Abstract
Bacteria synthesize guanosine tetra- and penta phosphate (commonly referred to as (p)ppGpp) in response to environmental stresses. (p)ppGpp reprograms cell physiology and is essential for stress survival, virulence and antibiotic tolerance. Proteins of the RSH superfamily (RelA/SpoT Homologues) are ubiquitously distributed and hydrolyze or synthesize (p)ppGpp. Structural studies have suggested that the shift between hydrolysis and synthesis is governed by conformational antagonism between the two active sites in RSHs. RelA proteins of γ-proteobacteria exclusively synthesize (p)ppGpp and encode an inactive pseudo-hydrolase domain. Escherichia coli RelA synthesizes (p)ppGpp in response to amino acid starvation with cognate uncharged tRNA at the ribosomal A-site, however, mechanistic details to the regulation of the enzymatic activity remain elusive. Here, we show a role of the enzymatically inactive hydrolase domain in modulating the activity of the synthetase domain of RelA. Using mutagenesis screening and functional studies, we identify a loop region (residues 114–130) in the hydrolase domain, which controls the synthetase activity. We show that a synthetase-inactive loop mutant of RelA is not affected for tRNA binding, but binds the ribosome less efficiently than wild type RelA. Our data support the model that the hydrolase domain acts as a molecular switch to regulate the synthetase activity.
Subject terms: Bacterial genetics, Ribosome, tRNAs, RNA sequencing
Sinha and Winther show that the Escherichia coli RelA inactive hydrolase domain modulates the activity of the synthetase domain. RelA produces (p)ppGpp in γ-proteobacteria; using mutagenesis screening and functional studies, the authors demonstrate that the H loop region in the RelA hydrolase domain acts as a molecular switch to regulate the synthetase domain activity of the enzyme.
Introduction
Bacteria have evolved intricate mechanisms and responses to adapt quickly to changing and stressful environments. One of such bacterial responses is the universal stringent response. The stringent response is induced in response to amino acid starvation1,2, fatty acid limitation3,4, iron limitation5, heat shock6, glucose starvation7, nitrogen starvation8, phosphate starvation9 and other stress conditions10. The stringent response reprograms cell physiology, which facilitates stress adaptation and survival under harsh environmental conditions11. Furthermore, the stringent response is essential for virulence and has been shown to mediate antibiotic tolerance12,13. Derivatives of GDP/GTP, guanosine tetra- and pentaphosphate (collectively referred to as (p)ppGpp or alarmones), are the effector molecules of the stringent response and are synthesized/hydrolyzed by the RSH superfamily (RelA/SpoT homologues) proteins. The most commonly distributed protein of this family is the bifunctional Rel protein, which has both (p)ppGpp synthetase and hydrolase activities.
In γ-proteobacteria such as in Escherichia coli, the rel gene has been duplicated to form relA and spoT14. The RelA protein has only (p)ppGpp synthetase activity but carries an inactive pseudo-hydrolase domain, whereas, SpoT is a weak (p)ppGpp synthetase and exhibits strong hydrolase activity11. Hence SpoT is essential for cell growth unless the RelA synthetase function is compromised, as SpoT is necessary for (p)ppGpp hydrolysis15. Weak SpoT-dependent (p)ppGpp synthesis has been reported under multiple starvation conditions; however, RelA exclusively synthesizes (p)ppGpp in response to amino acid starvation10,11. These metabolic cues are not mutually exclusive and accumulating evidence suggest that diverse starvation signals including glucose and fatty acid starvation can indirectly lead to conditions that trigger the RelA-dependent stringent response4,7,16,17.
RelA activation occurs when RelA binds with an uncharged tRNA at an empty A-site of a stalled ribosome, which leads to induction of (p)ppGpp synthesis18. Cryo-EM structures of RelA in complex with uncharged tRNA and the ribosome have revealed that the C-terminal Zinc-finger domain (ZFD) and RNA recognition motif (RRM) of RelA are responsible for ribosome binding at helix 38, the A-site finger of 23 S ribosomal RNA in the 50 S ribosomal subunit (Fig. 1a). The C-terminal TGS domain (ThrRS, GTPase, SpoT/RelA) is primarily involved in the recognition and binding to uncharged tRNA19–21. All three domains enclose the A-site tRNA, and expose the N-terminal synthetase (SYN) and inactive hydrolase (pseudo-HD) domains on the surface of the ribosome. Recently, it was demonstrated using an in vivo UV crosslinking and analysis of cDNAs (CRAC) approach that RelA interacts with the ribosome as a RelA•tRNA complex2. RelA is thought to bind with tRNA at ribosomal A-sites during amino acid starvation, when EF-Tu•GTP•tRNA ternary complexes are scarce18.
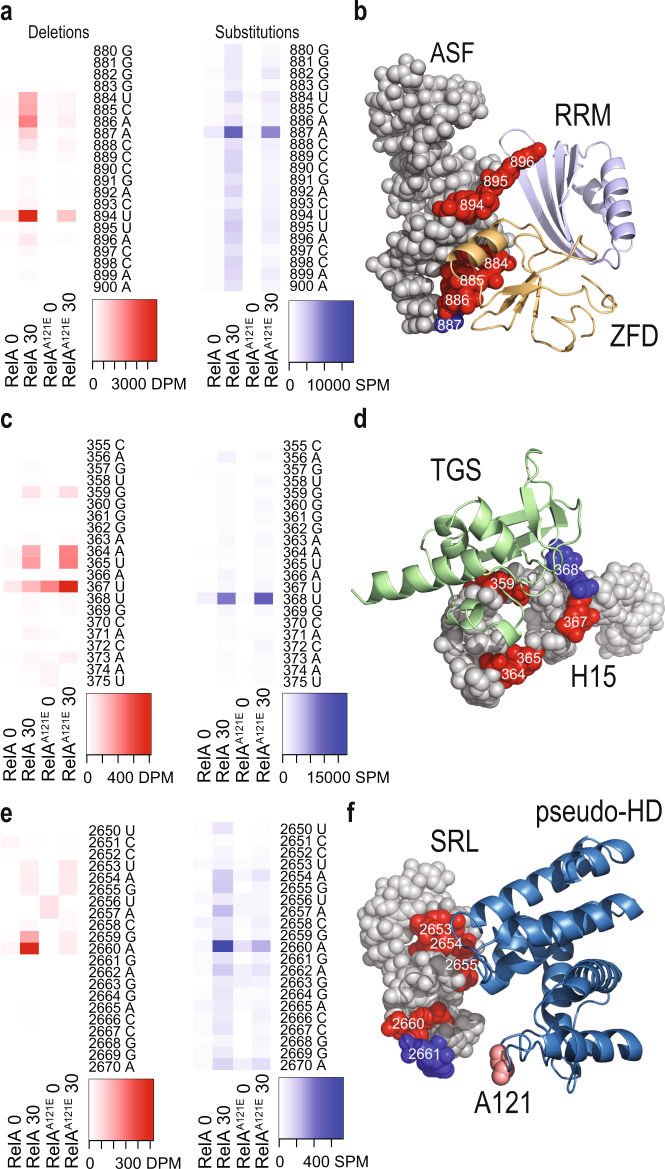
Fig. 1. Residues of 114–130 of the RelA pseudo-hydrolase domain form a loop that controls ppGpp synthesis.
