0.016 | 0.115 | |
| Curcumin obese | 0.06 ± 0.08 | 0.141 | 0.462 | |
| Synergistic obese | 0.03 ± 0.01 | 0.402 | 0.600 |
aSignificant difference between normal weight and obese rats in each group (control, mangosteen, curcumin, and synergistic).
bSignificant difference between each group and respective normal weight or obese control groups.
Effect of treatments on absolute and relative levels of dyslipidemia related variables
Dyslipidemia was measured by blood cholesterol, TAG, LDL-C, and HDL-C levels. Obese rats displayed significantly higher cholesterol, TAG, and LDL-C levels, and non-significantly lower HDL-C levels (Table 3). While MPE was ineffective in reducing dyslipidemia-related markers (total and LDL-C), it did induce a significant increase in HDL-C, an anti-atherogenic marker (p ˂ 0.026).
Table 3.
Independent and synergistic effects of curcumin and mangosteen extract on the lipid profiles of lean and HFD-induced obese rats.
| Parameters | Groups | Mean ± SD | p-valuea | p-valueb |
|---|---|---|---|---|
| CHOL (mg/dl) | Control lean | 74.26 ± 6.97 | ||
| Mangosteen lean | 105.51 ± 14.21 | 0.002 | ||
| Curcumin lean | 104.07 ± 9.84 | 0.001 | ||
| Synergistic lean | 88.87 ± 2.93 | 0.003 | ||
| Control obese | 148.00 ± 48.24 | 0.026 | ||
| Mangosteen obese | 182.22 ± 9.31 | 0.001 | 0.189 | |
| Curcumin obese | 123.41 ± 34.60 | 0.287 | 0.381 | |
| Synergistic obese | 145.97 ± 21.93 | 0.004 | 0.934 | |
| HDL-C (mg/dl) | Control lean | 54.95 ± 8.71 | ||
| Mangosteen lean | 72.29 ± 17.96 | 0.088 | ||
| Curcumin lean | 56.18 ± 11.46 | 0.853 | ||
| Synergistic lean | 68.57 ± 18.27 | 0.171 | ||
| Control obese | 51.45 ± 17.76 | 0.702 | ||
| Mangosteen obese | 55.08 ± 8.81 | 0.026 | 0.693 | |
| Curcumin obese | 50.16 ± 12.30 | 0.446 | 0.897 | |
| Synergistic obese | 50.94 ± 13.88 | 0.124 | 0.961 | |
| LDL-C (mg/dl) | Control lean | 11.12 ± 3.89 | ||
| Mangosteen lean | 31.88 ± 12.21 | 0.016 | ||
| Curcumin lean | 30.30 ± 10.07 | 0.009 | ||
| Synergistic lean | 13.19 ± 3.25 | 0.463 | ||
| Control obese | 65.05 ± 43.59 | 0.075 | ||
| Mangosteen obese | 95.94 ± 8.81 | 0.009 | 0.173 | |
| Curcumin obese | 49.35 ± 16.88 | 0.076 | 0.602 | |
| Synergistic obese | 58.45 ± 15.91 | 0.009 | 0.754 | |
| TAG (mg/dl) | Control lean | 182.29 ± 31.89 | ||
| Mangosteen lean | 194.60 ± 24.94 | 0.516 | ||
| Curcumin lean | 196.49 ± 18.04 | 0.411 | ||
| Synergistic lean | 215.54 ± 6.71 | 0.052 | ||
| Control obese | 223.07 ± 14.77 | 0.032 | ||
| Mangosteen obese | 222.53 ± 3.91 | 0.038 | 0.941 | |
| Curcumin obese | 197.22 ± 89.32 | 0.987 | 0.556 | |
| Synergistic obese | 250.27 ± 44.94 | 0.126 | 0.234 |
aSignificant difference between normal weight and obese rats in each group (control, mangosteen, curcumin, and synergistic).
bSignificant difference between each group and respective normal weight or obese control groups.
Obese rats also had higher total cholesterol/HDL-C and HDL-C/LDL-C values, clinically used measures that indicate a risk factor for cardiovascular disease. While normal weight rats recorded ratios of 1.52 ± 0.28 and 4.62 ± 1.21, respectively, obese rats recorded much higher total cholesterol/HDL-C (3.02 ± 0.78) concomitant with much lower HDL-C/LDL-C (1.15 ± 0.73) ratios (Table 4). Serum glucose was also measured in the eight experimental groups. While HFD-induced obesity did not lead to elevation of blood glucose, MPE was effective in reducing glucose levels in both normal weight and obese animals reporting values of 154.43 ± 34.27 and 169.67 ± 36.21 respectively which is much lower than control untreated lean and obese animals. The synergistic treatment domonstrate more potency in reducing blood glucose levels in HFT-induced obese rats recording value of 155.44 ± 74.38 (Table 4).
Table 4.
