Abstract
Prostate cancer (PCa) is the most commonly diagnosed solid tumor and the second leading cause of cancer-related deaths in U.S. men in 2020. Androgen-deprivation therapy (ADT) is the standard of care for metastatic PCa. Unfortunately, PCa relapse often occurs one to two years after initiation of ADT, resulting in the development of castration-resistant PCa (CRPCa), a lethal disease. While several anticancer agents such as docetaxel, abiraterone acetate, and enzalutamide are currently utilized to extend a patient’s life after development of CRPCa, patients will eventually succumb to the disease. Hence, while targeting androgen signaling and utilization of docetaxel remain the most crucial agents for many of these combinations, many studies are attempting to exploit other vulnerabilities of PCa cells, such as inhibition of key survival proteins, anti-angiogenesis agents, and immunotherapies. This review will focus on discussing recent advances on targeting therapy. Several novel small molecules will also be discussed.
Keywords: Prostate cancer, castration-resistant prostate cancer, androgen deprivation therapy, targeting therapy, combination treatment
Introduction
Prostate cancer (PCa) is the most commonly diagnosed solid tumor with 1 in 7 males developing the disease. It is also the second leading cause of cancer-related deaths in men in the United States in 2020 [1]. While nonmetastatic (M0) PCa has an excellent prognosis for patients [2], patients who develop metastatic (M1) PCa have a 5-year survival rate of only 31%. Due to the dependence of PCa cells on androgens for survival and growth [3], androgen-deprivation therapy (ADT), which blocks androgen receptor (AR) signaling, has been the standard of care for treating M1 PCa. Unfortunately, many patients will often remain on ADT for the rest of their lives [1,4].
PCa cells have many mechanisms by which they can overcome and become resistant to ADT or androgen biosynthesis inhibition, thus developing the castration-resistant (CR) phenotype. The CR phenotype results from the ability of the cells to bypass the need for normal physiological testosterone levels. For example, development of castration-resistance can arise from AR overexpression, mutation in the ligand-binding site of AR, constitutive AR activation through splice variants (e.g. AR-V7), or intra-tumoral androgen biosynthesis due to overexpression of CYP17A1. Some PCa tumors exhibit ligand-independent AR activation through activation of growth factor signaling pathways, such as ErbB-2, AKT, MAPK, etc. to promote progression and survival [5-17]. Moreover, neuroendocrine (NE)-like cells can support the CR phenotype of adenocarcinoma. While the authentic NE cell and its tumors may account for less than 5% of total PCa, NE-like PCa cells have been found in more than 40% of CRPCa tumors [18-21]. While, these NE-like PCa cells express NE biomarkers, they are derived from adenocarcinoma cells via trans-differentiation pathways during ADT. NE-Like cell are vital to prostate tumor survival by altering the microenvironment for promoting CRPCa cell growth [18-21].
ADT is a long-term treatment for metastatic PCa; unfortunately, PCa cells eventually develop resistance to this treatment. Currently, FDA-approved drugs such as docetaxel, abiraterone acetate, and enzalutamide that can treat CRPCa have a modest impact on extending survival in patients by only a few months (Figure 1). Finding alternative therapies or combinations of therapies that can directly target these mechanisms of resistance are necessary to improve patients’ clinical outcomes and potentially achieve longer term survival. Because targeting androgen signaling is the primary mechanism of CR PCa therapy, this review will briefly overview ADT and then focus on discussing recent advances on targeting other vulnerabilities of PCa cells, for example, inhibition of key survival proteins and anti-angiogenesis agents. We will also discuss several novel small molecules that are currently under investigation in preclinical models that could be utilized for the management of CRPCa.
Figure 1.
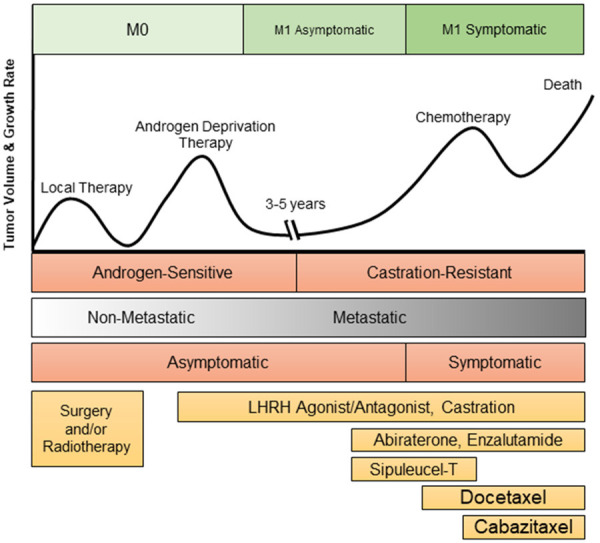
PCa Disease Progression and Current Treatment Options. PCa originates within the prostate and will often be detected before it invades into other areas of the body. Localized PCa is treated via surgery and/or radiotherapy. When the PCa is metastatic, the standard-of-care treatment is ADT. Three to five years after treatment with ADT, the PCa is likely to relapse, thus the development of CRPC. CRPC is initially treated with antiandrogens such as enzalutamide or abiraterone or immunotherapy Sipuleucel-T. Upon further progression of the disease, docetaxel and cabaitaxel may be utilized, in addition to abiraterone and enzalutamide if the patient has not previously been treatment with these agents. Unfortunately, these anti-cancer treatments utilized for CRPC will only prolong a patient’s life by several months before they succumb to the disease [120].
Single agent therapy with lifelong ADT
Due to the reliance of PCa cells on androgens for survival and proliferation, inhibition of the AR signaling pathway is the standard-of-care for the treatment of metastatic PCa. ADT can be carried out by one or more of the following methods: orchiectomy, chemical castration, and/or antiandrogen therapy. Chemical castration employs luteinizing hormone releasing hormone (LHRH) or gonadatropin releasing hormone (GnRH) agonists or antagonists to prevent androgen stimulation of PCa cells via the reduction of testosterone production in the testes [1,4].
Androgen targeted therapies (ATTs) can also be utilized in conjunction with chemical or surgical castration to further reduce the ability of cancer cells to produce or utilize androgen signaling. One class of ATT is the androgen biosynthesis inhibitor, such as abiraterone acetate, which is an effective treatment option for tumors that have obtained the ability for extragonadal and/or intratumoral androgen biosynthesis [23,24]. Another class of ATT is the AR antagonist that prevents AR nuclear translocation and DNA binding. First generation AR antagonists including bicalutamide, nilutamide, and flutamide are FDA-approved for the treatment of PCa. The second generation agent enzalutamide was FDA-approved for CRPCa treatments in 2012 [22,25-27]. Demonstrating the significantly superior activity of the second generation antiandrogens compared to first, the STRIVE trial (NCT01664923), in both M0 and M1 CRPCa patients, showed that enzalutamide had a significantly higher PFS at 19.4 months compared to 5.7 months with bicalutamide, and also increased time to PSA progression [28]. These results have led to the adoption of first-line enzalutamide (or abiraterone) over bicalutamide in treating PCa in recent years. More recently, nonsteroidal AR antagonist darolutamide has more recently become FDA-approved for the treatment of M0 CRPCa due to the ARAMIS trial (NCT02200614).
Although ADT is the gold standard treatment for metastatic PCa, hormone therapy usually fails as the cancers can overcome androgen signaling inhibition and continue to progress. The relapse can occur as early as <12 months after initiation of ADT [1,29]. Therefore, many completed and ongoing studies are looking to combination treatments with ATTs to reduce the risk of recurrence after ADT or to treat CRPCa. In the following sections, this review will focus on analyzing recent advances on targeting the vulnerabilities of PCa cells that could be the potential regiments for managing CRPCa.
Precision medicine combinations in prostate cancer
In the past fifteen years, precision medicine for treatment of multiple different types of cancers, including PCa, has begun to become a topic of interest for many clinicians and researchers. Some approaches of targeting therapies for CRPCa during ADT are discussed below.
Genetic polymorphisms in PCa
Genetic factors are shown to play a critical role in about 40% PCa risk. Many single nucleotide polymorphisms (SNPs) have been identified to be associated with increased disease risk in PCa, which have become strong predictors of PCa aggressiveness compared to PSA alone [30,31]. Importantly, the risk of aggressive PCa is predictably enhanced with compounding SNPs, particularly SNPs at 8q24 (MYC), 17q12, and 17q24.3. The potential utility of these specific SNPs in regards to targeted treatments for PCa are currently under investigation.
AR-V7 expression in PCa
As mentioned previously, AR amplification, mutation, and expression of alternative splice variants often occurs in PCa in attempt to evade ADT-mediated suppression of tumor growth. Although there are no current strategies that directly target AR amplification or mutation, AR-V7 has become an important biomarker for finding new treatments for CRPCa. Several treatment combinations have been proposed to tackle this common problem, including ATT, bromodomain extraterminal enhancer protein inhibitors (BETi), immune checkpoint inhibitors, and small molecule inhibitors.