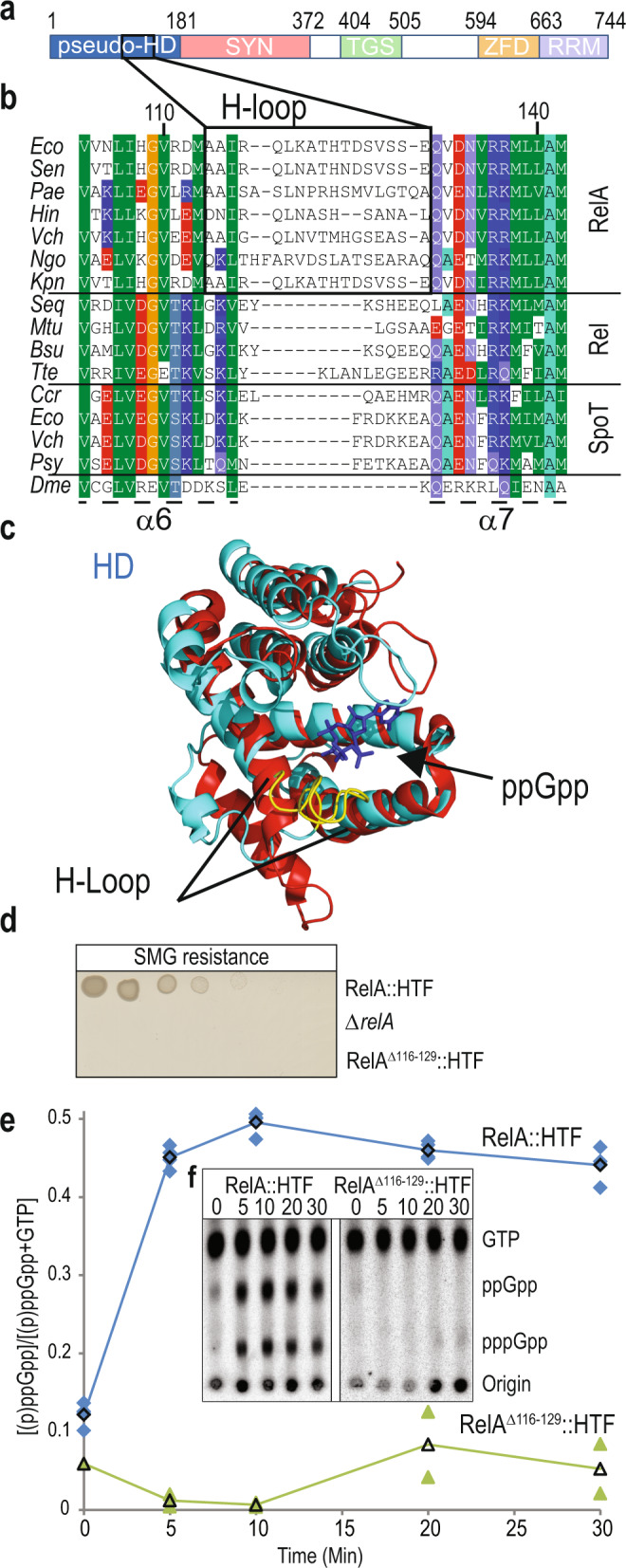
a Illustration of functional domains of RelA: HYD inactive hydrolase domain (residues 1–181), SYN synthetase domain (residues 182–372), TGS ThrRS, GTPase and SpoT-like domain (residues 404–505), ZFD Zinc-finger domain (residues 594–663) and RRM RNA recognition motif (residues 664–744). b Multiple sequence alignment of selected RelA, Rel and SpoT sequences. Eco Escherichia coli (RelA: NP_417264.1, SpoT: NP_418107.1), Sen Salmonella enterica (NP_461877.1), Pae Pseudomonas aeruginosa (NP_249625.1), Hin Hemophilus influenza (WP_011271992.1), Vch Vibrio cholera (RelA: WP_000226858.1, SpoT: WP_010895463.1), Ngo Neisseria gonorrhoeae (AP023075.1), Kpn Klebsiella pneumoniae (CP006918.1), Seq Streptococcus dysgalactiae subsp. equisimilis (Q54089), Mtu Mycobacterium tuberculosis (NP_217099), Bsu Bacillus subtilis (NP_390638),Tte Thermus thermophilus (WP_011173739.1), Ccr Caulobacter crescentus (WP_010919427.1), Psy Pseudomonas syringae (WP_003096603.1), Dme Drosophila melanogaster (NP_651682.1). Location of helices α6, α7 and the H-loop is indicated. c Structure of RelA pseudo-hydrolase domain (PDB: 5IQR, shown in cyan) superimposed onto RelTte hydrolase domain (HD, PDB: 6S2T, shown in red). Position of ppGpp in RelTte hydrolase active site is indicated in blue and the H-loop of RelA is shown in yellow. d Functional assay of stringent response in H-loop deletion mutant RelAΔ116–129. Escherichia coli K-12 MG1655 relA::HTF, MG1655 ΔrelA and MG1655 relAΔ116–129::HTF were grown overnight in LB medium at 37 °C. The cells were washed and serial diluted in phosphate buffered saline (PBS) and spotted onto MOPS MM SMG agar plates (SMG resistance) and plates were incubated at 30 °C (See Supplementary Fig. 1f for loading controls). e, f (p)ppGpp measurements in RelA H-loop mutant. Strains from d were grown exponentially in MOPS minimal medium at 30 °C containing [32P]-radiolabeled phosphate as described in methods. At time zero cells were starved for isoleucine by addition of 500 μg/mL L-Valine (final concentration). Samples were collected at time points indicated (min), precipitated in formic acid and spotted on a TLC plate. Nucleotides were separated using 1.5 M potassium phosphate pH 3.4 as solvent. e Quantification of (p)ppGpp for RelA::HTF (n = 4 biologically independent samples are shown with blue diamonds,) and RelAΔ116–129::HTF (n = 2 biologically independent samples are shown with Green triangles). The curves with black symbols indicate averaged values. f Representative TLC from e), positions of GTP, ppGpp and pppGpp are indicated. For TLCs of biological replicates see Supplementary Fig. 1i–n.
In bifunctional RSHs, including Rel, (p)ppGpp hydrolysis or synthesis is governed by conformational antagonism between the active sites of the hydrolase and synthase domains22–24. Specifically, switching between hydrolase domain ON or OFF determines if the synthetase domain will be OFF or ON. More recently, it has also been shown that the TGS domain of Bacillus subtilis Rel is directly involved in the repression of the synthetase domain, which keeps the enzyme in a hydrolase ON-state in the absence of deacylated tRNA and the vacant ribosomal A-site25.
The switch-ON signal for SpoT enzymes is still not clear, but for bifunctional Rel proteins, (p)ppGpp synthesis results from an accumulation of uncharged tRNA during amino acid starvation26–28. SpoT does not respond to amino acid starvation like Rel or RelA, its hydrolysis or synthesis activity is instead governed by the interaction with auxiliary protein regulators. These factors have been reported to either stimulate SpoT hydrolase or synthetase activity under diverse conditions either with high intracellular GTP levels or under fatty acid, carbon or phosphate starvation3,29–31. In all cases, direct interaction of regulators with specific domains of SpoT control switching of hydrolase/synthetase from ON/OFF to OFF/ON and vice versa. E. coli RelA contains a pseudo-HD, which is conserved but lacks the essential residues needed for (p)ppGpp hydrolysis14,32 (Supplementary Fig. 1a). Previous studies suggested that an interaction between the inactive pseudo-HD of RelA and the ribosome could alter the conformation of the SYN domain to regulate RelA synthetase activity2,20. Interestingly, mutations in the pseudo-HD of RelA that affects (p)ppGpp synthesis have previously been isolated, which indicates that the inactive hydrolase domain has a role in the regulation of RelA synthetase activity33. Moreover, degenerated inactive hydrolase domains are preserved in various RelA homologs suggesting its possible important regulatory role in RelA function14. However, mechanistic details of this regulation have remained elusive.
Here we identify a region in the inactive pseudo-HD (between α6 and α7), which regulates RelA synthetase activity. Interestingly, this region is extended in RelA homologs compared to Rel/SpoT and by mutagenesis of the loop we reveal several mutations that have deleterious or stimulatory effects on RelA (p)ppGpp synthetase activity. Importantly, while single point mutant (RelA121E) produced very little (p)ppGpp, ribosome interaction studies (CRAC) revealed that this mutant was still able to bind to tRNA and the ribosome, albeit less efficient to the latter. Based on these results, we propose here that the inactive hydrolase domain of RelA is important as a regulatory switch for RelA activation in vivo.
Results
The H-loop of RelA pseudo-hydrolase domain controls synthetase activity
To explore if the pseudo-HD is involved in RelA•Ribosome interaction and RelA activation, we selectively mutated residues in the hydrolase domain based on their vicinity to ribosomal RNA, when RelA is bound in the ribosomal A-site (Supplementary Fig. 1b–c). Alanine substitutions of these potential residues did not affect RelA activity, as revealed by growth on SMG plates (Supplementary Fig. 1d). RelA is essential for growth on SMG plates, which contain high concentrations of single carbon amino acids serine, methionine and glycine and leads to isoleucine starvation34. We used C-terminally HTF-tagged (six histidine, TEV protease cleavage site and three FLAG epitopes) functional RelA as described previously2. Mutations in the hydrolase domain have previously been reported to alter RelA (p)ppGpp synthetase activity and thereby permit deletion of the otherwise essential spoT locus33. Particularly, deletion of tryptophan 39 in α-Helix2 has previously been shown to decrease RelA activity (Supplementary Fig. 1d)33. This deletion is likely to result in larger structural changes in the hydrolase domain and was therefore not investigated further in our study, while point mutation (W39A) did not affect the RelA activity (Supplementary Fig. 1d).