Independent and synergistic effects of curcumin and mangosteen extract on cardiovascular risk factors in lean and HFD-induced obese rats.
| Parameters | Groups | Mean ± SD | p-valuea | p-valueb |
|---|---|---|---|---|
| CHOL/HDL-C | Control lean | 1.52 ± 0.28 | ||
| Mangosteen lean | 1.49 ± 0.24 | 0.877 | ||
| Curcumin lean | 1.73 ± 0.40 | 0.352 | ||
| Synergistic lean | 1.36 ± 0.32 | 0.416 | ||
| Control obese | 3.02 ± 0.78 | 0.010 | ||
| Mangosteen obese | 2.97 ± 0.85 | 0.005 | 0.934 | |
| Curcumin obese | 2.67 ± 1.31 | 0.165 | 0.628 | |
| Synergistic obese | 2.78 ± 0.67 | 0.003 | 0.620 | |
| HDL-C/LDL-C | Control lean | 4.61 ± 1.21 | ||
| Mangosteen lean | 2.54 ± 1.02 | 0.016 | ||
| Curcumin lean | 1.95 ± 0.40 | 0.009 | ||
| Synergistic lean | 5.35 ± 1.43 | 0.465 | ||
| Control obese | 1.15 ± 0.73 | 0.009 | ||
| Mangosteen obese | 0.57 ± 0.07 | 0.009 | 0.346 | |
| Curcumin obese | 1.21 ± 0.79 | 0.117 | 0.917 | |
| Synergistic obese | 0.95 ± 0.51 | 0.009 | 1.000 | |
| GLU (mg/dl) | Control lean | 232.55 ± 74.67 | ||
| Mangosteen lean | 154.43 ± 34.27 | 0.076 | ||
| Curcumin lean | 121.12 ± 8.90 | 0.009 | ||
| Synergistic lean | 162.08 ± 39.89 | 0.117 | ||
| Control obese | 206.94 ± 100.22 | 0.347 | ||
| Mangosteen obese | 169.67 ± 36.21 | 0.346 | 0.917 | |
| Curcumin obese | 174.43 ± 56.07 | 0.076 | 0.754 | |
| Synergistic obese | 155.44 ± 74.38 | 0.917 | 0.754 |
aSignificant difference between normal weight and obese rats in each group (control, mangosteen, curcumin, and synergistic).
bSignificant difference between each group and respective normal weight or obese control groups.
Correlations between all measured markers were also calculated. Negative correlations were observed between BMI, antioxidant status (GSH levels), and HDL-cholesterol (Table 5). BMI was also positively correlated with dyslipidemia-related markers CHOL, LDL, TAG, and CHOL/HDL-C with strong P values < 0.01 or 0.05.
Table 5.
Pearson’s correlation coefficients between measured variables.
| Parameters | R (correlation Coefficient) | p-value | Correlation |
|---|---|---|---|
| BMI with CHOL | 0.601** | 0.000 | Pa |
| BMI with HDL-C | − 0.381* | 0.015 | Nb |
| BMI with GSH | − 0.298* | 0.011 | Nb |
| BMI with CHOL/HDL-C | 0.645** | 0.000 | Pa |
| CHOL with TRIG | 0.507** | 0.001 | Pa |
| TRIG with CHOL/HDL-C | 0.384* | 0.014 | Pa |
| LDL-C with BMI | 0.634** | 0.000 | Pa |
| LDL-C with GSH | 0.343* | 0.030 | Na |
| LDL-C with CHOL | 0.955** | 0.000 | Pa |
| LDL-C with TRIG | 0.451** | 0.003 | Pa |
| LDL-C with HDL-C/LDL-C | − 0.927** | 0.000 | Nb |
**p < 0.01.
*p < 0.05.
aPositive correlation.
bNegative correlation.
ROC and AUC were calculated to measure specificity and sensitivity in the four obese groups. Among the measured variables, BMI was the most efficient variable either to predict obesity in HFD-fed mice or the therapeutic potency of curcumin and mangosteen (Table 6). With exception of glucose most of the measured variables recorded excellent ROCAUCs range between 0.8 and 1.0 with satisfactory specificity and sensitivity.
Table 6.
ROC of all parameters for obese rats.
