Abstract
The posterior lid margin, where the mucocutaneous junction (MCJ) between the eyelid skin and tarsal conjunctiva is located, plays a critical role in maintaining the homeostasis of the ocular surface. Posterior migration of the MCJ leads to lid-margin keratinization (LMK), which has a domino effect on the delicate balance of the ocular surface microenvironment. This occurs most commonly following Stevens-Johnson syndrome/toxic epidermal necrolysis and is not known to regress spontaneously or with medical therapy. Over time, LMK causes blink-related chronic inflammatory damage to the corneal surface which may have blinding consequences. Lid-margin mucous membrane grafting (MMG) is the only definitive therapy for LMK. Timely MMG can significantly alter the natural course of the disease and not only preserve but even improve vision in affected eyes. Literature searches were conducted on PubMed, using the keywords “mucous membrane grafts,” “lid margin keratinization,” “Stevens-Johnson syndrome,” “toxic epidermal necrolysis,” “lid related keratopathy,” and “lid wiper epitheliopathy”. This review, which is a blend of evidence and experience, attempts to describe the indications, timing, surgical technique, postoperative regimen, and clinical outcomes of MMG for LMK. The review also covers the possible complications and pearls on how they can be effectively managed, including how suboptimal cosmetic outcomes can be avoided. The authors hope that this review will aid ophthalmologists, including cornea and oculoplasty specialists, to learn and perform this vision-saving surgery better, with the aim of helping their patients with chronic ocular surface disorders, relieving their suffering, and improving their quality of life.
Keywords: Lid margin keratinization, lid-related keratopathy, Stevens-Johnson syndrome, toxic epidermal necrolysis
If the eyes are the windows to the human soul, then the eyelids are its gatekeepers. Eyelids not only play an important role in communicating human emotions as a part of facial expressions, but they also protect the more delicate inner structures of the eye from any external damage. The eyelid and posterior eyelid margin remain in close contact with the delicate ocular surface microenvironment and play an important role in maintaining its normal equilibrium.[1] Of particular importance is the mucocutaneous junction (MCJ) where the rough skin of the eyelid transitions into the smooth and delicate conjunctival surface [Fig. 1a].[1] The lid wiper, which is the part of the eyelid that is in contact with the globe, extends from the MCJ to the subtarsal fold superiorly and from the medial punctum to the lateral canthus horizontally.[2,3,4] Corneal epitheliopathy caused secondary to disturbance to the lid wiper is termed as lid wiper epitheliopathy (LWE), which occurs whenever the altered lid wiper moves against the ocular surface with each blink.[3] This can happen due to posterior migration of the MCJ [Fig. 1b] also known as lid margin keratinization (LMK). With every blink in eyes with LMK, this keratinized epithelium of the inner lid margin in both upper and lower lids cause progressive corneal epitheliopathy. Posterior migration of the MCJ and subsequent keratinization of the tarsal conjunctival epithelium causing keratopathy of varying degrees was first described in 1956.[5] Keratopathy due to LMK can result in epithelial defects, microbial keratitis, corneal vascularization, and corneal perforations, especially when coupled with moderate-severe dry eye.
Figure 1.
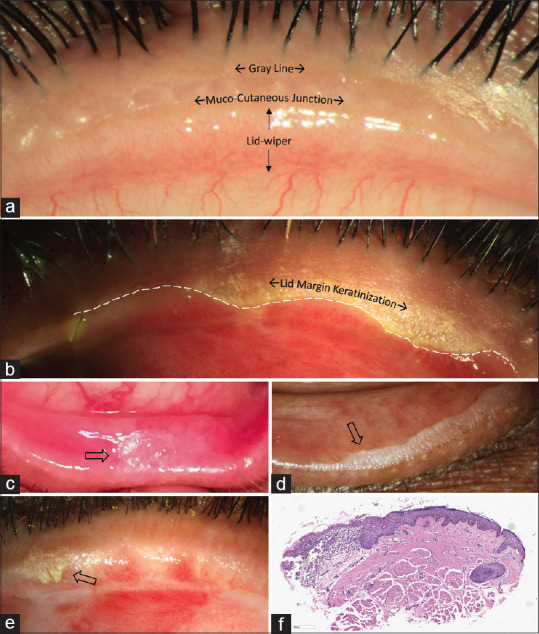
Normal appearance of posterior lid margin and lid margin keratinization (LMK). (a) Normal everted upper eyelid showing gray line, mucocutaneous junction (MCJ) and the lid-wiper. (b) Posteriorly migrated MCJ (white dotted line) with LMK in SJS. (c) Focal patch of LMK post plaque brachytherapy for a lid tumour. (d) LMK following chronic use of anti-glaucoma medications. (e) LMK in biopsy-proven pemphigoid. (f) Histopathological appearance of LMK, showing posterior migration of the keratinized epithelium with diffuse subepithelial lymphocytic infiltration (H and E stain; original magnification X10)