To find conserved possible regulatory regions in RelA pseudo-HD domain, we aligned sequences of N-terminal domains of RelA and Rel/SpoT from different species. Interestingly, the alignment revealed a short region (Residues 114–130) in the hydrolase domain between α6 and α7 unique only to RelA homologs (Fig. 1a–b and Supplementary Fig. 1a). This region forms an extended loop in RelA compared to Rel/SpoT and is predicted to be in the vicinity of the (p)ppGpp binding site in the hydrolase domain of RelTte of Thermus thermophilus24 (Fig. 1c and Supplementary Fig. 1e). For simplicity, we refer to this loop as the H-loop (Hydrolase-loop). Considering the conformational antagonism observed between the HYD and SYN domain of Rel proteins, we hypothesized that in RelA, this conserved loop could possibly regulate synthetase activity by indirectly changing the conformation of the SYN domain22,24.
Indeed, deletion of a part of the H-loop (Δ116–129) abolished the ability of RelAΔ116–129 to support growth on SMG plates (Fig. 1d and Supplementary Fig. 1f for untagged controls). Similar effect was also observed when assaying AT (3-amino-1,2,4-triazole) resistance, which causes histidine starvation (Supplementary Fig. 1g)35. The HTF tag allows us to compare protein expression by western blot analysis in response to amino acid starvation (see Supplementary Methods). The lack of growth on SMG plates cannot be explained by altered protein expression or stability, as both RelA::HTF and RelAΔ116–129::HTF are produced at comparable levels (Supplementary Fig. 1h, compare lane 3–4 and lane 11–12). To directly analyse synthetase activity, we measured (p)ppGpp accumulation after amino acid starvation. Consistent with the complementation results, MG1655 relA::HTF accumulated (p)ppGpp (~5-fold) in response to isoleucine starvation whereas no increase in (p)ppGpp was observed in MG1655 relAΔ116–129::HTF (Fig. 1e–f). In conclusion, these results reveal a role of the H-loop in RelA activation.
Single substitution mutations in the H-loop inhibit RelA synthetase activity
Deletion of a part of the H-loop (Δ116–129) can affect overall protein structure/function therefore, to investigate further the importance of the H-loop in regulating the synthetase activity, we implemented a genetic screen to isolate H-loop substitution mutants having altered ppGpp synthetase activity (Fig. 2a). Using error-prone PCR, randomized mutations were generated and inserted into the H-loop by λ-red recombination36. Mutants were isolated and screened on SMG plates. H-loop mutants showing growth defects were subsequently isolated and sequenced (Fig. 2b). Interestingly, a majority of substitutions (21 different, independently isolated mutants) were present in the start of the loop between residues 110–123 and had either one or two amino acid changes. Remarkably, substitutions primarily generated proline or charged amino acids such as glutamate or lysine. Four different mutants were selected from the primary screen (L119M, A121E, M113K and Q118L) and re-introduced at the E. coli chromosomal relA locus (Fig. 2c). As a control I116L was used, which has previously been isolated in the study by Montero et al.33. We observed that all the tested mutations affected RelA complementation on SMG plates, however, A121E drastically hampered RelA activity (Fig. 2c). Similar results were obtained when these mutations were introduced into untagged RelA (Supplementary Fig. 2a) or when assaying AT resistance (Supplementary Fig. 1g). Furthermore, comparable protein levels of RelA::HTF, RelAA121E::HTF and RelAI116L::HTF in response to amino acid starvation were confirmed by western analysis (Supplementary Fig. 1h, compare lanes 3–4 with 5–6 and 9–10). Consistent to their growth phenotype on SMG plates, MG1655 relAI116L::HTF and relAA121E::HTF showed reduced (p)ppGpp synthesis in response to isoleucine starvation as measured by thin layer chromatography (Fig. 2d, for TLCs see Supplementary Fig. 1i–l). Particularly, MG1655 relAA121E::HTF showed a little (p)ppGpp accumulation after 30 min (30% of wild-type after 30 min). In conclusion, we have isolated a hydrolase domain H-loop substitution mutant, RelAA121E which affects (p)ppGpp synthesis.
Fig. 2. Isolation of H-loop mutants with altered (p)ppGpp synthesis.
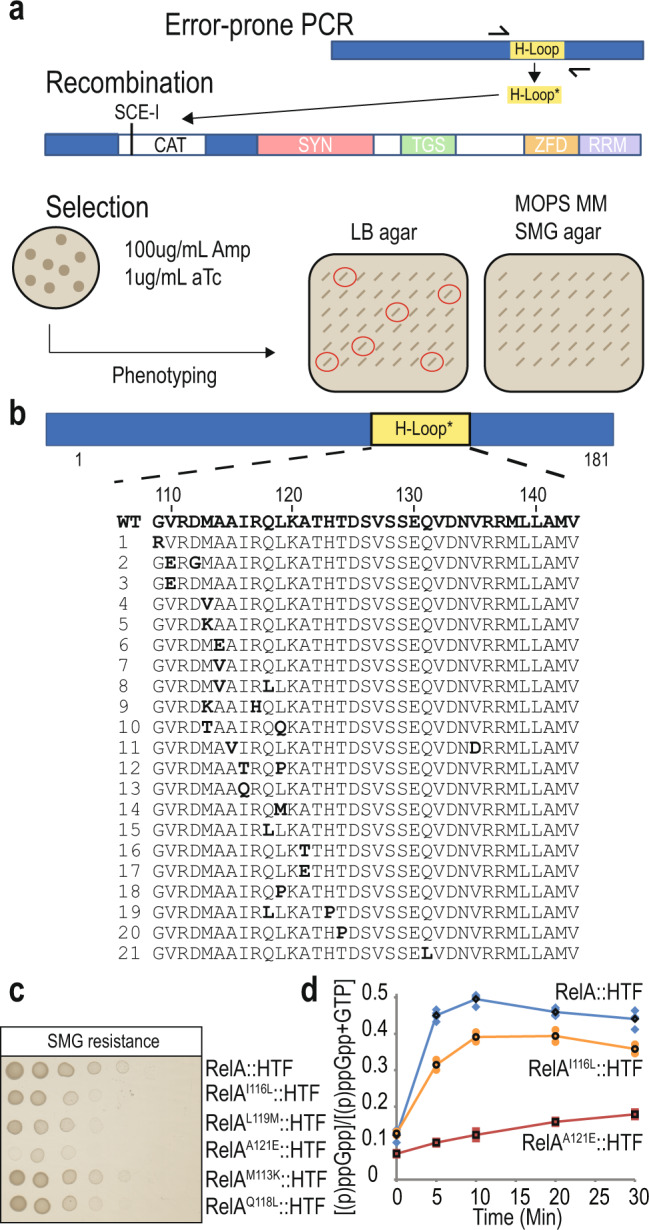
a Outline of the H-loop random mutagenesis screen. The H-loop was amplified using error-prone PCR as described in methods. The PCR product was then electroporated into MG1655 relAI116::cm::HTF containing plasmid pWRG99, which have previously been induced with 0.2% arabinose to express lambda recombinase36. After phenotypic expression at 37 °C, cells were plated on LB agar containing 100 μg/mL ampicillin (Amp) and 1 μg/mL anhydrotetracycline (aTc). Induction of the Sce-I restriction enzyme by aTc addition facilitated the site-directed replacement of the chloramphenicol resistance gene cassette (CAT) with the PCR product. Colonies were selected and re-streaked onto LB agar (loading control) and MOPS MM SMG plates to assay RelA functionality at 30 °C. Colonies that showed decreased growth on functional plates were sequenced (indicated with red circles). b Overview of the H-loop and substitution mutants isolated in a). Repeated mutations, nonsense and frame-shift mutations were excluded in this study. c Assaying the stringent response in selected substitution mutants. Substitution mutations I116L, L119M, A121E, M113K and Q118L were introduced by site-directed recombination in MG1655 relA::HTF as described in methods. The cells were grown in LB 37 °C, washed in PBS and spotted onto MOPS MM SMG plates (SMG resistance) followed by incubation at 30 °C (See Supplementary Fig. 2a for loading controls). d (p)ppGpp measurements in selected mutants in response to isoleucine starvation. MG1655 relA::HTF (blue diamonds), relAI116L::HTF (Orange circles) and relAA121E::HTF (red squares) were grown exponentially in MOPS minimal medium containing 32P-labeled phosphate. To induce isoleucine starvation, L-Valine was added, to a final concentration of 500 μg/mL. Samples were collected before (time zero) and after starvation, followed by precipitation and separation by thin layer chromatography. The mutant curves with black symbols are based on averaged quantifications from n = 2 biological independent samples see Supplementary Fig. 1i–l.