AR-V7 expression in PCa confers resistance to various ATTs [32,33] as well as taxanes [34] and is strongly correlated with poor survival [35]. Many preclinical studies have found success in inhibiting PCa growth through the combination of ATT with small molecule inhibitors. For example, due to the ability of BETi to block AR binding to chromatin [36], the utilization of these molecules in combination with enzalutamide has shown to enhance anticancer effects [37]. As such, several clinical trials are underway to examine the combination of ATT with BETi in AR-V7 positive metastatic CRPCa patients (Table 1). Surprisingly, niclosamide, a treatment used for tapeworms, can effectively inhibit AR-V7 [38]. The combination of this drug with bicalutamide or abiraterone can re-sensitize PCa cells to cell death that were previously resistant to abiraterone, bicalutamide, or enzalutamide treatments [39,40]. Similarly, bardoxolone-methyl (CDDO-Me) also inhibits both full length AR and AR-V7 and had additive efficacy in CRPCa cells when combined with enzalutamide [41]. Targeting AR and AR-V7 cofactor phosphatidylinositol-4-phosphate 5-kinase type 1 alpha (PIP5K1a) via its inhibitor ISA-2011B can also sensitize PCa cells to enzalutamide in vitro and in vivo [42]. On the other hand, the unique combination of imipridone ONC201 and mammalian target of rapamycin (mTOR) inhibitor everolimus shows synergy in AS and AI preclinical PCa models due to the ability of ONC201 to inhibit AR and AR-V7 activity [43]. It has also been noted that AR-V7 positive PCa tumors have a higher mutational burden [44], of which has been suggested to be sensitive to immune checkpoint inhibition in various tumors [45]. As such, AR-V7 positive tumors have been treated with multiple immunotherapies, such as anti-PD-1 nivolumab and anti-CTLA-4 ipilimumab with quite some success [44].
Table 1.
Ongoing clinical trials for ATT combination therapies in PCa
| Clinical Trial | Primary Anticancer Agent | Secondary Anticancer Agent | Phase | Current Status | Estimated Completion Date |
|---|---|---|---|---|---|
| LACOG-0415 (NCT02867020) | Abiraterone | Apalutamide | II | Recruiting | December 2020 |
| NCT03419234 | Abiraterone | Cabazitaxel | II | Recruiting | April 1, 2025 |
| NCT02703623 | Abiraterone, Apalutamide | Ipilimumab, Cabazitaxel, Carboplatin | II | Active, not recruiting | May 1, 2020 |
| NCT03072238 | Abiraterone | Ipatasertib | III | Active, not recruiting | November 17, 2023 |
| NCT01485861 | Abiraterone | Ipatasertib or Apitolisib | I/II | Recruiting | April 30, 2021 |
| LATITUDE (NCT01715285) | Abiraterone | LHRH agonists or castration | III | Active, not recruiting | December 31, 2022 |
| NCT03732820 | Abiraterone | Olaparib | III | Recruiting | August 17, 2022 |
| NCT01576172 | Abiraterone | Veliparib | II | Active, not recruiting | March 30, 2020 |
| HERO (NCT03085095) | ADT | Relugolix (Reluminia) | III | Active | November 1, 2021 |
| NCT02913196 | Apalutamide | Abiraterone, Docetaxel | I | Recruiting | June 2022 |
| NCT02106507 | Apalutamide | Everolimus | I | Active, not recruiting | April 2021 |
| NCT01251861 | Bicalutamide | MK2206 (AKT Inhibitor) | II | Active, not recruiting | June 19, 2020 |
| ARNEO (NCT03080116) | Degarelix (GnRH antiagonist) | Apalutamide | II | Recruiting | December 30, 2021 |
| IMbassador250 (NCT03016312) | Enzalutamide | Atezolizumab (Anti-PD-L1) | III | Active, not recruiting | Sept 30, 2020 |
| NCT02833883 | Enzalutamide | CC-115 (Dual DNA-dependent protein kinase and mTOR Inhibitor) | I | Active, not recruiting | July 2021 |
| NCT02207504 | Enzalutamide | Crizotinib (TKI) | I | Active, not recruiting | March 2021 |
| NCT02125084 | Enzalutamide | Everolimus (mTOR Inhibitor) | I | Active, not recruiting | January 2021 |
| NCT03834493 | Enzalutamide | Pembrolizumab | III | Recruiting | April 30, 2024 |
| NCT01867333 | Enzalutamide | PROSTVAC | II | Active, not recruiting | January 1, 2021 |
| NCT00450463 | Flutamide | PROSTVAC | II | Completed (No compiled results) | June 8, 2017 |
| NCT03488810 | LHRH, Radiation | Apalutamide | III | Not yet recruiting | June 15, 2026 |
To date, there are many active clinical trials analyzing the efficacy of combination trials in advanced PCa and CRPCa, many based on the preclinical studies mentioned above. Here we have listed some studies of interest that focus on combinations with ATTs will have results shortly.
DNA damage repair deficient PCa
Mutations and deletions in DNA damage repair proteins are seen in up to 20% of primary PCa. Commonly lost DNA damage repair genes include BRCA1, BRCA2, CDK12, ATM, FANCD2, among many others. Tumors with any of these genetic abnormalities will often receive radiation, and occasionally radiation plus ADT for patients with local disease, which appears to be effective [46,47]. Poly (ADP-ribose) polymerase (PARP) inhibition in post-abiraterone metastatic CRPCa patients was found to have some success, mainly in patients with mutations in DNA repair genes who experiences an increased survival of 6.3 months [48]. Two PARP inhibitors, Rucaparib and Olaparib, have since received FDA approval for treating metastatic CRPCa. Two pre-clinical models showed synergistic anticancer effects of Olaparib and enzalutamide in AR-sensitive and AR-independent cell lines and in xenograft models [49,50]. The combination of abiraterone and Olaparib in a phase II trial (NCT01972217) initially found a 5.6 month increase in PFS in metastatic CRPCa patients compared to abiraterone alone [51] (Table 2). Another phase III trial (NCT03732820) for this combination is currently ongoing. Several studies are also ongoing to determine the anti-cancer efficacy of Olaparib and Veliparib in PCa in combination with abiraterone or enzalutamide (Table 1). However, initial results suggest that PCa tumors with homologous recombination deficiency (HRD) are already more susceptible to androgen deprivation than those tumors who have intact DNA damage repair response and do not require PARP inhibitors [52]. HRD was shown to give metastatic CRPCa patients increased sensitivity to radionucleotide Radium-223 [53], therefore the combination of Olaparib and Radium-223 will be studied in phase II trials in attempt to further increase anti-tumor effects of this molecule. Chemotherapeutics, such as combinations of docetaxel and carboplatin, have also been utilized for these particular tumors with some efficacy in metastatic CRPCa patients with HRD [54,55]. Preclinical studies have suggested that there is synergistic anti-tumor effects in combining DNA repair protein inhibitors, specifically Olaparib and ATR-inhibitor AZD6738 (Ceralasertib) [56], which have spurred clinical trials in metastatic CRPCa, among several other cancers (Table 3).
Table 2.
Completed clinical trials of abiraterone combination treatments for CRPCa
| Clinical Trial | Secondary Anticancer Agent | Phase | N | Primary Outcome | Result | Reference |
|---|---|---|---|---|---|---|
| NCT01685125 | Dastinib (Src Inhibitor) | II | 26 | PFS | No Benefit | Dorff et al. 2019 [100] |
| NCT01485861 | Ipatasertib (AKT inhibitor) | II | 253 | Radiographic PFS | Increased PFS, time to PSA progression, and survival | De Bono et al. 2019 [77] |
| NCT01972217 | Olaparib | II | 142 | Radiographic PFS | Increased PFS by 5.6 months, more dramatic response in HHR mutant tumors | Clarke et al. 2018 [51] |
Many clinical trials have analyzed the toxicity and effectiveness of combination treatments with abiraterone for the potential of PCa and CRPCa therapy.
Table 3.
Current clinical trials analyzing the safety and efficacy of combinations treatments with small molecule inhibitors or radium 223
| Clinical Trial | Primary Anticancer Agent | Secondary Anticancer Agent | Phase | Current Status | Estimated Completion Date |
|---|---|---|---|---|---|
| NCT03840200 | Ipatasertib (AKT inhibitor) | Rucaparib (PARP inhibitor) | I/II | Recruiting | November 5, 2021 |
| NCT03874884 | Olaparib | 177Lu-PSMA-617 | II | Recruiting | October 2022 |
| TRAP (NCT03787680) | Olaparib | AZD6738 (ATM Inhibitor) | II | Recruiting | November 2025 |
| NCT02893917 | Olaparib | Cediranib (Anti-angiogenesis) | II | Active, not recruiting | December 2020 |
| NCT03810105 | Olaparib | Durvalumab | II | Recruiting | February 2021 |
| NCT03574571 | Radium 223 | Docetaxel | III | Recruiting | June 2023 |
| NCT03737370 | Radium 223 | Docetaxel | II | Recruiting | October 31, 2021 |
| PEACE III (NCT02194842) | Radium 223 | Enzalutamide | III | Recruiting | December 2025 |
| NCT02225704 | Radium 223 | Enzalutamide | II | Active, not recruiting | December 2022 |
| NCT04019327 | Talazoparib (PARP Inhibitor) | Temozolomide | I/II | Recruiting | July 2022 |
| NCT02711956 | ZEN003696 (BETi) | Enzalutamide | I/II | Active, not recruiting | October 2019 |
| NCT04471974 | ZEN003696 | Enzalutamide, Pembrolizuman | II | Not yet recruiting | August 31, 2025 |
Other various small molecule or Radium 223 combinations have emerged due to the fact that not many anti-cancer agents can effectively suppress CRPC for less than a year. PARP inhibitors have becoming an area of major interest as they have been shown to be quite effective in patients with mutations in DNA repair pathways.