| Parameters | Groups | AUC | Cut-off value | Sensitivity % | Specificity % | P value | 95% CI |
|---|---|---|---|---|---|---|---|
| BMI | Control obese | 1.000 | 0.705 | 100.0 | 100.0 | 0.009 | 1.000–1.000 |
| Mangosteen obese | 1.000 | 0.695 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 1.000 | 0.755 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Synergistc obese | 1.000 | 0.660 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| MDA | Control obese | 0.800 | 102.640 | 80.0 | 80.0 | 0.117 | 0.494–1.106 |
| Mangosteen obese | 0.800 | 98.195 | 80.0 | 100.0 | 0.117 | 0.449–1.151 | |
| Curcuma obese | 0.520 | 110.025 | 40.0 | 100.0 | 0.917 | 0.110–0.930 | |
| Synergistc obese | 0.760 | 90.030 | 100.0 | 60.0 | 0.175 | 0.436–1.084 | |
| GSH | Control obese | 0.680 | 148.060 | 100.0 | 60.0 | 0.347 | 0.301–1.059 |
| Mangosteen obese | 1.000 | 149.995 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 0.840 | 135.745 | 80.0 | 80.0 | 0.076 | 0.580–1.100 | |
| Synergistc obese | 0.580 | 162.795 | 80.0 | 60.0 | 0.676 | 0.195–0.965 | |
| GST | Control obese | 0.600 | 0.911 | 40.0 | 100.0 | 0.602 | 0.219–0.981 |
| Mangosteen obese | 0.600 | 2.417 | 60.0 | 100.0 | 0.602 | 0.171–1.029 | |
| Curcuma obese | 0.700 | 1.228 | 80.0 | 60.0 | 0.296 | 0.358–1.042 | |
| Synergistc obese | 0.960 | 1.432 | 100.0 | 80.0 | 0.016 | 0.843–1.077 | |
| Vit. C | Control obese | 0.840 | 0.028 | 80.0 | 80.0 | 0.076 | 0.580–1.100 |
| Mangosteen obese | 0.960 | 0.035 | 100.0 | 80.0 | 0.016 | 0.843–1.077 | |
| Curcuma obese | 0.780 | 0.024 | 60.0 | 100.0 | 0.144 | 0.467–1.093 | |
| Synergistc obese | 0.660 | 0.032 | 60.0 | 80.0 | 0.403 | 0.304–1.016 | |
| CHOL | Control obese | 1.000 | 85.105 | 100.0 | 100.0 | 0.009 | 1.000–1.000 |
| Mangosteen obese | 1.000 | 146.145 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 0.640 | 126.625 | 60.0 | 100.0 | 0.465 | 0.238–1.042 | |
| Synergistc obese | 1.000 | 109.055 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| HDL-C | Control obese | 0.560 | 58.545 | 60.0 | 80.0 | 0.754 | 0.168–0.952 |
| Mangosteen obese | 0.920 | 56.185 | 80.0 | 100.0 | 0.028 | 0.738–1.102 | |
| Curcuma obese | 0.640 | 60.955 | 100.0 | 40.0 | 0.465 | 0.277–1.003 | |
| Synergistc obese | 0.800 | 59.835 | 80.0 | 80.0 | 0.117 | 0.494–1.106 | |
| LDL-C | Control obese | 0.840 | 29.425 | 80.0 | 100.0 | 0.076 | 0.544–1.136 |
| Mangosteen obese | 1.000 | 65.210 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 0.840 | 44.870 | 80.0 | 100.0 | 0.076 | 0.544–1.136 | |
| Synergistc obese | 1.000 | 27.970 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| TRIG | Control obese | 1.000 | 204.290 | 100.0 | 100.0 | 0.009 | 1.000–1.000 |
| Mangosteen obese | 0.960 | 211.535 | 100.0 | 80.0 | 0.016 | 0.843–1.077 | |
| Curcuma obese | 0.600 | 226.895 | 60.0 | 100.0 | 0.602 | 0.171–1.029 | |
| Synergistc obese | 0.800 | 242.755 | 80.0 | 100.0 | 0.117 | 0.449–1.151 | |
| CHOL/HDL-C | Control obese | 1.000 | 1.950 | 100.0 | 100.0 | 0.009 | 1.000–1.000 |
| Mangosteen obese | 1.000 | 1.800 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 0.800 | 1.970 | 80.0 | 80.0 | 0.117 | 0.494–1.106 | |
| Synergistc obese | 1.000 | 1.790 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| HDL-C /LDL-C | Control obese | 1.000 | 2.470 | 100.0 | 100.0 | 0.009 | 1.000–1.000 |
| Mangosteen obese | 1.000 | 1.065 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| Curcuma obese | 0.800 | 1.305 | 80.0 | 100.0 | 0.117 | 0.449–1.151 | |
| Synergistc obese | 1.000 | 2.605 | 100.0 | 100.0 | 0.009 | 1.000–1.000 | |
| GLU | Control obese | 0.680 | 152.088 | 60.0 | 100.0 | 0.347 | 0.301–1.059 |
| Mangosteen obese | 0.680 | 145.168 | 80.0 | 60.0 | 0.347 | 0.328–1.032 | |
| Curcuma obese | 0.840 | 141.216 | 60.0 | 100.0 | 0.076 | 0.580–1.100 | |
| Synergistc obese | 0.520 | 139.069 | 80.0 | 40.0 | 0.917 | 0.135–0.905 |
Multiple regression analysis was also performed using GSH levels as the dependent variable. Interestingly, we observed that MDA as measure of oxidative stress together with dyslipidemia-related independent variables greatly contributed to GSH depletion (Table 7).
Table 7.