RelAA121E substitution mutant interacts with uncharged tRNA and the ribosome
We have implemented a crosslinking methodology, crosslinking and analysis of cDNA (CRAC) (Supplementary Fig. 3a), which allows mapping of protein-RNA interactions with single nucleotide resolution, in living cells2,37. We applied this approach to investigate the interaction of the H-loop RelA mutant with the tRNA and the ribosome. In response to isoleucine starvation, wild-type RelA crosslinking increases with uncharged isoleucine tRNA, tRNAileTUV, and the ribosome2. Using false discovery rate analysis (FDR) we previously identified three sites on the ribosome, which showed statistically increased crosslinking with RelA after isoleucine starvation. These sites are: the A-site finger (ASF) of 23 S rRNA, Helix 15 (H15) of 16 S rRNA and the Sarcin-Ricin Loop (SRL) of 23 S rRNA (Fig. 3a). Consistent with the cryo-EM structure of RelA bound to the ribosome along with the uncharged tRNA19–21, we observed crosslinking between the ZFD and RRM domains of RelA with the ASF of 23 S rRNA, and the TGS domain of RelA with H15 of 16 S rRNA2. These interactions are consistent with the RelA accommodation in the A-site during isoleucine starvation. Here we performed CRAC to compare interactions of the RelA::HTF and RelAA121E::HTF with the tRNA and the ribosome.
Fig. 3. RelAA121E displays decreased interaction with the ribosome in response to isoleucine starvation.
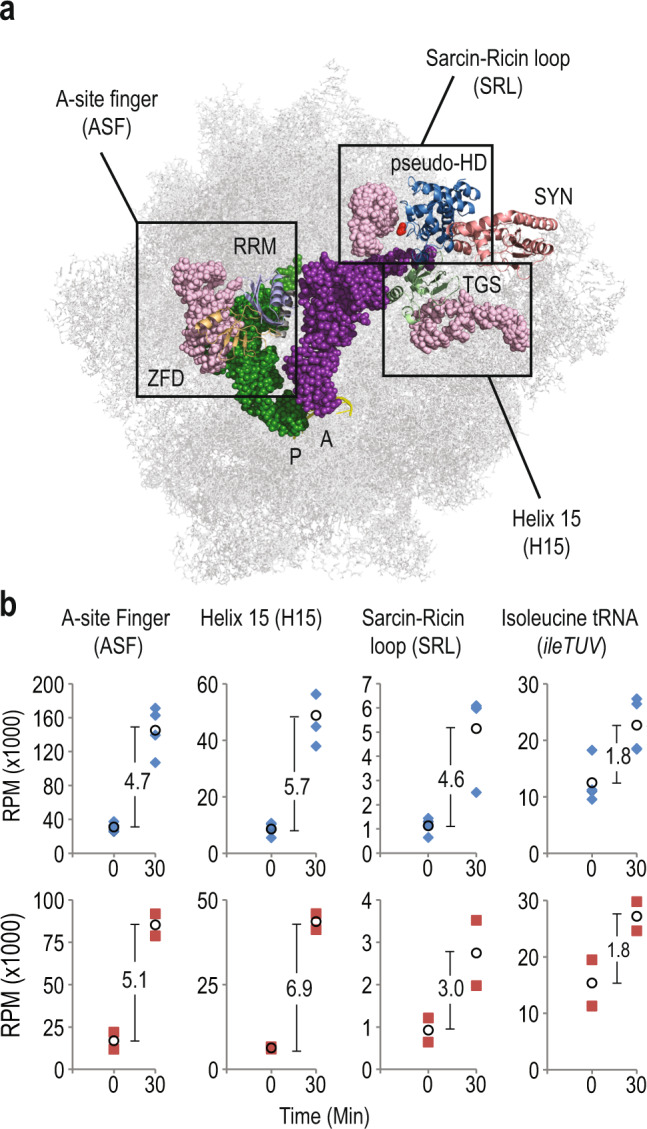
a Structure of RelA bound to tRNA and the ribosome (PDB: 5IQR). Functional domains have been coloured as in Fig. 1a and A- and P-site tRNAs are indicated in magenta and green respectively. Previously identified RelA ribosome interaction sites using false discovery rate (FDR) analysis2: A-site Finger (ASF, nt 834–927 in 23 S rRNA), Sarcin-Ricin Loop (SRL, nt 2652–2673 in 23 S rRNA) of 50 S ribosomal subunit and Helix 15 (H15, nt 328–407 in 16 S rRNA) of 30 S ribosomal subunit are indicated with boxes in light pink. Location of A121 in the hydrolase domain is indicated in red. b Plots of normalized cDNA coverage (Reads per Million, RPM) with ribosome and tRNAileTUV binding sites obtained from CRAC analysis. MG1655 relA::HTF (blue diamonds) and MG1655 relAA121E::HTF (red squares) were analysed before (0) and 30 min after isoleucine starvation (30). Average coverage between biological independent replicates (n = 4 for MG1655 relA::HTF and n = 2 for MG1655 relAA121E::HTF) are indicated with black circles and fold change between conditions are indicated between the data points (For replicate plots and data points see Supplementary Fig. 3b–d and h).
The main interaction site for RelA C-terminal ZFD and RRM domains is helix 38 of 23 S rRNA A-site finger (ASF), which bridges the A-site between the ribosomal subunits2,19,20,38 (Fig. 3a). RelA and RelAA121E showed similar fold increase in interaction with ASF after isoleucine starvation, 4.7- and 5.1-fold respectively (Fig. 3b and Supplementary Fig. 3b and 3e). However, the number of normalized cDNA reads in RelAA121E is only 59% of the wild-type, which indicates weaker binding to the ribosome. RelAA121E also showed weaker interaction with the Sarcin-Ricin Loop (SRL) of 23 S rRNA i.e. 53% of the wild-type and lower fold increase in response to starvation (3-fold compared to 4.6-fold) indicating decreased interaction at this site by the mutant RelA protein (Fig. 3b and Supplementary Fig. 3c). Interestingly, the interaction of RelAA121E TGS domain with Helix 15 (H15) of 16 S rRNA was less affected and only showed 10% lower number normalized cDNA reads after 30 min of amino acid starvation (Fig. 3b and Supplementary Fig. 3c and 3f). The TGS interaction occurs with the ribosome when RelA binds with the uncharged tRNA in the ribosomal A-site21. We did not observe any difference in the binding with uncharged isoleucine tRNA (tRNAIleTUV) between RelA::HTF and RelAA121E::HTF in response to isoleucine starvation (Fig. 3b and Supplementary Fig. 3d and 3g). This suggests that the mutant protein binds efficiently to tRNA, similar to wild-type protein. In RelA::HTF, (p)ppGpp accumulated within 5 min of isoleucine starvation and we therefore performed CRAC also at this time-point (Supplementary Fig. 3e–g). However, we did not observe any difference in the enrichment patterns of the ribosomal RNAs (23 S and 16 S) and isoleucine tRNA between 5 min and 30 min of starvation in both the strains. Based on these results, we conclude that RelAA121E::HTF is still able to bind to uncharged tRNA and the ribosome in response to isoleucine starvation. However, we did observe a lower number of cDNA reads from the ribosomal RNA suggesting a weaker binding to the ribosome and in particular a weaker interaction with the ASF and SRL in the mutant. The results indicate that the intact pseudo-HD domain of RelA is necessary for efficient ribosome binding and is important for (p)ppGpp synthesis.