ERG-positive PCa
Gene fusion events are also common in PCa and often include ETS transcription factors ERG or ETV. The fusion of TMPRSS2 and ERG oncogenes occurs in up to 50% of PCa [57,58]. Although a frequent mutation, several studies have shown mixed associations between TMPRSS2-ERG, Gleason grade, and patient outcome [59-61]. While other ETS fusion proteins can occur in PCa, most have a frequency of less than 1%, thus they are rarely studied [62]. One method to treat PCa with TMPRSS2-ERG fusions is through inhibition of ETS co-factors, such as PARP or histone deacetylase 1 (HDAC1), often in combination with ADT. Unfortunately, these phase I and II trials for patients with metastatic CRPCa have not achieved much success compared to ADT alone [52,63,64]. As such, novel inhibitors of ERG are under development and show some promise. For example, selective ERG inhibitor ERGi-USU effectively inhibited ERG-positive VCaP xenograft tumor growth alone, but had additive effects when cells were treated in combination with enzalutamide [65]. This same group found that dual inhibition of NOTCH (GSI-1) and AR (bicalutamide, enzalutamide, or abiraterone) signaling also synergistically suppressed ERG-positive PCa cells [66]. Additionally, silencing TMPRSS2-ERG mRNA through Arg-Gly-Asp (RGD)-peptide-coated liposomal siRNA nano-vectors has shown to enhance PCa sensitivity to docetaxel in vivo [67].
PTEN-Null PCa
Phosphatase and tensin homolog (PTEN) deletion is another common genetic alteration in PCa that positively correlates with TMPRSS2-ERG fusions. The loss of PTEN has a strong relationship with aggressive PCa and increased mortality rates, especially when the tumor exhibits no ERG overexpression [68,69]. Because the loss of PTEN leads to increased activation of the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway, this pathway is the most common target for PTEN-null PCa [70], however there has been little success in these monotherapies due to crosstalk activation of AR [71]. Preclinical models demonstrated that inhibition of PI3K or AKT with small molecules AZD8186 or AZD5363, respectively, in combination with ADT resulted in enhanced growth suppression of cells and xenograft PCa tumors [72-74]. While the combinations of dual PI3K/mTOR inhibitor BEZ235 and abiraterone or pan-class I PI3K inhibitor buparlisib and enzalutamide caused many adverse side effects in phase I and II trials [75,76], other clinical trials were more successful. For example, phase II clinical studies of abiraterone acetate in combination with AKT inhibitor ipatasertib have shown efficacy against metastatic CRPC, particularly in patients with PTEN loss [77] (Table 2). Similarly, inhibition of mTOR can sensitize PTEN-null PCa cells to radiation in vitro [78]. The combination of olaparib and PI3K inhibitor BKM120 [79] or radiation [80] can also be utilized to effectively suppress the growth of PTEN/TP53-deficient PCa cells in vivo. Further studies on the combination of PI3K pathway inhibitors are warranted. Nevertheless, while AKT inhibitors may inhibit PCa tumorigenicity, treatments of those compounds can cause an adverse elevation of PSA levels [81]. Therefore, alternative biomarkers to determine the success of this treatment should be developed.
MYC amplified or dysregulated PCa
MYC encodes a transcription factor oncogene that drives many cancers by increasing expression of pro-proliferative genes. MYC is amplified in about 8% of primary PCa tumors and can predict biochemical recurrence [82,83]. MYC dysregulation can also occur upon loss of prostate-specific transcription factor NKX.3.1, which competes for MYC binding spots [84]. Importantly, amplification or dysregulation of MYC results in the CR phenotype [85,86]. Targeting MYC transcription through BETi has shown some efficacy in preclinical models through interrupting AR-mediated transcription of MYC [87,88]. Importantly, combination treatment of PI3K and BET inhibitors showed strong anti-cancer effects against a murine model of PCa with PTEN deletion and forced MYC expression [89]. New evidence has also demonstrated that SPOP mutants, which are commonly seen in PCa, are resistant to BETi [90]. This has been attributed to increased AR and PI3K signaling [91], therefore this type of therapy would be beneficial for a limited patient population.
Targeting MYC interacting proteins PIM or Pol I has become an interesting strategy to prevent MYC function in PCa. Inhibition of PIM (AZD1208) or Pol I (CX-5461) alone have shown to effectively suppress MYC-driven cancers in preclinical studies [92,93], therefore the combination of these two inhibitors was attempted in PCa. In vivo studies from a MYC overexpression and PTEN-deficient mouse model and patient derived xenografts (PDX) showed effective suppression of tumor growth as well as induced cell death with their combination [94]. Additionally, attempts have been made to target MYC mRNA and expression. In a study by Leonetti et al., it was demonstrated that utilization of of bcl-2 (G3139) and c-myc (INX-6295) antisense olidodeoxynucleotides in combination with docetaxel effectively promoted tumor regression PC3 PCa xenograft growth, in addition to increasing survival [95]. Another preclinical study by Ciccarelli et al. showed that in vitro and in vivo PCa cells with overexpression of MYC could be sensitized to radiation by MEK/ERK inhibitors, which down regulated MYC protein levels [96].
Combinations of standard treatments with novel small molecule inhibitors
Expanding the toolbox of anticancer agents for CRPCa is currently ongoing with some novel small molecules showing to be quite effective against this disease. While most of these molecules can be effective alone, they are often analyzed for their anti-tumor effects in combination with other standard-of-care therapies for PCa.
Although many small molecule inhibitors have been combined with docetaxel, mixed results have been obtained. Due to the frequent alterations in kinase signaling pathways upon progression to the CR phenotype [7,8,97], inhibition of tyrosine kinases and their corresponding downstream molecules was attempted for a treatment for CRPCa. Phase I/II trials (NCT00439270) showed that dasatinib (Sprycel), a Src and BCR-ABL tyrosine kinase inhibitor (TKI), in combination with docetaxel was well tolerated by patients [98]; however the phase III READY trial (NCT00744497) demonstrated that there was no improvement in patient survival [99] (Table 4). Similarly, the combination of dasatinib and abiraterone also did not show any benefit to patients [100] (Table 4). Phase III TRAPEZE trial (NCT00554918) combining docetaxel with strontium-89, zoledronic acid, or both showed significantly reduced bone metastasis, while no effect on overall patient survival upon treatment with zoledronic acid and docetaxel [101]. The SYNERGY trial (NCT01188187) demonstrated that the combination of docetaxel and custirsen, an antisense oligonucleotide that inhibits production of resistance-associated chaperone protein Clusterin, also does not improve overall patient survival [102] (Table 4).
Table 4.
Clinical trials of combination therapies for CRPCa with taxanes
| Clinical Trial | Primary Anti-Cancer Agent | Secondary Anticancer Agent | Phase | N | Primary Outcome | Result | Reference |
|---|---|---|---|---|---|---|---|
| SYNERGY (NCT01188187) | Docetaxel | Custirsen | III | 1022 | Overall Survival | No Survival Benefit | Chi et al. 2017 [102] |
| READY (NCT00744497) | Docetaxel | Dastinib (Src Inhibitor) | III | 1522 | Overall Survival | No Survival Benefit | Araujo et al. 2013 [99] |
| TRAPEZE (NCT00554918) | Docetaxel | Strontium-89 or Zolendronic Acid (Osteoclast Inhibitor) | II | 757 | PFS and Cost-Efficacy | St-89 improved PFS, ZA Reduced Bone Metastasis, No Survival Benefit | James et al. 2016 [101] |
Several other combinations with docetaxel have been analyzed for their efficacy against CRPC. However, docetaxel continues to have associated toxicities that result in reduced survival or lower the quality of life.