Multiple regression analysis using GSH levels as the dependent variable.
| Predictor variable | Coefficient | SE | p-value | Adjusted R2 | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| LDL-C | 1.196 | 0.285 | 0.000 | 0.298 | 0.618 | 1.773 |
| LDL-C | 2.355 | 0.391 | 0.983 | 0.482 | 1.563 | 3.146 |
| HDL-C/LDL-C | 25.584 | 6.716 | 0.000 | 11.976 | 39.191 | |
| LDL-C | 2.231 | 0.350 | 0.000 | 0.589 | 1.521 | 2.941 |
| HDL-C/LDL-C | 26.558 | 5.990 | 0.000 | 14.409 | 38.707 | |
| MDA | − 1.509 | 0.463 | 0.002 | − 2.448 | − 0.570 | |
Discussion
A diet high in fat leads to obesity in both humans and animals19,20. In both rats and mice, a positive relationship has been demonstrated between the levels of fat in the diet and body weight or fat gain; also, rats that consumed diets containing high quantities of fat gained weight faster than those on diets containing minimal fat21–25.
The recorded increase of BMI in obese rats is in agreement with previous studies26, in which they reported that BMI in obese rats is usually higher than 0.75 gm/cm2. The final weight and weight gain in obese rats were also coupled with high BMI, in agreement with the findings of Picklo et al.27, who showed the obesogenic effect of a saturated lipid diet in animal models. Unexpectedly, while both MPE and curcumin were effective in lowering the BMI of obese animals, both also induced weight gain in lean animals (Table 1 and Fig. 1). This is similar to the findings of Husen et al.28, who reported an increase in body weight following mangosteen extract and curcumin after the decrease in body weight induced by STZ treatment.
The elevated levels of ROS after high caloric intake or inflammation can later result in increase of BMI or incidence of obesity. Adipose tissue can undergo pathological alterations induced by inflammation or oxidative stress, which in turn enhances the secretion of adipokines and affects the peripheral tissues that produce ROS, further promoting oxidative stress and the inflammatory response29,30. The concentration of the serum MDA can be used as an indicator of oxidative stress. MDA is one of the final products of the peroxidation of polyunsaturated fatty acids (PUFA). The concentration of MDA can be used as an indicator of cell or tissue damage due to the increase in lipid peroxidation. The unexpected non-significant increase of MDA and decrease in GSH levels, GST activity, and vitamin C levels in HFD-induced obese rats might be attributed to the addition of coconut oil to the high saturated fat diet given in our paradigm as an inducer of obesity. Multiple studies demonstrate the anti-oxidative effects of virgin coconut oil, supporting this hypothesis31,32. Table 2 also shows the independent and synergistic effects of MPE and curcumin on oxidative stress-related variables. While MPE did not demonstrate anti-oxidative effects in normal weight rats, it did induce a significant increase in GSH levels in obese rats. GST functions as antioxidant, and enzymes can catalyze the detoxification of xenobiotics via conjugation with GSH.
The diet-induced obesity animal model is one of the most common and reliable models used in obesity studies due to its similarity in modeling the most common route of obesity in humans, as well as related metabolic effects. As shown previously, this HFD models obesity via increased food intake, body weight gain, body fat accumulation, BMI increase, defects in antioxidant levels, and disruption in the lipid profile19,25.
While LDL-C is responsible for the delivery of cholesterol to peripheral tissues, HDL-C mediates the inverse process of cholesterol transport from peripheral tissues33. The non-significant decrease of HDL-C in obese rats reported in the present study may be related to the anti-oxidative effect of coconut oil, a component of the HFD used here31,32.
In general, ingestion of coconut oil can increase HDL-C34. It has been proposed that lauric acid, the main constituent of coconut oil, is the cornerstone of this pathway. Lauric acid accounts for 50% of the content of coconut oil. Although lauric acid is considered a medium-chain fatty acid (MCFA), 70% of lauric acid is transported as a long-chain fatty acid (LCFA), while the other 30% remains as a MCFA. Thus, there are two ways of transporting lauric acid in the body. When lauric acid reaches the liver, it serves as a substrate in the production of apoA1 and apoB, further contributing to the formation of both HDL-C and LDL-C35.
Studies show that in contrast, to LDL-C, HDL-C may play an anti-atherogenic and anti-thrombotic role by protecting LDL-C particles against lipid peroxidation and reducing the deleterious effects of oxidized LDL-C35. Based on this report, the non-significant decrease of HDL-C in obese rats may be related to the presence of coconut oil in the HFD36.
Moreover, our data is consistent with those of Wihastuti et al.37, who showed that 400 mg/kg body weight MPE affected LDL-C but no other lipid marker, and significantly reduced H2O2 levels and NF-κB expression. At concentrations of 800 mg/kg body weight, this extract was most effective in improving the lipid profile; this suggests that although in the present study MPE did induce a hypo-lipidemic effect in obese rats, the anti-atherogenic effects likely occurred via its anti-oxidative and anti-inflammatory effects37.