RelAA121E crosslinks to the same RNA sites as RelA
After confirmation that both RelA::HTF and RelAA121E::HTF bind to similar sites on the ribosome, we analysed the crosslinking pattern at the specific residue level. In CRAC method, crosslinking pattern can easily be scored as reverse transcription mutations (RT-mutations) that occur at high frequency at the crosslinking sites. The most common mutations observed are deletions or substitutions in the cDNA at the crosslinking sites2,39. Crosslink mediated increase in both deletions and substitutions were clearly observed in the ASF region of 23 S rRNA in response to isoleucine starvation (Supplementary Fig. 4a–d for all RT-mutation heatmaps). We primarily observed substitutions at A887 and deletions at U884-C888 showing crosslinking pattern of the ZFD domain (Fig. 4a–b). These mutations were similar for both RelA and RelAA121E, suggesting similar binding of the ZFD domain to the ASF. The signature of RRM domain interactions with ASF is deletions at position U894-A896 (Fig. 4a–b)2. Though the number of cDNA reads from RelAA121E aligning to ASF crosslinking is lower than the RelA, the crosslinking patterns were the same. Furthermore, crosslinking of the TGS domain to Helix 15 of 16 S rRNA resulted in substitutions at U368 and deletions at position G359, A364, U365 and A367 (Fig. 4c–d and Supplementary Fig. 4e–h). At Helix 15, we observed slightly lower crosslinking with RelAA121E as compared to RelA, but with similar crosslinking pattern. Another crosslinking site in the ribosome is the SRL of 23 S rRNA, which showed increased amounts of substitutions at A2660 and deletions at U2653-G2655, G2659 and A2660 (Fig. 4e–f and Supplementary Fig. 4i–l). Again, the crosslinking pattern is the same between the two strains, but the number of reads was lower in RelAA121E. Additionally, as expected from the enrichment data, crosslinking to tRNAileTUV was similar for both RelA and RelAA121E (Supplementary Fig. 4m–n). Taken together, the crosslinking data indicate that RelA and RelAA121E bind to uncharged tRNA and to the ribosome in a similar manner but mutant protein interacts less efficiently with the ribosome compared to wild-type protein. Importantly, the interactions with ASF and SRL are affected indicating that these interactions might play an important role for SYN domain activation and (p)ppGpp synthesis.
Fig. 4. RelAA121E ribosome interaction is analogous to RelA.
RT-mutations (deletion and substitutions) in cDNA reads obtained with CRAC analysis of MG1655 encoding relA::HTF or relAA121E::HTF before and 30 min after isoleucine starvation (here named 0 or 30, respectively). Heatmaps show nucleobase positions with increased error-frequencies caused by RelA•RNA crosslinking. Deletions per million, DPM, or Substitutions per million, SPM, are indicated in red and blue respectively. a RT-mutations in the A-site finger (ASF) of 23 S rRNA (nt 880–900). b Close-up on the ASF (PDB: 5IQR) with positions with significant number of RT-mutations. Deletions or substitutions are shown in red and blue, respectively. ZFD and RRM domains of RelA are coloured in pale orange and pale blue. c RT-mutations in helix 15 (H15) of 16 S rRNA (nt 355–375). d Close-up on H15 (PDB: 5IQR) with crosslinking sites. TGS domain of RelA is displayed in pale green. e RT-mutations in the Sarcin-Ricin Loop (SRL) of 23 S rRNA (nt 2650–2670). f Close-up on SRL (PDB: 5IQR) with crosslinking sites. The pseudo-HD of RelA is shown in blue and the position of mutated alanine 121 is highlighted in salmon. RT-mutations from biological replicates see Supplementary Fig. 4a–n.
Alanine substitutions in the H-loop stimulate (p)ppGpp synthesis
The data presented above provided evidence that the H-loop of the pseudo-HD is important for the regulation of the SYN domain and (p)ppGpp synthesis. Moreover, single or double substitutions in the H-loop decreased ribosome binding and affected for (p)ppGpp synthesis. To investigate further the role of the H-loop, we made site-directed substitutions of four residues of the H-loop into alanine, which would likely affect the structure of the loop. The mutated residues were arginine 117, glutamine 118, lysine 120 and histidine 123 (Mutant is referred to as RelAQUAD::HTF, Fig. 5a).
Fig. 5. Alanine substitutions in the H-loop leads to increased (p)ppGpp synthesis.
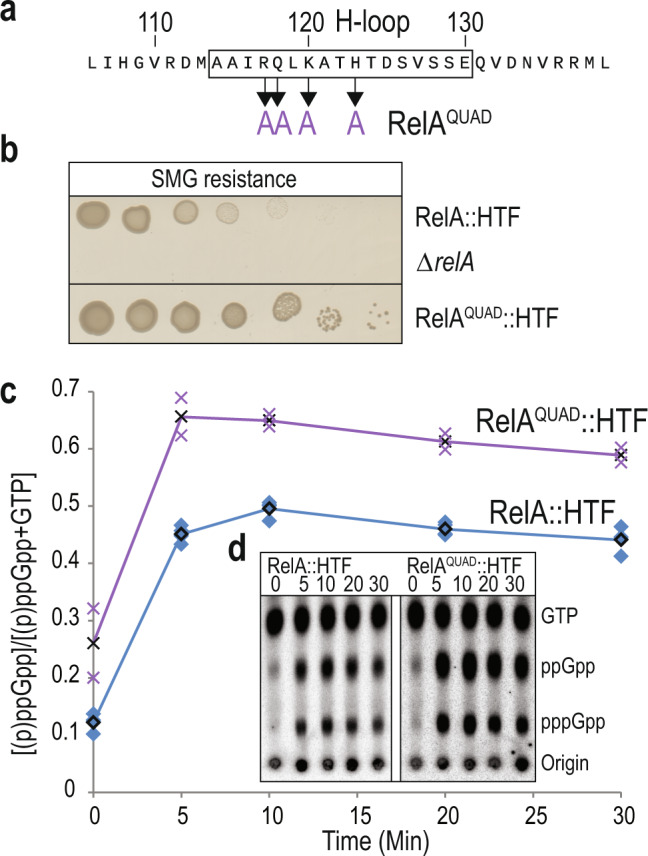
a Primary sequence of the H-loop region (indicated within a box) of RelA. Arrows indicate positions which have been substituted to alanine in RelAQUAD (R117A, Q118A, K120A and H123A). b Functional assay of RelAQUAD. Cell cultures of MG1655 relA::HTF, MG1655 ΔrelA and MG1655 relAQUAD::HTF, were washed in PBS, serial diluted and spotted onto MOPS MM SMG plates (SMG resistance). The plates were incubated ON at 30 °C (See Supplementary Fig. 1f for loading controls). c (p)ppGpp measurements of strains in b). Cells were grown exponentially in MOPS minimal medium containing 32P-labeled phosphate. Isoleucine starvation was induced by addition of L-valine, to a final concentration of 500 μg/mL. Samples were collected before (time zero) and after starvation, precipitated and separated by thin layer chromatography. The RelAQUAD::HTF curve with black crosses is based on the average of n = 2 biological independent samples see Supplementary Fig. 1m and 1n. d Representative TLC from a, positions of GTP, ppGpp and pppGpp are indicated.
Surprisingly, the mutant showed increased survival on SMG plates as compared to wild-type (Fig. 5b). The stimulatory effect of the RelAQUAD mutant was independent of the HTF tag, as a similar effect was observed with the untagged protein (Supplementary Fig. 1f). A similar effect was also observed when assaying AT resistance (Supplementary Fig. 1g). In an attempt to explain this surprising effect, (p)ppGpp synthesis was measured by TLC after isoleucine starvation (Fig. 5c–d). Interestingly, while RelA showed an approximately 5-fold increase in (p)ppGpp synthesis, RelAQUAD::HTF only showed about a 3-fold increase in response to isoleucine starvation. The strain expressing RelAQUAD::HTF on the other hand, already had an elevated basal level of (p)ppGpp prior to starvation (about 2-fold higher than the basal level of RelA). The RelAQUAD::HTF protein levels were comparable to wild-type RelA before and after starvation and hence the higher basal level of (p)ppGpp is due to the higher basal synthetase activity of the mutant protein rather than the amount of protein per se. (compare lanes 7–8, Supplementary Fig. 1h). This suggests that the RelAQUAD mutant intrinsically produces more (p)ppGpp than the wild-type RelA. This feature surprisingly did not affect growth on LB plates, but supported better survival and growth on SMG and AT plates. In conclusion, four alanine substitutions in the H-loop of RelA increased basal level of (p)ppGpp synthesis, but decreased the fold induction of (p)ppGpp synthesis in response to isoleucine starvation. The increased (p)ppGpp synthesis by the RelAQUAD mutant even in the absence of a starvation signal further demonstrates the importance of the H-loop in regulation of the SYN domain of RelA.
Discussion
Here, we have established a new fundamental function of the N-terminal inactive pseudo-hydrolase domain (pseudo-HD) of RelA for the activation of the synthetase domain (SYN). By genetic, biochemical and in vivo crosslinking analysis, we show that the pseudo-HD domain is important for regulating RelA synthetase activity. Further, we identify a loop between helix α6 and α7 (Residues 114–130), which is divergent and longer in hydrolase inactive RelA homologues as compared to hydrolase active Rel/SpoT (Fig. 1b). Random mutagenesis in this loop led to the identification of residues, which when mutated, affects RelA synthetase activity (Fig. 2b). Introduction of multiple alanine substitutions in the loop (RelAQUAD) has an stimulatory effect on (p)ppGpp synthesis and increases the basal level before isoleucine starvation (Fig. 5b–d). Surprisingly, a single point mutant RelAA121E in the pseudo-HD severely affects SYN domain function and (p)ppGpp synthesis (Fig. 2c–d).