As for pre-clinical models, one promising small molecule is Aneustat (OMN54), a multivalent botanical drug currently undergoing a phase I clinical trial (NCT01555242) for advanced cancers, primarily lymphomas. Interestingly, preclinical studies revealed that treatment of PCa mouse xenografts with docetaxel and Aneustat dramatically reduced PCa tumor growth with potential synergistic effects [103]. Additionally, fatty acid binding protein 5 (FABP5) inhibitors with docetaxel or cabazitaxel show synergistic cytotoxic effects in vitro and in vivo [104]. Inclusion of ERK inhibitors can be an alternate approach to reduce taxane toxicity. Because ERK inhibitors are shown to increase the potency of docetaxel inhibition of CRPCa cells [97], ERK inhibitors may be included with docetaxel under ADT, which will allow to reduce the docetaxel dosage, as well as its cytotoxicity, while accomplish a similar therapeutic index [97]. Another study aimed at targeting the PI3K/mTOR survival pathway in combination with docetaxel through N-(2-hydroxypropyl)methacrylamide (HPMA) drug conjugates. They found increased solubility and anti-tumor effects with the HPMA conjugate combinations against PC-3 xenograft tumors, in addition to a reduction in the cancer stem cell population [105]. Because focal adhesion kinase (FAK) expression positively correlates with advanced disease, inhibition of this protein via defactinib in combination with docetaxel enhanced cancer cell death in CRPCa and docetaxel-resistant CRPCa preclinical models [106]. Another combination of docetaxel with additional anti-microtubule agent mebendazole was found to be effective against PCa through a drug screen. Interestingly, further analysis showed enhanced anti-tumor activity; unfortunately, potential toxicities due to excessive disruption of the microtubule network were not reported in this study [107].
Other successes with docetaxel have been found through inhibition of specific receptors. Docetaxel nanoparticles in combination with a receptor activator of nuclear factor κB ligand (RANKL) monoclonal antibody, denosumab, led to an increase in survival and reduction in tumor burden and bone metastasis in PCa xenograft animal models [108]. Other preclinical studies have found success by inhibition of endothelin-1 (ET-1) binding to its receptor Endothelin A (ETA) through ETA antagonist ABT-627 to reduce in vitro and in vivo LNCaP and C4-2b PCa cell growth [109]. Meanwhile, early ex vivo studies showed that the combination of docetaxel and dopamine D2 receptor agonist bromocriptine did effectively reduce tumor growth and bone metastasis in PCa xenograft models [110], suggesting another novel treatment combination for utilization in PCa. Future clinical trials of these new combinations may provide an answer. As of late, many clinical trials have aimed to tackle late stage disease through combination therapies with docetaxel. Examples include docetaxel plus radioligand therapy, immunotherapies, or small molecule inhibitors (Table 5).
Table 5.
Current clinical trials for CRPCa of combination therapies with taxanes
| Clinical Trial | Primary Anticancer Agent | Secondary Anticancer Agent | Phase | Current Status | Estimated Completion Date |
|---|---|---|---|---|---|
| upFrontPSMA (NCT04343885) | Docetaxel | 177Lu-PSMA-617 | II | Recruiting | April 2024 |
| TheraP (NCT03392428) | Post-Docetaxel | 177Lu-PSMA-617 | II | Active, not recruiting | January 2021 |
| NCT02218606 | Cabazitaxel | Abiraterone | II | Active, not recruiting | August 2020 |
| NCT03110588 | Cabazitaxel | Abiraterone and Enzalutamide | I | Recruiting | August 1, 2023 |
| NCT01555242 | Docetaxel | Aneustat | I | Completed (No compiled results) | January 2014 |
| ProCAID (NCT02121639) | Docetaxel | AZD5363 (AKT Inhibitor) | I/II | Active, not recruiting | February 2020 |
| NCT03218826 | Docetaxel | AZD8186 (PI3K Inhibitor) | I | Recruiting | April 1, 2021 |
| NCT01505868 | Cabazitaxel | Carboplatin | I/II | Active, not recruiting | July11, 2030 |
| NCT02522715 | Cabazitaxel | Enzalutamide | I/II | Active, not recruiting | August 31, 2025 |
| PROSTRATEGY (NCT03879122) | Docetaxel/ADT | Nivolummab, Ipilimumab | II/III | Active, not recruiting | December 31, 2023 |
| NCT03834506 | Docetaxel | Pembrolizumab | III | Recruiting | February 28, 2023 |
| NCT02649855 | Docetaxel | PROSTVAC | II | Active, not recruiting | January 1, 2021 |
| NCT01420250 | Cabazitaxel | Radiation (IMRT), Bicalutamide | I | Active, not recruiting | January 1, 2021 |
| NCT02494921 | Docetaxel | Ribociclib (CDK4/6 Inhibitor) | I/II | Active, not recruiting | June 30, 2021 |
Similar to combination therapies with ATTs, many trials are looking to combinations with docetaxel to effectively suppress CRPCa. The intent is to find other therapies that can reduce the amount of taxane necessary for cancer treatment to prevent the toxicities commonly associated with these types of agents.
Other combinations with two non-standard PCa treatments have also been analyzed in preclinical and clinical studies. While in vitro results of DNA damaging agent temozolomide in combination with olaparib have shown to have enhanced anti-tumor efficacy [111], a phase 1 trial (NCT01085422) of veliparib and temozolomide showed little synergy in M1 CRPCa patients [112]. It is important to note that although these particular results showed no survival benefit, clinical studies continue to analyze the efficacy of PARP inhibitors with temozolomide combinations in CRPCa (Table 3). Similarly, anti-angiogenesis agent cediranib showed modest activity alone in M1 CRPC patients [113], therefore, this drug is now being explored in combination therapy with olaparib in PCa (Table 3), as ovarian cancer patients have benefited from this combination [114].
It is noteworthy that statin derivative SVA is of particular interest. In addition to functioning as a single agent with minimal toxicity, SVA exhibits an added effect when combined with docetaxel (Figure 2A and 2B) and novel anti-microtubule CIL-102 derivatives (Figure 2C and 2D). Those novel CIL-102 derivatives are shown to have increased selectivity over CRPC cells with low toxicity toward non-cancerous cells, and also effectively inhibit the tumorigenicity of both CRPCa and docetaxel-resistant CRPC [115]. Thus, these compounds have great potential for utilization in treating docetaxel-resistant CRPCa as a single agent as well as in combination with other types of therapies.
Figure 2.
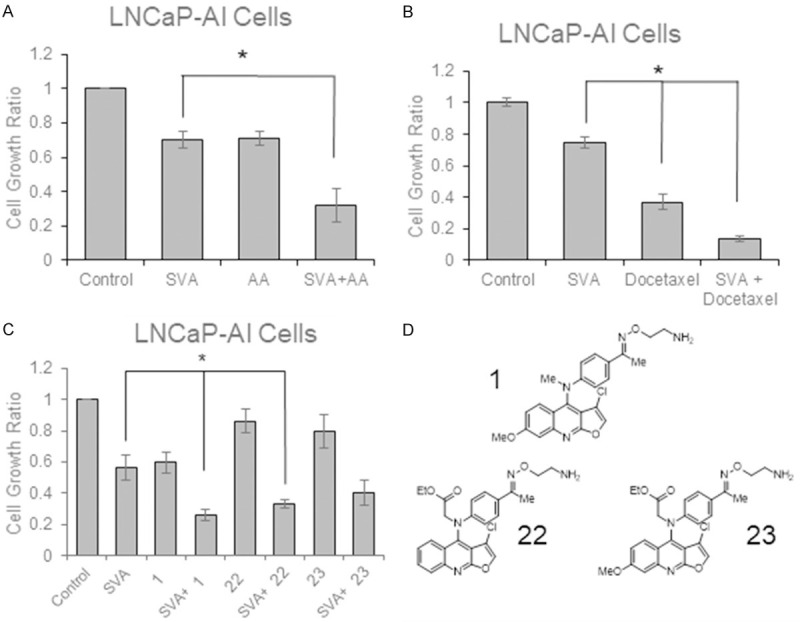
Combination treatments with Simvastatin Derivative SVA. A. LNCaP-AI cells were plated for 3 days in regular medium before being adjusted to steroid-reduced conditions for 2 days. Cells were then treated with 2.5 μM SVA and/or 5 μM abiraterone acetate for 3 days in steroid-reduced conditions. Cell number was determined with trypan blue exclusion dye assay. Results presented are mean ± SE. n=3. *P<0.05 (Unpublished data). B. LNCaP-AI cells were plated for 3 days in regular medium before being adjusted to steroid-reduced conditions for 2 days. Cells were then treated with 2.5 μM SVA and/or 1 nM docetaxel for 3 days in steroid-reduced conditions. Cell number was determined with trypan blue exclusion dye assay. Results presented are mean ± SE. n=3. *P<0.05 (Unpublished data) C. LNCaP-AI cells were plated for 3 days in regular medium before being adjusted to steroid-reduced conditions for 2 days. Cells were then treated with 5 μM SVA and/or 500 nM of the CIL-102 derivatives 1, 22, or 23 for 3 days in steroid-reduced conditions. Cell number was determined with trypan blue exclusion dye assay. Results presented are mean ± SE. n=3. *P<0.05 (Unpublished data). D. Chemical structures of CIL-102 derivatives 1, 22, and 23.