This contradiction may also be attributed to the fact the amount of total xanthone in the MPE is strongly affected by the extraction capacity of the solvent to recover different phenolic constituents from various fruit origins, as well as the methods of transportation and storage38–40. Aisha et al.41 have reported that toluene is the most efficient extraction solvent for MPE compared to 75% ethanol and methanol42.
Obesity is a risk factor for the development of cardiovascular disease, diabetes mellitus, hyperlipidemia, and arteriosclerosis43. To treat and prevent obesity and obesity-related complications, an increasing number of people use hypoglycemia-inducing or weight-loss drugs. However, the long-term use of these drugs can damage the liver and kidney. Unsurprisingly, finding safe and effective weight-loss- and hypoglycemia-inducing agents is becoming increasingly urgent.
Table 4 reveals the level of serum glucose in the studied rats. While obesity itself did not induce elevation of blood glucose, MPE was effective in reducing glucose levels in both normal weight and obese animals. This is consistent with the previous work of Taher et al.44, which demonstrated that orally administered MPE at various doses demonstrated a hypoglycemic effect in streptozotocin (STZ)-induced diabetic rats and normoglycemic rats. Moreover, curcumin demonstrates hypoglycemic effects in both obese and normal weight rats; in obese rats, the synergistic effects of both of these extracts was much higher than each extract independently. This is also supported by Rivera-Mancía et al.45 and Sohaei et al.46, who showed the hypoglycemic effects of curcumin. As previously described, the use of curcumin in vitro and in animal models of diabetes revealed a variety of potential mechanisms of action to treat diabetes mellitus; however, clinical trials in humans have thus far been inconsistent with these findings.
Pearson’s correlation coefficient (PCC) is a statistical metric that measures the strength and direction of a linear relationship between two or more random variables47. Table 5 indicates the correlations between all variables measured in this study. It can be seen that obesity (BMI), antioxidant status (GSH levels), and dyslipidemia-related markers (CHOL, TAG, CHOL/HDL-C) were negatively or positively correlated in a manner that demonstrates the negative impact of oxidative stress and dyslipidemia on obesity.
Abruzzo et al.48 highlighted the advantage of using ROC curves as an outstanding statistical tool for the identification of biomarkers that are sufficiently sensitive and specific for the early diagnosis of obesity. Although its utility in prediction, risk valuation, and assessment of therapeutic interventions still requires further validation, ROC curves emphasize the most significant statistical differences between patients and controls and even animal models of diseases49. The AUC provides a useful measure to evaluate the predictive value of biomarkers. While an AUC value near 1 designates an excellent predictive marker, a curve that lies adjacent to the diagonal (AUC = 0.5) indicates no diagnostic usefulness. AUC values close to 1.00 are always accompanied by satisfactory values of specificity and sensitivity50.
Table 6 demonstrates the ROC curves with AUC, specificity, and sensitivity in obese rats. Among the measured variables, BMI showed excellent predictive value as a marker of obesity, with AUC between 0.8 and 1 with satisfactory specificity and sensitivity. The other measured variables demonstrate relatively less predictive ability with any specificity and sensitivity.
Conclusion
The present study ascertained the efficiency of a HFD in inducing obesity in rats, and the effectiveness of MPE and curcumin independently or synergistically in treating obesity-induced atherogenesis.
Methods
Preparation of mangosteen extract
Mangosteen fruits were obtained from a local market in Riyadh, and authenticated by a taxonomist from the Department of Botany, College of Science, King Saud University, Riyadh. 500 g of the dried pericarp of mangosteen was soaked in 1.5 L of 99.7% methanol at room temperature for 72 h. Extracts were filtered through Whatman No. 1 filter paper, and solvent was evaporated using a rotary evaporator at 60 °C. The resulting viscous concentrate was freeze-dried to ensure complete removal of solvent, and stored at − 20 °C for further use. GC–MS analysis for Garcinia mangostina was done and show 99–95% similarity in the database for fatty acids. Major components of the extract are listed in Table S1 shown below.
Preparation of curcumin
Fresh curcumin was obtained from a local market in Riyadh, and dried after proper cleaning using a drying oven with an air fan at 50 °C for 3–5 h. Curcumin was then ground and sieved, and the remaining soft powder was stored at − 80 °C until further use.
Animals
All experimental procedures were carried out in Experimental Surgery and Animal Laboratory Prince Naif Health Research Center (PNHRC) after being approved by the Ethical Committee of Scientific Research at KSU. All animals hosted in in polypropylene cages in an environmentally controlled clean air room, with a temperature of (25 °C ± 1 °C) a 12 h light/12 h dark cycle and a relative humidity of 50 ± 5%.