Recently, it has been shown in the RelTte of Thermus thermophiles, that the loop between α6 and α7 changes conformation and moves away from hydrolase active site to make hydrolase active site free for ppGpp hydrolysis and goes towards it to block the hydrolase active site24. This conformational change is mediated by binding of ppGpp to the hydrolase active site of the protein. Additionally, binding of ppGpp to the hydrolase domain directly precludes the binding of GDP/ATP in the synthetase domain to prevent ppGpp hydrolysis and synthesis happening simultaneously24. Similar observations had also been reported for Relseq of Streptococcus dysgalactiae, here the binding of pGpp in the hydrolase domain does not prevent binding of GTP or GDP in the synthetase domain, but prevents binding of ATP and (p)ppGpp synthesis22,23. Recently it was shown that the TGS domain of RelBsu is involved in the regulation of the synthetase domain25. Upon binding of RelBsu to the ribosome along with uncharged tRNA, the TGS domain moves away from the synthetase domain with structural changes in the N-terminal domains including α7 of the hydrolase domain. Moreover, substitutions in α7 (R125E/M127E) were observed to decrease hydrolase activity and increase synthetase activity25. Thus, in all cases the hydrolase domain seems to control (p)ppGpp synthesis.
In E. coli RelA, the hydrolase domain is inactive and crucial residues for hydrolytic function including the conserved HDxxED motif is absent (Supplementary Fig. 1a). However, our results show that the hydrolase domain still plays a similar role of regulating SYN domain function and that might be the reason for it to be preserved in the RelA even with the extended H-loop between helix α6 and α7.
The single point mutant RelAA121E identified in our study is severely affected for (p)ppGpp synthesis (Fig. 2c–d). This mutant was still able to bind to the ribosome and uncharged tRNA in response to amino acid starvation, which is consistent with the fact that the substitution is present in the pseudo-HD, distant from ribosome and tRNA binding domains (Fig. 3a–b). The similar binding pattern with the ribosome and tRNA of this mutant confirms that the mutant protein is structurally similar to wild-type RelA protein and this single point mutation has not affected protein structure drastically. Interestingly, while crosslinking to Helix 15 of 16 S rRNA is similar between RelA and RelAA121E, crosslinking to the ASF and the SRL is slightly affected (59% and 53%, respectively, Fig. 3b). Thus far, we know that the ZFD and RRM domains are responsible for the binding to ASF; however, the mechanistic details of RelA crosslinking to SRL is still not clear2,19,20,38. Due to the location of the SRL in the vicinity of the pseudo-HD and the dynamic nature of the N-terminus, we earlier suggested that the pseudo-HD interacts with SRL and could promote RelA activation2. Although crosslinking with the SRL was found to be lower in the RelAA121E, the crosslinking pattern is similar to wild-type suggesting that the H-loop is perhaps not responsible for this interaction (Fig. 4e, f). Previously, it has been shown that cleavage between G2661 and A2662 of SRL by α-sarcin toxin decreases GTPase activity of EF-G when bound to the ribosome, but does not affect RelA (p)ppGpp synthesis in vitro and argued that SRL is not needed for RelA acitvity38. In addition interaction with the SRL was also not observed by RelBsu from Bacillus subtilis25. Nevertheless, it has previously been shown that ribosomal protein L11 and the SRL contacts with the RelA bound tRNA at the elbow and acceptor stem to stabilize the distorted configuration (Supplementary Fig. 1b)20. Therefore, the RelA-SRL interaction might be dynamic and occur during binding of RelA to the ribosome to help RelA to be stabilized on the ribosome.
We argue that the most probable explanation is that the RelAA121E mutant is locked in a conformation on the ribosome that does not allow the SYN domain to have sufficient flexibility to reach a fully active configuration. It should be noted that RelAA121E is not a null mutant and it can still synthesize ppGpp but with reduced efficiency (Fig. 2d). Another possibility is that the (p)ppGpp synthesis could stabilize the binding of RelA to the A-site and promote increased synthesis by a mechanism similar to positive allosteric feed-back38,40,41. In either case, RelA needs the fully functional H-loop to bind efficiently to the ribosome and to switch ON (p)ppGpp synthetase activity of the SYN domain.
In conclusion, we present robust evidence demonstrating that the inactive HYD domain of RelA plays a regulatory role in controlling (p)ppGpp synthetase activity of the SYN domain. Our data thus unravel a distinct layer of RelA synthetase activity regulation. We believe that the ON/OFF switch earlier proposed for Rel proteins might be a well-conserved regulatory mechanism for RSH proteins, which has evolved differently for monofunctional and bifunctional proteins.
Methods
Strains and plasmids
Strains constructed and plasmids used in this study are described in Supplementary Methods. Strains, plasmids and oligonucleotides are listed in Supplementary Table 1.
Media and growth conditions
Escherichia coli K-12 strains were routinely grown in liquid LB complex medium or on solid LB agar medium at 30 or 37 °C. For amino acid starvation experiments the bacterial cells were grown in MOPS (morpholinepropanesulfonic acid) minimal medium at 30 °C or 37 °C supplemented with 0.2% glucose, with all nucleobases (10 µg ml−1 of each) and 1.32 mM K2HPO442. In liquid medium, isoleucine starvation was induced by addition of L-Valine to a final concentration of 500 μg/mL43. For functional studies on solid medium, isoleucine starvation was induced by the addition of single carbon amino acids: Serine, Methionine and Glycine (SMG), to a final concentration of 100 μg/mL on MOPS minimal medium (MM) agar plates34. In addition, RelA functionality was also assayed by 3-amino-1,2,4-triazole (AT) resistance35. AT resistance was assayed on M9 minimal medium agar containing 0.2% glucose, all amino acids except histidine, 1 mM adenine, 1 mM thiamine with or without 15 mM 3-amino-1,2,4-triazole. To select for resistance cassette on chromosome and plasmid, liquid or solid media were supplemented with 10/25 μg/mL chloramphenicol or 100 μg/mL ampicillin. When stated, 0.2% arabinose or 1 μg/mL anhydrotetracycline was added to induce transcription from arabinose or tetracycline inducible promoters.
(p)ppGpp measurements
(p)ppGpp measurements during isoleucine starvation was performed as described previously by Michael Cashel42. Overnight cultures of relevant strains were diluted 100-fold in 5 ml of MOPS minimal medium supplemented with 0.2% glucose and all nucleobases (10 µg ml−1 of each), and incubated at 30 °C with shaking. At OD600 ~ 0.5, cells were diluted 10-fold to an OD600 of ~0.05 and were left to grow with shaking at 30 °C with H332PO4 (100 µCi/ml). After ~2 generations (OD600 of ~0.2), amino acid starvation was induced by the addition of valine (500 μg/ml). Fifty-microliter samples were withdrawn before and 5, 10, 20 and 30 min after addition of valine. The reactions were stopped by the addition of 10 μl of ice-cold 2 M formic acid and centrifuged at maximum speed for 1 h at 4 °C. 10 μl of each reaction mixture was loaded on polyethyleneimine (PEI) cellulose thin layer chromatography (TLC) plates (purchased from GE Healthcare) and separated by chromatography in 1.5 M potassium phosphate at pH 3.4. The TLC plates were revealed by phosphorimaging (GE Healthcare) and analysed using the ImageJ software44. The increase in the level of (p)ppGpp was quantified as the fraction of (p)ppGpp of (p)ppGpp+GTP.
Random mutagenesis screening using error-prone PCR
To screen for RelA hydrolase mutants with altered synthetase activity the loop region was amplified from relA using oligos loop-mut-f and loop-mut-rv using the DreamTaq polymerase (Thermo) According to Rasila et al.45. Hundred microlotre PCR was prepared containing 10 U DreamTaq polymerase, 10 μl 10X Dream tag buffer, 200 μM of each dNTP, 0.3 μM of each primer, colony DNA as template and 2–4 μl mutagenesis buffer (4 mM dTTP, 4 mM dCTP, 2.5 mM MnCl2, 27.5 mM MgCl2). The PCR product was purified and electroporated into recombination competent MG1655 relAI116::cm::HTF containing plasmid pWRG99. After 1 h of phenotypic expression cells were serially diluted and plated on LB plates containing 100 μg/mL ampicillin and 1 μg/mL anhydrotetracycline. Positive Sce-I resistant clones were re-streaked on LB plates and MOPS MM SMG plates at 30 °C.