Development of more novel compounds for CRPCa treatment is equally important in this aspect. Additional novel compounds, for example, imidazopyridine derivatives [116] and pregnene analogs [117], have been shown to possess anti-tumor effect on various CRPCa cells under ADT conditions. More recently phase II and III clinical studies have begun to show the efficacy of novel compounds to treat advanced PCa including radioactive compound 177Lu-PSMA617 [118] in post-docetaxel CRPCa patients and GnRH antagonist Relugolix (Relumina) [119]. Importantly, imidazopyridine compounds exhibit activities toward CRPCa cells as well as NE-like PCa cells at a clinically achievable concentrations (Figure 3). The potential of these novel small compound inhibitors deserve further investigation for their utilities in advanced PCa therapy.
Figure 3.
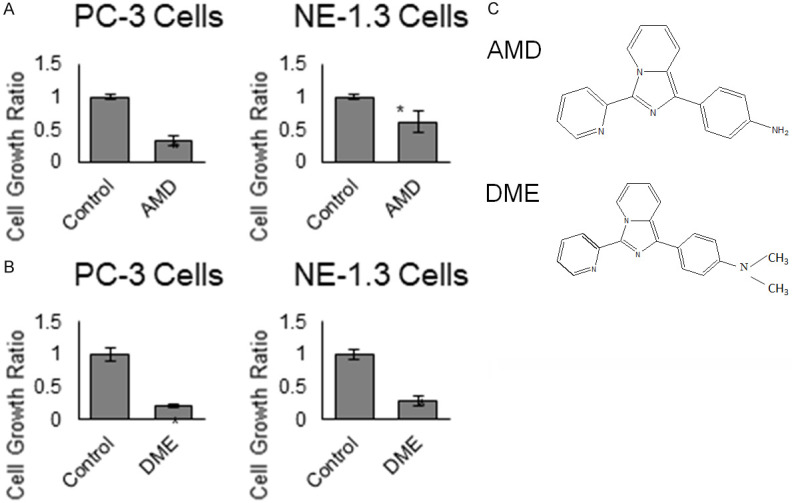
Novel Imidazopyridine derivatives on neuroendocrine-like prostate cancer cells. PC-3 and NE-1.3 cells were cultured in steroid-reduced conditions and treated with 10 µM of each compound (A) AMD and (B) DME for 3 days. Both treated and control cells were harvested and cell numbers were determined by trypan blue dye exclusion assay. Results presented are mean ± SE. n=3, *P<0.05. (C) Chemical structures of AMD and DME. (Unpublished data).
Conclusion and prospective
In summary, many attempts have been made to reduce the lethality of CRPCa. The search for improved treatment strategies continues as current therapies are combined together or with new therapeutic agents, as well as precision medicine for specific genetic abnormalities, such as expression of AR-V7. Currently, there has been some progress made in extending a CR PCa patients lifespan, but combinations thus far have not been shown to be safe and effective options to further improve outcomes in CRPCa.
We propose that the next immediate step in the management and treatment of PCa is to make CRPCa as a chronic disease, thus reducing the lethality of this specific type of cancer. Importantly, further studies of combination treatments utilizing ADT, immunotherapy, and docetaxel, as well as studies directed towards precision medicine, are warranted in preclinical and clinical settings. Additionally, the discovery and development of novel compounds, for example, SVA, CIL-102, and imidazopyridine derivatives, as single agents and/or combination usages with efficacy toward CRPCa as well as NE-like PCa cells is imperative. An important treatment combination to be developed is one which targets both the adenocarcinoma and the neuroendocrine PCa cell populations while spare the normal cells from cytotoxicity. Nevertheless, advancements in immunotherapy and the synthesis of novel anticancer agents provide new methods and combinations for the treatment of CRPCa.
Acknowledgements
This work was supported in part by the National Institute of Health [R03 CA230950, R01 CA233664, F32 CA250129], Department of Defense PCRP Grants [PC121645 and PC141559], the University of Nebraska Medical Center Graduate Studies Fellowship, the Fred and Pamela Buffett Cancer Center Support Grant [P30 CA036727], the UNMC Cancer Biology Training Grant [T32CA009476], and the UNMC Purdue Pharma Scholars Award. The work is also supported in part by the University of Colorado Training Program in Cancer Biology [T32CA190216-04].
Disclosure of conflict of interest
None.
Abbreviations
- ADT
Androgen-deprivation therapy
- AI
Androgen-independent
- AR
Androgen receptor
- AS
Androgen-sensitive
- ATT
Androgen targeted therapies
- CDDO-Me
Bardoxolone-methyl
- BETi
Bromodomain extraterminal inhibitors
- CRPC
Castration-resistant prostate cancer
- CTLA-4
Cytotoxic T-lymphocyte Associated Protein 4
- ETA
Endothelin Receptor A
- ET-1
Endothelin-1
- FABP5
fatty acid binding protein 5
- FAK
Focal adhesion kinase
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GnRH
Gonadatropin releasing hormone
- GR
Glucocorticoid receptor
- HDAC1
Histone deacetylase 1
- HRD
Homologous recombination deficiency
- IGF
Insulin-like growth factor
- LHRH
Luteinizing hormone releasing hormone
- mTOR
Mammalian target of rapamycin
- mHSPC
Metastatic hormone-sensitive prostate cancer
- mCRPC
metastatic castration-resistant prostate cancer
- NE
Neuroendocrine
- NHEJ
Nonhomologous end-joining
- PAcP
Prostatic acid phosphatase
- PARP
Poly (ADP-ribose) polymerase
- PCa
Prostate cancer
- PDX
Patient-derived xenografts
- PFS
Progression-free survival
- PI3K
Phosphoinositide 3-kinase
- PIP5K1a
Phosphatidylinositol-4-phosphate 5-kinase type 1 alpha
- PMSA
Prostate-specific membrane antigen
- PSA
Prostate-specific antigen
- PTEN
Phosphatase and tensin homolog
- RANKL
Receptor activator of nuclear factor κB ligand
- SNPs
Single nucleotide polymorphisms
- SVA
Simvastatin hydroxyl acid
- TKI
Tyrosine kinase inhibitor
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR, D’Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Kane CJ, Kawachi MH, Kuettel M, Lee RJ, Meeks JJ, Penson DF, Plimack ER, Pow-Sang JM, Raben D, Richey S, Roach M 3rd, Rosenfeld S, Schaeffer E, Skolarus TA, Small EJ, Sonpavde G, Srinivas S, Strope SA, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C, Clark PJ. Quantitative Studies of Prostatic Secretion: Ii. The effect of castration and of estrogen injection on the normal and on the hyperplastic prostate glands of dogs. J Exp Med. 1940;72:747–762. doi: 10.1084/jem.72.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Geller J, Albert J, Kirshner M. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metab. 1985;61:129–133. doi: 10.1210/jcem-61-1-129. [DOI] [PubMed] [Google Scholar]
- 5.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 6.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 7.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Igawa T, Yuan TC, Zhang XQ, Lin FF, Lin MF. ErbB-2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene. 2003;22:781–796. doi: 10.1038/sj.onc.1206066. [DOI] [PubMed] [Google Scholar]
- 9.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 10.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muniyan S, Chen SJ, Lin FF, Wang Z, Mehta PP, Batra SK, Lin MF. ErbB-2 signaling plays a critical role in regulating androgen-sensitive and castration-resistant androgen receptor-positive prostate cancer cells. Cell Signal. 2015;27:2261–2271. doi: 10.1016/j.cellsig.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DR, Ingersoll MA, Lin MF. ErbB-2 signaling in advanced prostate cancer progression and potential therapy. Endocr Relat Cancer. 2019;26:R195–R209. doi: 10.1530/ERC-19-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepe P, Verzoni E, Miodini P, Claps M, Ratta R, Martinetti A, Mennitto R, Sottotetti E, Procopio G, Cappelletti V, Daidone MG. Could circulating tumor cells and ARV7 detection improve clinical decisions in metastatic castration-resistant prostate cancer? The Istituto Nazionale dei Tumori (INT) experience. Cancers (Basel) 2019;11:980. doi: 10.3390/cancers11070980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho Y, Dehm SM. Androgen receptor rearrangement and splicing variants in resistance to endocrine therapies in prostate cancer. Endocrinology. 2017;158:1533–1542. doi: 10.1210/en.2017-00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lallous N, Volik SV, Awrey S, Leblanc E, Tse R, Murillo J, Singh K, Azad AA, Wyatt AW, LeBihan S, Chi KN, Gleave ME, Rennie PS, Collins CC, Cherkasov A. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 2016;17:10. doi: 10.1186/s13059-015-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 19.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12(Suppl 2):S141–144. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 20.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, Karan D, Batra SK, Lin MF. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 21.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 22.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung D, Krivoshik A, Sternberg CN. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 27.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, Iversen P, Evans CP, Kim CS, Kimura G, Miller K, Saad F, Bjartell AS, Borre M, Mulders P, Tammela TL, Parli T, Sari S, van Os S, Theeuwes A, Tombal B. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–154. doi: 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, Dunshee C, Wang F, Wu K, Krivoshik A, Phung D, Higano CS. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J. Clin. Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 29.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 30.Seibert TM, Fan CC, Wang Y, Zuber V, Karunamuni R, Parsons JK, Eeles RA, Easton DF, Kote-Jarai Z, Al Olama AA, Garcia SB, Muir K, Grönberg H, Wiklund F, Aly M, Schleutker J, Sipeky C, Tammela TL, Nordestgaard BG, Nielsen SF, Weischer M, Bisbjerg R, Røder MA, Iversen P, Key TJ, Travis RC, Neal DE, Donovan JL, Hamdy FC, Pharoah P, Pashayan N, Khaw KT, Maier C, Vogel W, Luedeke M, Herkommer K, Kibel AS, Cybulski C, Wokolorczyk D, Kluzniak W, Cannon-Albright L, Brenner H, Cuk K, Saum KU, Park JY, Sellers TA, Slavov C, Kaneva R, Mitev V, Batra J, Clements JA, Spurdle A, Teixeira MR, Paulo P, Maia S, Pandha H, Michael A, Kierzek A, Karow DS, Mills IG, Andreassen OA, Dale AM PRACTICAL Consortium*. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ. 2018;360:j5757. doi: 10.1136/bmj.j5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Røder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC COGS-Cancer Research UK GWAS-ELLIPSE (part of GAME-ON) Initiative; Australian Prostate Cancer Bioresource; UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology; UK ProtecT (Prostate testing for cancer and Treatment) Study Collaborators; PRACTICAL (Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome) Consortium. Kote-Jarai Z, Easton DF. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45:385–91. 391e1–2. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, McLaughlin B, Wahl J, Greene SB, Heller G, Marrinucci D, Fleisher M, Dittamore R. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1441–1449. doi: 10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan SC, Selth LA, Li Y, Nyquist MD, Miao L, Bradner JE, Raj GV, Tilley WD, Dehm SM. Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015;43:5880–5897. doi: 10.1093/nar/gkv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, Plymate SR, Navone NM, Wang S, Feng FY, Chinnaiyan AM. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of prostate cancer. Mol Cancer Res. 2016;14:324–331. doi: 10.1158/1541-7786.MCR-15-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, Gao AC. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3198–3210. doi: 10.1158/1078-0432.CCR-13-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Armstrong C, Zhu Y, Lou W, Gao AC. Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget. 2016;7:32210–32220. doi: 10.18632/oncotarget.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C, Armstrong CM, Lou W, Lombard AP, Cucchiara V, Gu X, Yang JC, Nadiminty N, Pan CX, Evans CP, Gao AC. Niclosamide and bicalutamide combination treatment overcomes enzalutamide- and bicalutamide-resistant prostate cancer. Mol Cancer Ther. 2017;16:1521–1530. doi: 10.1158/1535-7163.MCT-16-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khurana N, Chandra PK, Kim H, Abdel-Mageed AB, Mondal D, Sikka SC. Bardoxolone-Methyl (CDDO-Me) suppresses androgen receptor and its splice-variant AR-V7 and enhances efficacy of enzalutamide in prostate cancer cells. Antioxidants (Basel) 2020;9:68. doi: 10.3390/antiox9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarwar M, Semenas J, Miftakhova R, Simoulis A, Robinson B, Gjorloff Wingren A, Mongan NP, Heery DM, Johnsson H, Abrahamsson PA, Dizeyi N, Luo J, Persson JL. Targeted suppression of AR-V7 using PIP5K1alpha inhibitor overcomes enzalutamide resistance in prostate cancer cells. Oncotarget. 2016;7:63065–63081. doi: 10.18632/oncotarget.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lev A, Lulla AR, Ross BC, Ralff MD, Makhov PB, Dicker DT, El-Deiry WS. ONC201 targets AR and AR-V7 signaling, reduces pSA, and synergizes with everolimus in prostate cancer. Mol Cancer Res. 2018;16:754–766. doi: 10.1158/1541-7786.MCR-17-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudadi K, Suzman DL, Anagnostou V, Fu W, Luber B, Wang H, Niknafs N, White JR, Silberstein JL, Sullivan R, Dowling D, Harb R, Nirschl TR, Veeneman BA, Tomlins SA, Wang Y, Jendrisak A, Graf RP, Dittamore R, Carducci MA, Eisenberger MA, Haffner MC, Meeker AK, Eshleman JR, Luo J, Velculescu VE, Drake CG, Antonarakis ES. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9:28561–28571. doi: 10.18632/oncotarget.25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, Spitz A, DeCarli M, Sinibaldi V, Pratz CF, Lu C, Silberstein JL, Luo J, Schweizer MT, Drake CG, Carducci MA, Paller CJ, Antonarakis ES, Eisenberger MA, Denmeade SR. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19:76–86. doi: 10.1016/S1470-2045(17)30906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Q, Tong D, Liu G, Yi Y, Xu J, Yang X, Wang L, Zhang J, Ye J, Zhang Y, Yuan G, Wang P, Chen R, Guan Y, Yi X, Zhang D, Jiang J. A novel BRCA2 mutation in prostate cancer sensitive to combined radiotherapy and androgen deprivation therapy. Cancer Biol Ther. 2018;19:669–675. doi: 10.1080/15384047.2018.1451278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O, Crabb S, Robinson A, McLaren D, Birtle A, Tanguay J, Miranda S, Figueiredo I, Seed G, Bertan C, Flohr P, Ebbs B, Rescigno P, Fowler G, Ferreira A, Riisnaes R, Pereira R, Curcean A, Chandler R, Clarke M, Gurel B, Crespo M, Nava Rodrigues D, Sandhu S, Espinasse A, Chatfield P, Tunariu N, Yuan W, Hall E, Carreira S, de Bono JS. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:162–174. doi: 10.1016/S1470-2045(19)30684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asim M, Tarish F, Zecchini HI, Sanjiv K, Gelali E, Massie CE, Baridi A, Warren AY, Zhao W, Ogris C, McDuffus LA, Mascalchi P, Shaw G, Dev H, Wadhwa K, Wijnhoven P, Forment JV, Lyons SR, Lynch AG, O’Neill C, Zecchini VR, Rennie PS, Baniahmad A, Tavare S, Mills IG, Galanty Y, Crosetto N, Schultz N, Neal D, Helleday T. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8:374. doi: 10.1038/s41467-017-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Karanika S, Yang G, Wang J, Park S, Broom BM, Manyam GC, Wu W, Luo Y, Basourakos S, Song JH, Gallick GE, Karantanos T, Korentzelos D, Azad AK, Kim J, Corn PG, Aparicio AM, Logothetis CJ, Troncoso P, Heffernan T, Toniatti C, Lee HS, Lee JS, Zuo X, Chang W, Yin J, Thompson TC. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10:eaam7479. doi: 10.1126/scisignal.aam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Flechon A, Redfern C, Goessl C, Burgents J, Kozarski R, Hodgson D, Learoyd M, Saad F. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 52.Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, Siddiqui J, Wu YM, Robinson D, Lonigro RJ, Cao X, Tomlins SA, Mehra R, Cooney KA, Montgomery B, Antonarakis ES, Shevrin DH, Corn PG, Whang YE, Smith DC, Caram MV, Knudsen KE, Stadler WM, Feng FY, Chinnaiyan AM. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J. Clin. Oncol. 2018;36:991–999. doi: 10.1200/JCO.2017.75.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isaacsson Velho P, Qazi F, Hassan S, Carducci MA, Denmeade SR, Markowski MC, Thorek DL, DeWeese TL, Song DY, Tran PT, Eisenberger MA, Antonarakis ES. Efficacy of radium-223 in bone-metastatic castration-resistant prostate cancer with and without homologous repair gene defects. Eur Urol. 2019;76:170–176. doi: 10.1016/j.eururo.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beltran H, Eng K, Mosquera JM, Sigaras A, Romanel A, Rennert H, Kossai M, Pauli C, Faltas B, Fontugne J, Park K, Banfelder J, Prandi D, Madhukar N, Zhang T, Padilla J, Greco N, McNary TJ, Herrscher E, Wilkes D, MacDonald TY, Xue H, Vacic V, Emde AK, Oschwald D, Tan AY, Chen Z, Collins C, Gleave ME, Wang Y, Chakravarty D, Schiffman M, Kim R, Campagne F, Robinson BD, Nanus DM, Tagawa ST, Xiang JZ, Smogorzewska A, Demichelis F, Rickman DS, Sboner A, Elemento O, Rubin MA. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015;1:466–474. doi: 10.1001/jamaoncol.2015.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lloyd RL, Wijnhoven PWG, Ramos-Montoya A, Wilson Z, Illuzzi G, Falenta K, Jones GN, James N, Chabbert CD, Stott J, Dean E, Lau A, Young LA. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene. 2020;39:4869–4883. doi: 10.1038/s41388-020-1328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin MA, Girelli G, Demichelis F. Genomic correlates to the newly proposed grading prognostic groups for prostate cancer. Eur Urol. 2016;69:557–560. doi: 10.1016/j.eururo.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 58.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 59.Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS Transatlantic Prostate Group. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW, Eastham JA, Scardino PT, Scher HI, Tickoo SK, Reuter VE, Gerald WL. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, Martin NE, Kunz L, Penney KL, Ligon AH, Suppan C, Flavin R, Sesso HD, Rider JR, Sweeney C, Stampfer MJ, Fiorentino M, Kantoff PW, Sanda MG, Giovannucci EL, Ding EL, Loda M, Mucci LA. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gasi Tandefelt D, Boormans J, Hermans K, Trapman J. ETS fusion genes in prostate cancer. Endocr Relat Cancer. 2014;21:R143–152. doi: 10.1530/ERC-13-0390. [DOI] [PubMed] [Google Scholar]