Forty male albino Wistar rats, weighing 100 ± 20 g of four weeks age, The animals had free access food (standard laboratory animal feed pellets) and water for 4 groups normal weight and HFD for another four groups to induce obesity. The diet was prepared in collaboration with (PNHRC), Medicine college in King Khalid Hospital (KKH), in (KSU), HFD (45% kcal from fat) was prepared by making the composition of the British company. Test Diet replacing the pig fat in the composition of the company with 50% hydrogenated fats (Butter oil, Palm oil) and 50% coconut oil due as pork fat is forbidden according to the Saudi Food and Drug Regulations SFD (Diet formulation is shown in Table S2). All experiments were performed in accordance with the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of King Saud University (reference no: KSU-SE-18-17).
Animal groups and diets
Animals were divided into eight groups of 5 animals each. Group 1 serves as the control group, as animals were fed a normal diet. Group 2 received a normal diet and 400 mg/kg body weight/day water extract of mangosteen pericarp for 6 weeks. Group 3 rats received a normal diet and curcumin at 80 mg/kg body weight/day for 6 weeks. Group 4 received a normal diet and both MPE (400 mg/kg body weight/day) and curcumin (80 mg/kg body weight/day) for 6 weeks. Group 5 was fed with a high-fat diet (HFD) for 4 weeks. Group 6 rats were fed a HFD for 4 weeks followed by 400 mg/kg body weight/day water extract of mangosteen pericarp for 6 weeks51. Group 7 received HFD for 4 weeks, followed by 80 mg/kg body weight/day curcumin for 6 weeks52. Group 8 animals received a HFD for 4 weeks, followed by both MPE (400 mg/kg body weight/day) and curcumin (80 mg/kg body weight/day) for 6 weeks. At the end of the experiment, animals were anesthetized with 5.0% of sevoflurane and 100% oxygen, the flow rate of sevoflurane was determined as the following formula: flow rate (ml/min) = 0.5 × body weight (g). Blood was collected from the eye and heart using hematocrit capillaries (75 mm/75 μl). Our study was carried out in compliance with the ARRIVE guidelines.
Collection of serum
Serum was collected from blood samples by centrifugation for 15 min at 4000 rpm. Serum was further utilized in the analysis of the following biochemical parameters.
Levels of serum lipid fractions
Cholesterol, triglyceride, HDL, and LDL levels were quantitatively determined using commercial kits from United Diagnostics Industry Company (UDIC), Riyadh, Saudi Arabia.
Levels of serum glucose
Quantitative determination of glucose levels was made via the use of Trinder glucose oxidase I activity test using a kit from, a product of United Diagnostics Industry Company (UDIC), Riyadh, Saudi Arabia.
Level of serum oxidative stress markers
Lipid oxidation was estimated according to the method reported by Begonaruizlarrea et al.53. In this method, lipid peroxidation was determined by measuring the thiobarbituric acid reactive sbstances (TBARS), mainly malondialdehyde (MDA).
Vitamin C was estimated using the method described by Jagota and Dani54. In this method, ascorbic acid was reacted Folin phenol reagent to develop a color which is directly proportional to vitamin C concentration. The absorption maximum of the color developed by the interaction of ascorbic acid with Folin reagent is 760 nm. Glutathione levels were evaluated per the method described by Beutler et al.55. The method based on the development of a relatively stable yellow color when 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) was added to sulphahydryl compounds. Glutathione S-transferase (GST) activity was determined by method reported by Habig and Pabst [56]. The reaction is measured by observing the conjugation of 1-chloro, 2, 4-dinitrobenzene (CDNB) with reduced (GSH). This was done by watching an increase in absorbance at 340 nm.
Statistical analysis
Data are expressed as means ± standard deviation (SD). To compare results between groups, one-way analysis of variance (ANOVA) tests were used. Significance was assigned at the level of p < 0.05. The receiver operating characteristics curve (ROC) was analyzed, and the area under the curve (AUC), cutoff values, and degrees of sensitivity and specificity were calculated.
Supplementary Information
Acknowledgements
The authors thank the Deanship of Scientific Research for funding and supporting this research through the DSR Graduate Students Research Support (GSR) initiative. The authors also extend their gratitude to the RSSU at King Saud University for their technical support.
Author contributions
R.S.M.L.: aquistion of the data. H.A.A.: supervise the practical work. A.T.A.: performing the experimental work. M.N.A-.M: co-drafted the manuscript. R.S.B.: aquistion of the data and revised the manuscript. A.E-.A.: supervise the experimental work and drafted the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86545-z.