RelA-RNA interactions by UV crosslinking and analysis of cDNAs
Crosslinking and analysis of cDNAs (CRAC) was performed essentially as previously described in Winther et al.2 (Supplementary Fig. 3a shows an overview). MG1655 relA::HTF and MG1655 relAA121E::HTF were grown overnight (ON) MOPS minimal medium supplemented with 0.2% glucose and all nucleobases (10 µg ml−1 of each) at 30 °C. The ON cultures where then diluted to OD600 = 0.005 into two flasks containing 2 L MOPS minimal medium and incubated with shaking at 30 °C. At OD600 = 0.2 one culture was UV crosslinked in a W5 crosslinking unit (Van Remmen UV techniek) by irradiation with 1800 mJ of UV-C for 100 s. The other culture was starved for Isoleucine by addition of 500 μg/mL L-Valine for 5 or 30 min before exposure to UV. After UV irradiation the cultures were separated into 1 L aliquots, harvested and the pellet washed in ice-cold 1XPBS (Phosphate Buffered Saline, Oxoid) before rapid freezing in liquid nitrogen. The pellets were stored at −80 °C before proceeding with purification. Pellets were dissolved 1 mL Lysis buffer (50 mM Tris-HCl pH 7.8, 150 mM NaCl, 0.1% NP-40, 5 mM β-Mercaptoethanol and Complete protease inhibitor) and lysed by vortexing 5 × 1 min with 3 mL 0.5 mm Zirconia beads (Thistle Scientific). Lysates were cleared by centrifugation and incubated with 200 μL anti-FLAG M2 affinity gel (Sigma–Aldrich) for 2 h at 4 °C. The resin was washed twice with Wash buffer (50 mM Tris-HCl pH 7.8, 0.1% NP-40, 5 mM β-Mercaptoethanol and 1 M (high salt) or 150 mM NaCl (low salt), respectively. The resin was resuspend in 600 μL low salt Wash buffer and RelA was cleaved from the resin by treatment with 5 μL HaloTEV protease (promega) for 2 h at 18 °C. Crosslinked RNA in the cleaved sample (500 μL) was trimmed using 1 μL (0.7U) RNaseIT (Agilent Technologies) for 5 min at 37 °C and stopped by addition of guanidine-HCl to a final concentration of 6 M. The trimmed sample was subsequently bound to 100 μL Ni-NTA superflow agarose (QIAGEN) overnight at 4 °C in Denaturing buffer (50 mM Tris-HCl pH 7.8, 300 mM NaCl, 6 M guanidine-HCl, 0.1% NP-40, 5 mM β-Mercaptoethanol) with 10 mM Imidazole. The resin was then washed twice in Denaturing buffer and three times in Reaction buffer (50 mM Tris-HCl pH 7.8, 10 mM MgCl2 and 5 mM β-Mercaptoethanol supplemented with 0.5% NP-40. First the crosslinked RNA was dephosphorylated using 0.1U/μL FastAP (Thermofischer) in Reaction buffer containing 1U/μL RNAsin (Promega) for 45 min at 37 °C and stopped by washing once with Denaturing buffer and three times with Reaction buffer supplemented with 0.5% NP-40. 1 mM of 3’-end mirCat-33 linker (see Supplementary Table 1) was ligated to the RNA using T4 RNA Ligase I (New England Biolabs) in Reaction buffer containing 1U/μL RNAsin for 6 h at 25 °C. The reaction was stopped by washing once with Denaturing buffer and three times with Reaction buffer supplemented with 0.5% NP-40. The RNA was then 5’-end phosphorylated using T4 polynucleotide kinase (Thermofischer) and 0.5 μCi/μL [γP32]-ATP for 40 min in Reaction buffer at 37 °C. ATP was added to a final concentration of 1.25 mM and incubation was continued for 20 min. The reaction was stopped by washing once in Denaturing buffer and three times with Reaction buffer supplemented with 0.5% NP-40. Barcoded 5’-linker (1.25 mM of L5Aa, L5Ab, L5Ad, L5Bb, L5Bc or L5Bd in Supplementary Table 1) was ligated to the sample RNA using T4 RNA ligase I in Reaction buffer containing 1U/μL RNAsin overnight at 16 °C. The resin washed three times in Wash buffer (50 mM Tris-HCl pH 7.8, 50 mM NaCl, 0.1% NP-40 and 5 mM β-Mercaptoethanol) and the RelA-RNA complex eluted twice with 200 μL Elution buffer (50 mM Tris-HCl pH 7.8, 50 mM NaCl, 150 mM Imidazole, 0.1% NP-40 and 5 mM β-Mercaptoethanol). The eluate was then precipitated using trichloroacetic acid and the precipitate dissolved in 1× LDS loading buffer (Life technologies) before separation on 4–12% NuPAGE gradient gel (Life technologies) in 1× MOPS running buffer (Life technologies). RelA-RNA complexes were transferred to a Hybond C+ extra membrane (Amersham) and extracted by incubation with 100 mg Proteinase K (Thermofischer) in 400 μL Wash buffer containing 1% SDS and 5 mM EDTA for 2 h at 55 °C. The RNA was isolated by phenol:chloroform:isoamylalcohol and chloroform extraction followed by ethanol precipitation at −80 °C for 30 min. The RNA was the converted to cDNA using Superscript III reverse transcriptase (Invitrogen) and 1 mM 33-rev oligo (see Supplementary Table 1) at 50 °C for 1 h followed by incubation with 0.5U/μL T4 RNase H at 37 °C. The libraries were generated by PCR using LA Takara taq polymerase (Clontech) and oligos P5 and PE (see Supplementary Table 1) size selected on a agarose gel and extracted using the MINelute extraction kit (QIAGEN).
The DNA libraries were sequenced on the Illumina MiSeq platform (50 bp single-end reads) and the sequencing output analysed using the pyCRAC software package46. We have previously adapted this approach for RelA2. FastQ files were demultiplexed using pyBacodeFilter.py and the reads 3’-end trimmed using the cutadapt tool. The reads were then collapsed based on the read sequence and the random triplet sequence in the 5’-end linker using pyFastDuplicateRemover.py. The cDNA reads were aligned to reference genome (MG1655 E. coli K-12 NC_000913.3) using Bowtie 2. To eliminate the possibility of alignment to identical or highly similar rRNA and tRNA we masked these in the reference genome (alaX, alaU, alaV, argY argZ, argQ, asnU, asnV, asnW, aspU, aspV, glnW, gltU, gltV, gltW, glyW, glyX, glyY, ileU, ileV, leuQ, leuV, leuP, lysQ, lysV, lysW, lysY, lysZ, metW, metZ, metY, pheV, serX, tyrU, tyrV, valW, valU, valX, valY, valZ, rrlA, rrlC-rrlH, rrsA, rrsC-rrsH, rrfA and rrfC-rrfH) using the Bedtools Maskfasta option. Previously we have identified regions of significant enrichment after isoleucine starvation by False Discovery Rate (FDR) analysis using pyCalculateFDR.py2. Selected regions including the A-site finger (ASF, nucleotide 834–927 in 23 S rRNA), Sarcin-Ricin Loop (SRL, nucleotide 2652–2673 in 23 S rRNA), Helix 15 (H15, nucleotide 328–407 in 16 S rRNA) and tRNAIleTUV (nucleotide 16–56 in ileT) were used to calculate the normalized cDNA coverage. Deletions and substitutions introduced in the cDNA reads were counted in selected regions showing significant enrichment after isoleucine starvation. cDNA reads and crosslinking sites were visualized by plots and heatmaps in R.
Statistics and reproducibility
All experimental results are based on two to four independent biological replicates and are defined in the figure legends.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Sine Lo Svenningsen and Kenn Gerdes for critical reading of the manuscript and in providing important suggestions. This work was supported by grants from the Novo Nordisk Foundation and the Danish National Research Foundation (DNRF120).
Author contributions
K.S.W. conceived and initiated the study. K.S.W. and A.K.S. performed the experiments and analysed the data. K.S.W. and A.K.S. wrote the paper.
Data availability
The datasets generated during and/or analysed in the current study are available GEO depository with accession number GSE150416, GSM29129892, GSM29129912, GSM29129902 and GSM29129922. All other data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-01963-z.