- 63.Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, Pili R, Zwiebel J, Scher H, Hussain M. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer. 2009;115:5541–5549. doi: 10.1002/cncr.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S, Riggs CE Jr, Higano C, Stadler WM, McCulloch W, Dearnaley D, Parker C, de Bono JS. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC) Ann Oncol. 2010;21:109–113. doi: 10.1093/annonc/mdp270. [DOI] [PubMed] [Google Scholar]
- 65.Mohamed AA, Xavier CP, Sukumar G, Tan SH, Ravindranath L, Seraj N, Kumar V, Sreenath T, McLeod DG, Petrovics G, Rosner IL, Srivastava M, Strovel J, Malhotra SV, LaRonde NA, Dobi A, Dalgard CL, Srivastava S. Identification of a small molecule that selectively inhibits erg-positive cancer cell growth. Cancer Res. 2018;78:3659–3671. doi: 10.1158/0008-5472.CAN-17-2949. [DOI] [PubMed] [Google Scholar]
- 66.Mohamed AA, Tan SH, Xavier CP, Katta S, Huang W, Ravindranath L, Jamal M, Li H, Srivastava M, Srivatsan ES, Sreenath TL, McLeod DG, Srinivasan A, Petrovics G, Dobi A, Srivastava S. Synergistic activity with NOTCH inhibition and androgen ablation in ERG-positive prostate cancer cells. Mol Cancer Res. 2017;15:1308–1317. doi: 10.1158/1541-7786.MCR-17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao L, Kahraman N, Yan G, Wang J, Ozpolat B, Ittmann M. Targeting the TMPRSS2/ERG fusion mRNA using liposomal nanovectors enhances docetaxel treatment in prostate cancer. Prostate. 2020;80:65–73. doi: 10.1002/pros.23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, Hicks JL, Wilson KM, Rider JR, Sesso HD, Fiorentino M, Flavin R, Finn S, Giovannucci EL, Loda M, Stampfer MJ, De Marzo AM, Mucci LA, Lotan TL. A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J Natl Cancer Inst. 2016;108:djv346. doi: 10.1093/jnci/djv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W, Xie CC, Thomas CY, Kim ST, Lindberg J, Egevad L, Wang Z, Zhang Z, Sun J, Sun J, Koty PP, Kader AK, Cramer SD, Bova GS, Zheng SL, Gronberg H, Isaacs WB, Xu J. Genetic markers associated with early cancer-specific mortality following prostatectomy. Cancer. 2013;119:2405–2412. doi: 10.1002/cncr.27954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Laskey J, Bettano KA, Kasibhatla S, Reilly JF, Sur C, Majumder PK. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–7472. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
- 71.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marques RB, Aghai A, de Ridder CMA, Stuurman D, Hoeben S, Boer A, Ellston RP, Barry ST, Davies BR, Trapman J, van Weerden WM. High efficacy of combination therapy using PI3K/AKT inhibitors with androgen deprivation in prostate cancer preclinical models. Eur Urol. 2015;67:1177–1185. doi: 10.1016/j.eururo.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 73.Thomas C, Lamoureux F, Crafter C, Davies BR, Beraldi E, Fazli L, Kim S, Thaper D, Gleave ME, Zoubeidi A. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 2013;12:2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, Scher HI, Barry ST, Sawyers CL, Chandarlapaty S, Rosen N. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. 2015;27:109–122. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei XX, Hsieh AC, Kim W, Friedlander T, Lin AM, Louttit M, Ryan CJ. A Phase I study of abiraterone acetate combined with BEZ235, a dual PI3K/mTOR inhibitor, in metastatic castration resistant prostate cancer. Oncologist. 2017;22:503–e543. doi: 10.1634/theoncologist.2016-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Armstrong AJ, Halabi S, Healy P, Alumkal JJ, Winters C, Kephart J, Bitting RL, Hobbs C, Soleau CF, Beer TM, Slottke R, Mundy K, Yu EY, George DJ. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer. 2017;81:228–236. doi: 10.1016/j.ejca.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, Arranz Arija JA, Shih KC, Radavoi GD, Xu N, Chan WY, Ma H, Gendreau S, Riisnaes R, Patel PH, Maslyar DJ, Jinga V. Randomized Phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. 2019;25:928–936. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]
- 78.Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, Gi YJ, Lu B. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez-Billalabeitia E, Seitzer N, Song SJ, Song MS, Patnaik A, Liu XS, Epping MT, Papa A, Hobbs RM, Chen M, Lunardi A, Ng C, Webster KA, Signoretti S, Loda M, Asara JM, Nardella C, Clohessy JG, Cantley LC, Pandolfi PP. Vulnerabilities of PTEN-TP53-deficient prostate cancers to compound PARP-PI3K inhibition. Cancer Discov. 2014;4:896–904. doi: 10.1158/2159-8290.CD-13-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van de Ven AL, Tangutoori S, Baldwin P, Qiao J, Gharagouzloo C, Seitzer N, Clohessy JG, Makrigiorgos GM, Cormack R, Pandolfi PP, Sridhar S. Nanoformulation of OLAPARIB Amplifies PARP INhibition and SEnsitizes PTEN/TP53-DEFICIENT PROSTate CANCER TO RADiation. Mol Cancer Ther. 2017;16:1279–1289. doi: 10.1158/1535-7163.MCT-16-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ingersoll MA, Miller DR, Martinez O, Wakefield CB, Hsieh KC, Simha MV, Kao CL, Chen HT, Batra SK, Lin MF. Statin derivatives as therapeutic agents for castration-resistant prostate cancer. Cancer Lett. 2016;383:94–105. doi: 10.1016/j.canlet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fromont G, Godet J, Peyret A, Irani J, Celhay O, Rozet F, Cathelineau X, Cussenot O. 8q24 amplification is associated with Myc expression and prostate cancer progression and is an independent predictor of recurrence after radical prostatectomy. Hum Pathol. 2013;44:1617–1623. doi: 10.1016/j.humpath.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 83.Hawksworth D, Ravindranath L, Chen Y, Furusato B, Sesterhenn IA, McLeod DG, Srivastava S, Petrovics G. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010;13:311–315. doi: 10.1038/pcan.2010.31. [DOI] [PubMed] [Google Scholar]
- 84.Anderson PD, McKissic SA, Logan M, Roh M, Franco OE, Wang J, Doubinskaia I, van der Meer R, Hayward SW, Eischen CM, Eltoum IE, Abdulkadir SA. Nkx3.1 and Myc crossregulate shared target genes in mouse and human prostate tumorigenesis. J Clin Invest. 2012;122:1907–1919. doi: 10.1172/JCI58540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–1731. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao L, Schwartzman J, Gibbs A, Lisac R, Kleinschmidt R, Wilmot B, Bottomly D, Coleman I, Nelson P, McWeeney S, Alumkal J. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLoS One. 2013;8:e63563. doi: 10.1371/journal.pone.0063563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke J, Wilder-Romans K, Dhanireddy S, Engelke C, Iyer MK, Jing X, Wu YM, Cao X, Qin ZS, Wang S, Feng FY, Chinnaiyan AM. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wyce A, Degenhardt Y, Bai Y, Le B, Korenchuk S, Crouthame MC, McHugh CF, Vessella R, Creasy CL, Tummino PJ, Barbash O. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget. 2013;4:2419–2429. doi: 10.18632/oncotarget.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stratikopoulos EE, Dendy M, Szabolcs M, Khaykin AJ, Lefebvre C, Zhou MM, Parsons R. Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell. 2015;27:837–851. doi: 10.1016/j.ccell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai X, Gan W, Li X, Wang S, Zhang W, Huang L, Liu S, Zhong Q, Guo J, Zhang J, Chen T, Shimizu K, Beca F, Blattner M, Vasudevan D, Buckley DL, Qi J, Buser L, Liu P, Inuzuka H, Beck AH, Wang L, Wild PJ, Garraway LA, Rubin MA, Barbieri CE, Wong KK, Muthuswamy SK, Huang J, Chen Y, Bradner JE, Wei W. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat Med. 2017;23:1063–1071. doi: 10.1038/nm.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blattner M, Liu D, Robinson BD, Huang D, Poliakov A, Gao D, Nataraj S, Deonarine LD, Augello MA, Sailer V, Ponnala L, Ittmann M, Chinnaiyan AM, Sboner A, Chen Y, Rubin MA, Barbieri CE. SPOP mutation drives prostate tumorigenesis In Vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell. 2017;31:436–451. doi: 10.1016/j.ccell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kirschner AN, Wang J, van der Meer R, Anderson PD, Franco-Coronel OE, Kushner MH, Everett JH, Hameed O, Keeton EK, Ahdesmaki M, Grosskurth SE, Huszar D, Abdulkadir SA. PIM kinase inhibitor AZD1208 for treatment of MYC-driven prostate cancer. J Natl Cancer Inst. 2015;107:dju407. doi: 10.1093/jnci/dju407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K, Huser N, Proffitt C, Bliesath J, Haddach M, Schwaebe MK, Ryckman DM, Rice WG, Schmitt C, Lowe SW, Johnstone RW, Pearson RB, McArthur GA, Hannan RD. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rebello RJ, Kusnadi E, Cameron DP, Pearson HB, Lesmana A, Devlin JR, Drygin D, Clark AK, Porter L, Pedersen J, Sandhu S, Risbridger GP, Pearson RB, Hannan RD, Furic L. The dual inhibition of RNA Pol I transcription and PIM kinase as a new therapeutic approach to treat advanced prostate cancer. Clin Cancer Res. 2016;22:5539–5552. doi: 10.1158/1078-0432.CCR-16-0124. [DOI] [PubMed] [Google Scholar]