References
- 1.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015;13(10):423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherer PE, Hill JA. Obesity, diabetes, and cardiovascular diseases. Circ. Res. 2016;118(11):1703–1705. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Boccellino M, et al. AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress. PLoS ONE. 2018;13(10):e0202297. doi: 10.1371/journal.pone.0202297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent HK, Taylo RAG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 5.Vanacore A, et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J Cell Physiol. 2018;233(12):9345–9353. doi: 10.1002/jcp.26427. [DOI] [PubMed] [Google Scholar]
- 6.Akbari F. A systematic review on diet quality among Iranian Youth: focusing on reports from Tehran and Isfahan. Arch Iran Med. 2014;17(8):574–584. [PubMed] [Google Scholar]
- 7.Azadbakht L, Akbari F. Diet quality among Iranian adolescents needs improvement. Public Health Nutr. 2015;18(4):615–621. doi: 10.1017/S1368980014000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia L, et al. Associationbetween diet quality and obesity indicators among the working-age adults in Inner Mongolia, Northern China: a cross-sectional study. BMC Public Health. 2020;20(1):1165. doi: 10.1186/s12889-020-09281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell NJ, Stock MJ. The development of obesity in animals: The role of dietary factors. Clin. Endocrinol. Metab. 1984;13(3):437–449. doi: 10.1016/S0300-595X(84)80032-8. [DOI] [PubMed] [Google Scholar]
- 10.Pandita A, Sharma D, Pandita D, et al. Childhood obesity: Prevention is better than cure. Diabetes Metab. Syndr. Obes. Targets Ther. 2016;9:83–89. doi: 10.2147/DMSO.S90783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizat WM, et al. Recent updates on metabolite composition and medicinal benefits of mangosteen plant. Peer J. 2019;7:e6324. doi: 10.7717/peerj.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CY, et al. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J. Agric. Food Chem. 2009;57(15):6661–6667. doi: 10.1021/jf9015202. [DOI] [PubMed] [Google Scholar]
- 13.Chae HS, et al. Mangosteen extract prevents dextran sulfate sodium-induced colitis in mice by suppressing NF-κB activation and inflammation. J. Med. Food. 2017;20(8):727–733. doi: 10.1089/jmf.2017.3944. [DOI] [PubMed] [Google Scholar]
- 14.Mamat SF, et al. Metabolomics analysis of mangosteen (Garcinia mangostana Linn.) fruit pericarp using different extraction methods and GC-MS. Plant OMICS. 2018;11(2):89–97. doi: 10.21475/poj.11.02.18.pne1191. [DOI] [Google Scholar]
- 15.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa) J. Altern. Complement Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 16.Di Pierro F, et al. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome: Preliminary study. Eur. Rev. Med. 2015;19(21):4195–4202. [PubMed] [Google Scholar]
- 17.Ghazimoradi M, et al. The effects of curcumin and curcumin–phospholipid complex on the serum pro-oxidant–antioxidant balance in subjects with metabolic syndrome. Phytother. Res. 2017;31(11):1715–1721. doi: 10.1002/ptr.5899. [DOI] [PubMed] [Google Scholar]
- 18.Sasidharan SR, et al. An experimental approach for selecting appropriate rodent diets for research studies on metabolic disorders. BioMed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/752870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 20.Warwick ZS, Schiffman SS. Role of dietary fat in calorie intake and weight gain. Neurosci. Biobehav. Rev. 1992;16(4):585–596. doi: 10.1016/S0149-7634(05)80198-8. [DOI] [PubMed] [Google Scholar]
- 21.Bourgeois F, Alexiu A, Lemonnter D. Dietary-induced obesity: Effect of dietary fats on adipose tissue cellularity in mice. Br. J. Nutr. 1983;49(1):17–26. doi: 10.1079/BJN19830006. [DOI] [PubMed] [Google Scholar]
- 22.Boozer CN, et al. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am. J. Physiol. Endocrinol. Metab. 1995;268(4):E546–E550. doi: 10.1152/ajpendo.1995.268.4.E546. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Ikemoto S, Ezaki O. Effect of the fat/carbohydrate ratio in the diet on obesity and oral glucose tolerance in C57BL/6J mice. J. Nutr. Sci. Vitaminol. Tokyo. 1999;45(5):583–593. doi: 10.3177/jnsv.45.583. [DOI] [PubMed] [Google Scholar]
- 24.Ghibaudi L, et al. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague-Dawley rats. Obes. Res. 2002;10(9):956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 25.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23(2):270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 26.Novelli ELB, et al. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007;41(1):111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 27.Picklo MJ, et al. Comparative effects of high oleic acid vs high mixed saturated fatty acid obesogenic diets upon PUFA metabolism in mice. Prostaglandins Leukot Essent Fatty Acids. 2017;1:11925–11937. doi: 10.1016/j.plefa.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Husen, S.A., et al. Activity assay of mangosteen (Garcinia mangostana L.) pericarp extract for decreasing fasting blood cholesterol level and lipid peroxidation in type-2 diabetic mice. AIP Conf Proc.;1888 (2017).