References
- 1.Cashel M, Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969;221:838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- 2.Winther KS, Roghanian M, Gerdes K. Activation of the stringent response by loading of RelA-tRNA complexes at the ribosomal A-site. Mol. Cell. 2018;70:95–105 e104. doi: 10.1016/j.molcel.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Battesti A, Bouveret E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 2006;62:1048–1063. doi: 10.1111/j.1365-2958.2006.05442.x. [DOI] [PubMed] [Google Scholar]
- 4.Sinha AK, Winther KS, Roghanian M, Gerdes K. Fatty acid starvation activates RelA by depleting lysine precursor pyruvate. Mol. Microbiol. 2019;112:1339–1349. doi: 10.1111/mmi.14366. [DOI] [PubMed] [Google Scholar]
- 5.Vinella D, Albrecht C, Cashel M, D’Ari R. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 2005;56:958–970. doi: 10.1111/j.1365-2958.2005.04601.x. [DOI] [PubMed] [Google Scholar]
- 6.Gallant J, Palmer L, Pao CC. Anomalous synthesis of ppGpp in growing cells. Cell. 1977;11:181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- 7.Hansen MT, Pato ML, Molin S, Fill NP, von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J. Bacteriol. 1975;122:585–591. doi: 10.1128/JB.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villadsen IS, Michelsen O. Regulation of PRPP and nucleoside tri and tetraphosphate pools in Escherichia coli under conditions of nitrogen starvation. J. Bacteriol. 1977;130:136–143. doi: 10.1128/JB.130.1.136-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bougdour A, Gottesman S. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc. Natl Acad. Sci. USA. 2007;104:12896–12901. doi: 10.1073/pnas.0705561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronneau S, Hallez R. Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol. Rev. 2019;43:389–400. doi: 10.1093/femsre/fuz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015;13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother. 2012;67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 13.Thompson A, et al. The bacterial signal molecule, ppGpp, mediates the environmental regulation of both the invasion and intracellular virulence gene programs of Salmonella. J. Biol. Chem. 2006;281:30112–30121. doi: 10.1074/jbc.M605616200. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE. 2011;6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao H, et al. Residual guanosine 3’,5’-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 1991;266:5980–5990. doi: 10.1016/S0021-9258(19)67694-5. [DOI] [PubMed] [Google Scholar]
- 16.Murray HD, Schneider DA, Gourse RL. Control of rRNA expression by small molecules is dynamic and nonredundant. Mol. Cell. 2003;12:125–134. doi: 10.1016/S1097-2765(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 17.Gentry DR, Cashel M. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol. Microbiol. 1996;19:1373–1384. doi: 10.1111/j.1365-2958.1996.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 18.Haseltine WA, Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl Acad. Sci. USA. 1973;70:1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown A, Fernandez IS, Gordiyenko Y, Ramakrishnan V. Ribosome-dependent activation of stringent control. Nature. 2016;534:277–280. doi: 10.1038/nature17675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loveland, A. B. et al. Ribosome*RelA structures reveal the mechanism of stringent response activation. elife10.7554/eLife.17029 (2016). [DOI] [PMC free article] [PubMed]
- 21.Arenz S, et al. The stringent factor RelA adopts an open conformation on the ribosome to stimulate ppGpp synthesis. Nucleic Acids Res. 2016;44:6471–6481. doi: 10.1093/nar/gkw470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogg T, Mechold U, Malke H, Cashel M, Hilgenfeld R. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response [corrected] Cell. 2004;117:57–68. doi: 10.1016/S0092-8674(04)00260-0. [DOI] [PubMed] [Google Scholar]
- 23.Mechold U, Murphy H, Brown L, Cashel M. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J. Bacteriol. 2002;184:2878–2888. doi: 10.1128/JB.184.11.2878-2888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamman H, et al. A nucleotide-switch mechanism mediates opposing catalytic activities of Rel enzymes. Nat. Chem. Biol. 2020 doi: 10.1038/s41589-020-0520-2. [DOI] [PubMed] [Google Scholar]
- 25.Pausch P, et al. Structural basis for regulation of the opposing (p)ppGpp synthetase and hydrolase within the stringent response Orchestrator Rel. Cell Rep. 2020;32:108157. doi: 10.1016/j.celrep.2020.108157. [DOI] [PubMed] [Google Scholar]
- 26.Krasny L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004;23:4473–4483. doi: 10.1038/sj.emboj.7600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avarbock D, Avarbock A, Rubin H. Differential regulation of opposing RelMtb activities by the aminoacylation state of a tRNA.ribosome.mRNA.RelMtb complex. Biochemistry. 2000;39:11640–11648. doi: 10.1021/bi001256k. [DOI] [PubMed] [Google Scholar]
- 28.Gratani FL, et al. Regulation of the opposing (p)ppGpp synthetase and hydrolase activities in a bifunctional RelA/SpoT homologue from Staphylococcus aureus. PLoS Genet. 2018;14:e1007514. doi: 10.1371/journal.pgen.1007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wout P, et al. The Escherichia coli GTPase CgtAE cofractionates with the 50S ribosomal subunit and interacts with SpoT, a ppGpp synthetase/hydrolase. J. Bacteriol. 2004;186:5249–5257. doi: 10.1128/JB.186.16.5249-5257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park YH, Lee CR, Choe M, Seok YJ. HPr antagonizes the anti-sigma70 activity of Rsd in Escherichia coli. Proc. Natl Acad. Sci. USA. 2013;110:21142–21147. doi: 10.1073/pnas.1316629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germain E, et al. YtfK activates the stringent response by triggering the alarmone synthetase SpoT in Escherichia coli. Nat. Commun. 2019;10:5763. doi: 10.1038/s41467-019-13764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemeyer EA, Richter D. Mechanism of the in vitro breakdown of guanosine 5’-diphosphate 3’-diphosphate in Escherichia coli. Proc. Natl Acad. Sci. USA. 1978;75:4180–4183. doi: 10.1073/pnas.75.9.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montero M, et al. Systematic production of inactivating and non-inactivating suppressor mutations at the relA locus that compensate the detrimental effects of complete spot loss and affect glycogen content in Escherichia coli. PLoS ONE. 2014;9:e106938. doi: 10.1371/journal.pone.0106938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uzan M, Danchin A. Correlation between the serine sensitivity and the derepressibility of the ilv genes in Escherichia coli relA- mutants. Mol. Gen. Genet. 1978;165:21–30. doi: 10.1007/BF00270372. [DOI] [PubMed] [Google Scholar]
- 35.Gropp M, Strausz Y, Gross M, Glaser G. Regulation of Escherichia coli RelA requires oligomerization of the C-terminal domain. J. Bacteriol. 2001;183:570–579. doi: 10.1128/JB.183.2.570-579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank K, Hensel M, Gerlach RG. Rapid and highly efficient method for scarless mutagenesis within the Salmonella enterica chromosome. PLoS ONE. 2011;6:e15763. doi: 10.1371/journal.pone.0015763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tree JJ, Granneman S, McAteer SP, Tollervey D, Gally DL. Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli. Mol. Cell. 2014;55:199–213. doi: 10.1016/j.molcel.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudrin P, et al. The ribosomal A-site finger is crucial for binding and activation of the stringent factor RelA. Nucleic Acids Res. 2018;46:1973–1983. doi: 10.1093/nar/gky023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmqvist E, et al. Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. EMBO J. 2016;35:991–1011. doi: 10.15252/embj.201593360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shyp V, et al. Positive allosteric feedback regulation of the stringent response enzyme RelA by its product. EMBO Rep. 2012;13:835–839. doi: 10.1038/embor.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takada H, et al. The C-terminal RRM/ACT domain is crucial for fine-tuning the activation of ‘Long’ RelA-SpoT homolog enzymes by ribosomal complexes. Front. Microbiol. 2020;11:277. doi: 10.3389/fmicb.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cashel M. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants. Methods Mol. Genet. 1994;3:341–356. [Google Scholar]
- 43.Leavitt RI, Umbarger HE. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J. Bacteriol. 1962;83:624–630. doi: 10.1128/JB.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasila TS, Pajunen MI, Savilahti H. Critical evaluation of random mutagenesis by error-prone polymerase chain reaction protocols, Escherichia coli mutator strain, and hydroxylamine treatment. Anal. Biochem. 2009;388:71–80. doi: 10.1016/j.ab.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Webb S, Hector RD, Kudla G, Granneman S. PAR-CLIP data indicate that Nrd1-Nab3-dependent transcription termination regulates expression of hundreds of protein coding genes in yeast. Genome Biol. 2014;15:R8. doi: 10.1186/gb-2014-15-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed in the current study are available GEO depository with accession number GSE150416, GSM29129892, GSM29129912, GSM29129902 and GSM29129922. All other data are available from the corresponding author on reasonable request.