- 95.Leonetti C, Biroccio A, D’Angelo C, Semple SC, Scarsella M, Zupi G. Therapeutic integration of c-myc and bcl-2 antisense molecules with docetaxel in a preclinical model of hormone-refractory prostate cancer. Prostate. 2007;67:1475–1485. doi: 10.1002/pros.20636. [DOI] [PubMed] [Google Scholar]
- 96.Ciccarelli C, Di Rocco A, Gravina GL, Mauro A, Festuccia C, Del Fattore A, Berardinelli P, De Felice F, Musio D, Bouche M, Tombolini V, Zani BM, Marampon F. Disruption of MEK/ERK/c-Myc signaling radiosensitizes prostate cancer cells in vitro and in vivo. J Cancer Res Clin Oncol. 2018;144:1685–1699. doi: 10.1007/s00432-018-2696-3. [DOI] [PubMed] [Google Scholar]
- 97.Zelivianski S, Spellman M, Kellerman M, Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Davis TL, Hauke R, Lin MF. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–485. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 98.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, Gallick GE, Trudel GC, Paliwal P, Agrawal S, Logothetis CJ. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1-2 study. Cancer. 2012;118:63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Araujo JC, Trudel GC, Saad F, Armstrong AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev VB, Efstathiou E, Oudard S, Morris MJ, Sizer B, Goebell PJ, Heidenreich A, de Bono JS, Begbie S, Hong JH, Richardet E, Gallardo E, Paliwal P, Durham S, Cheng S, Logothetis CJ. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dorff TB, Quinn DI, Pinski JK, Goldkorn A, Sadeghi S, Tsao-Wei D, Groshen S, Kuhn P, Gross ME. Randomized phase II trial of abiraterone alone or with dasatinib in men with metastatic castration-resistant prostate cancer (mCRPC) Clin Genitourin Cancer. 2019;17:241–247. e241. doi: 10.1016/j.clgc.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, Collins S, Daunton A, McLaren D, O’Sullivan J, Parker C, Porfiri E, Staffurth J, Stanley A, Wylie J, Beesley S, Birtle A, Brown J, Chakraborti P, Hussain S, Russell M, Billingham LJ. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic Acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2:493–499. doi: 10.1001/jamaoncol.2015.5570. [DOI] [PubMed] [Google Scholar]
- 102.Chi KN, Higano CS, Blumenstein B, Ferrero JM, Reeves J, Feyerabend S, Gravis G, Merseburger AS, Stenzl A, Bergman AM, Mukherjee SD, Zalewski P, Saad F, Jacobs C, Gleave M, de Bono JS. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2017;18:473–485. doi: 10.1016/S1470-2045(17)30168-7. [DOI] [PubMed] [Google Scholar]
- 103.Qu S, Wang K, Xue H, Wang Y, Wu R, Liu C, Gao AC, Gout PW, Collins CC, Wang Y. Enhanced anticancer activity of a combination of docetaxel and Aneustat (OMN54) in a patient-derived, advanced prostate cancer tissue xenograft model. Mol Oncol. 2014;8:311–322. doi: 10.1016/j.molonc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carbonetti G, Converso C, Clement T, Wang C, Trotman LC, Ojima I, Kaczocha M. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. Prostate. 2020;80:88–98. doi: 10.1002/pros.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou Y, Yang J, Zhang R, Kopecek J. Combination therapy of prostate cancer with HPMA copolymer conjugates containing PI3K/mTOR inhibitor and docetaxel. Eur J Pharm Biopharm. 2015;89:107–115. doi: 10.1016/j.ejpb.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, Kench JG, Stricker PD, Haynes AM, Centenera MM, Butler LM, Shreeve SM, Horvath LG, Daly RJ. Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate. 2018;78:308–317. doi: 10.1002/pros.23476. [DOI] [PubMed] [Google Scholar]
- 107.Rushworth LK, Hewit K, Munnings-Tomes S, Somani S, James D, Shanks E, Dufes C, Straube A, Patel R, Leung HY. Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment. Br J Cancer. 2020;122:517–527. doi: 10.1038/s41416-019-0681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vijayaraghavalu S, Gao Y, Rahman MT, Rozic R, Sharifi N, Midura RJ, Labhasetwar V. Synergistic combination treatment to break cross talk between cancer cells and bone cells to inhibit progression of bone metastasis. Biomaterials. 2020;227:119558. doi: 10.1016/j.biomaterials.2019.119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, Cher M, Sarkar FH. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–3826. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 110.Yang Y, Mamouni K, Li X, Chen Y, Kavuri S, Du Y, Fu H, Kucuk O, Wu D. Repositioning dopamine D2 receptor agonist bromocriptine to enhance docetaxel chemotherapy and treat bone metastatic prostate cancer. Mol Cancer Ther. 2018;17:1859–1870. doi: 10.1158/1535-7163.MCT-17-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palma JP, Wang YC, Rodriguez LE, Montgomery D, Ellis PA, Bukofzer G, Niquette A, Liu X, Shi Y, Lasko L, Zhu GD, Penning TD, Giranda VL, Rosenberg SH, Frost DJ, Donawho CK. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15:7277–7290. doi: 10.1158/1078-0432.CCR-09-1245. [DOI] [PubMed] [Google Scholar]
- 112.Hussain M, Carducci MA, Slovin S, Cetnar J, Qian J, McKeegan EM, Refici-Buhr M, Chyla B, Shepherd SP, Giranda VL, Alumkal JJ. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32:904–912. doi: 10.1007/s10637-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dahut WL, Madan RA, Karakunnel JJ, Adelberg D, Gulley JL, Turkbey IB, Chau CH, Spencer SD, Mulquin M, Wright J, Parnes HL, Steinberg SM, Choyke PL, Figg WD. Phase II clinical trial of cediranib in patients with metastatic castration-resistant prostate cancer. BJU Int. 2013;111:1269–1280. doi: 10.1111/j.1464-410X.2012.11667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, Rimel BJ, Buss MK, Nattam SR, Hurteau J, Luo W, Curtis J, Whalen C, Kohn EC, Ivy SP, Matulonis UA. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol. 2019;30:551–557. doi: 10.1093/annonc/mdz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller DR, Tzeng CC, Farmer T, Keller ET, Caplan S, Chen YS, Chen YL, Lin MF. Novel CIL-102 derivatives as potential therapeutic agents for docetaxel-resistant prostate cancer. Cancer Lett. 2018;436:96–108. doi: 10.1016/j.canlet.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ingersoll MA, Lyons AS, Muniyan S, D’Cunha N, Robinson T, Hoelting K, Dwyer JG, Bu XR, Batra SK, Lin MF. Novel imidazopyridine derivatives possess anti-tumor effect on human castration-resistant prostate cancer cells. PLoS One. 2015;10:e0131811. doi: 10.1371/journal.pone.0131811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdul-Rida NA, Farhan AM, Al-Masoudi NA, Saeed BA, Miller D, Lin MF. A novel pregnene analogs: synthesis, cytotoxicity on prostate cancer of PC-3 and LNCPa-AI cells and in silico molecular docking study. Mol Divers. 2020 doi: 10.1007/s11030-020-10038-w. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 118.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, Iravani A, Kong G, Ravi Kumar A, Murphy DG, Eu P, Jackson P, Scalzo M, Williams SG, Sandhu S. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 119.Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, Akaza H, Bossi A, van Veenhuyzen DF, Selby B, Fan X, Kang V, Walling J, Tombal B HERO Study Investigators. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 120.Crawford ED, Petrylak D, Sartor O. Navigating the evolving therapeutic landscape in advanced prostate cancer. Urol Oncol. 2017;35S:S1–S13. doi: 10.1016/j.urolonc.2017.01.020. [DOI] [PubMed] [Google Scholar]