- 29.Hensley K, et al. Therapeutic applications of reactive oxygen and nitrogen species in human disease. Free Radic. Biol. Med. 2000;28(10):1456–1462. doi: 10.1016/S0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 30.Cristancho A, Lazar M. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durašević S, et al. The protective role of virgin coconut oil on the alloxan-induced oxidative stress in the liver, kidneys and heart of diabetic rats. Food Funct. 2019;10(4):2114–2124. doi: 10.1039/C9FO00107G. [DOI] [PubMed] [Google Scholar]
- 32.Famurewa AC, et al. Antioxidant, anti-inflammatory, and antiapoptotic effects of virgin coconut oil against antibiotic drug gentamicin-induced nephrotoxicity via the suppression of oxidative stress and modulation of iNOS/NF-ĸB/caspase-3 signaling pathway in Wistar rats. J. Food Biochem. 2020;44(1):1–10. doi: 10.1111/jfbc.13100. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YZ, et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108(6):661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 34.Santos HO, et al. Coconut oil intake and its effects on the cardiometabolic profile-A structured literature review. Prog. Cardiovasc. Dis. 2019;62(5):436–443. doi: 10.1016/j.pcad.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Navab M, et al. Thematic review series: The Pathogenesis of Atherosclerosis The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid. Res. 2004;45(6):993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Eyres L, et al. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016;74(4):267–280. doi: 10.1093/nutrit/nuw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wihastuti TA, et al. Study of the inhibition effect of ethanolic extract of mangosteen pericarp on atherogenesis in hypercholesterolemic rat. Asian Pac. J. Trop. Dis. 2015;5(10):830–834. doi: 10.1016/S2222-1808(15)60940-9. [DOI] [Google Scholar]
- 38.Suttirak W, Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014;51(12):3546–3558. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kusmayadi A, et al. The effect of solvents and extraction time on total xanthone and antioxidant yields of mangosteen peel (Garcinia mangostana L.) extract. Drug Invent. Today. 2018;10(12):2572–2576. [Google Scholar]
- 40.Sungpud C, et al. Tuning of virgin coconut oil and propylene glycol ratios for maximizing the polyphenol recovery and in vitro bioactivities of mangosteen (Garcinia mangostana L.) pericarp. Process Biochem. 2019;87:179–186. doi: 10.1016/j.procbio.2019.08.023. [DOI] [Google Scholar]
- 41.Aisha AFA, Abu-Salah KM, Ismail Z, Majid AMSA. Determination of total xanthones in Garcinia mangostana fruit rind extracts by ultraviolet (UV) spectrophotometry. Med. Plants Res. 2013;7(1):29–35. [Google Scholar]
- 42.Mohammad NA, et al. Optimization of the antioxidant-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon. 2019;5(10):e02571. doi: 10.1016/j.heliyon.2019.e02571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christopher P, Cannon M. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone. 2008;9(2):24–41. doi: 10.1016/S1098-3597(09)62037-8. [DOI] [PubMed] [Google Scholar]
- 44.Taher MT, et al. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement Altern. Med. 2016;16(1):135. doi: 10.1186/s12906-016-1118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera-Mancía S, Trujillo J, Chaverri JP. Utility of curcumin for the treatment of diabetes mellitus: Evidence from preclinical and clinical studies. J. Nutr. Intermed. Metab. 2018;14:29–41. doi: 10.1016/j.jnim.2018.05.001. [DOI] [Google Scholar]
- 46.Sohaei S, et al. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther. Med. 2019;47:102201. doi: 10.1016/j.ctim.2019.102201. [DOI] [PubMed] [Google Scholar]
- 47.Lee Rodgers J, Nicewander WA. Thirteen ways to look at the correlation coefficient. Am. Stat. 1988;42(1):59–66. doi: 10.1080/00031305.1988.10475524. [DOI] [Google Scholar]
- 48.Abruzzo PM, et al. Perspective biological markers for autism spectrum disorders: advantages of the use of receiver operating characteristic curves in evaluating marker sensitivity and specificity. Dis. Mark. 2015;1:1–16. doi: 10.1155/2015/329607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Ansary A, Hassan WM, Daghestani M, Al-Ayadhi L, Ben Bacha A. Preliminary evaluation of a novel nine-biomarker profile for the prediction of autism spectrum disorder. PLoS ONE. 2020;15(1):e0227626. doi: 10.1371/journal.pone.0227626. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Metz CE. Basic principles of ROC analysis. Semin. Nucl. Med. 1978;8(4):283–298. doi: 10.1016/S0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 51.Wihastuti TA, Sargowo D, Tjokroprawiro A, Permatasari N, Widodo MA, Soeharto S. Vasa vasorum anti-angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition by mangosteen pericarp ethanolic extract (Garcinia mangostana Linn) in hypercholesterol-diet-given Rattus norvegicus Wistar strain. Vasc. Health Risk Manag. 2014;21(10):523–531. doi: 10.2147/VHRM.S61736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding L, et al. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 2016;304:99–109. doi: 10.1016/j.taap.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Begonaruizlarrea M, et al. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383–388. doi: 10.1016/0039-128X(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 54.Jagota SK, Dani HM. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982;127(1):178–182. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- 55.Beutler E, Duron O, Kelly BM. Improved methods for determination of blood Gluthatione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 56.Habig WH, Pabst MJ. (1974) Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;25:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.