The etiologies for LMK mentioned previously include Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), radiation therapy to the lid after lid carcinomas, and chronic allergic reaction to topical medications [Fig. 1b-d].[5] It can also rarely occur in mucous membrane pemphigoid (MMP) [Fig. 1e] or other auto-immune causes of chronic cicatrizing conjunctivitis and rarely following severe chemical burns. Histologically, LMK is characterized by a transition in epithelium from the stratified squamous non-keratinized epithelium of palpebral conjunctiva to stratified squamous keratinized epithelium of the skin secondary to MCJ migration posteriorly [Fig. 1f]. Clinically, LMK can appear as a focal or diffuse area of white coarse material deposited on the skin margin, sometimes extending on the tarsal conjunctiva. In more subtle cases, LMK can be easily discerned with fluorescein staining. It is important to realize that LMK does not regress spontaneously, nor is there reliable medical therapy for it. Although there are some reports of treatment by topical retinoic acid, the role of topical retinoids in ocular surface disease is highly controversial.[6,7] Lid-margin mucous membrane grafting (MMG) is the only treatment that directly addresses the pathology and restores a normally functioning posterior lid margin. It is a surgical procedure that replaces the keratinized lid margin and adjacent scarred tarsal conjunctival epithelium with normal healthy mucosa from the oral cavity. In this review, we describe the indications, the surgical technique, outcomes, limitations, and the impact of MMG.
Method of Literature Search
In February 2020, literature searches for the components of this review were completed using PubMed. The following keywords and their iterations were used for the searches, “mucous membrane grafts,” “lid margin keratinization,” “Stevens-Johnson syndrome,” “toxic epidermal necrolysis,” “lid related keratopathy,” and “lid wiper epitheliopathy”. These were entered into PubMed revealing 7862 related articles. Given the volume of literature recovered, our inclusion criteria included publication in the English language, and we also included articles that only included mainly the surgery “mucous membrane grafts,” the diagnosis of “lid margin keratinization,” and had to include “Stevens-Johnson syndrome” or “toxic epidermal necrolysis” as one of the etiologies. Articles were carefully read, and case series from single institutions were carefully screened to ensure only the largest studies were included. This considerably narrowed our search, and the final articles were included for this literature review.
Peri-Operative Considerations and Surgical Technique
Ideal source of mucosal grafts for the posterior lid margin
Donor mucosal sites with non-keratinized epithelium are preferred as substitutes for conjunctival epithelium at the lid margin. Oral mucosa, specifically lip (labial) mucosa is preferred primarily because of ease of access. Also, the same donor site can undergo repeated harvesting without causing major complications. Other possible sources that can be used are buccal, nasal, rectal, and vaginal mucosa.[8] Hard palate mucosa has keratinized epithelium and is avoided in indications where MMG is being done to provide a smooth lid margin.[9]
Indications of MMG for lid margin keratinization
The most common indication mentioned in literature is SJS/TEN.[5,10,11,12,13,14,15,16] In the chronic phase, various ocular complications emerge. LMK is one such common early manifestation that occurs as early as 3 months after acute SJS/TEN, is bilateral, and can occur in all four lids.[17] LMK causes progressive keratopathy leading to eventual LSCD.[14,15] SJS/TEN was found to be the third most common cause of bilateral LSCD in patients presenting to a tertiary care ophthalmic center.[18] In another recent paper, two-thirds of patients presented more than a year after acute SJS, 99% without prior AMT, with low vision or blindness in 60% of eyes.[15] Other etiologies of LMK such as irradiation to the eye or the head and neck region for carcinomas[5,19,20] are very rare. So, for all practical purposes, this review focuses on MMG for LMK in SJS/TEN.
Preoperative considerations
Recipient eye
1) Firstly, it is important to differentiate an eye with chronic sequelae of SJS/TEN from an eye in the chronic stage of MMP. A detailed history should be elicited and a careful ocular examination is warranted.[21] If a surgical procedure is planned in a case that could potentially be MMP, it may be disastrous if the patient has not been administered systemic immunosuppression first.[22]
2) Once a diagnosis of chronic SJS/TEN is established, the next step is performing differential fluorescein staining [Fig. 2] to establish that the keratopathy is secondary to lid changes. If staining on the cornea corresponds to the area of LMK on the eyelid, this is termed as lid-related keratopathy.[15] If staining is diffuse or restricted to the inter-palpebral area, then this is termed as non-lid-related keratopathy and could be secondary to dry eye. Usually, there is always some overlap between these two [Fig. 2], however, MMG is indicated only if the keratopathy is attributable to LMK.
Figure 2.
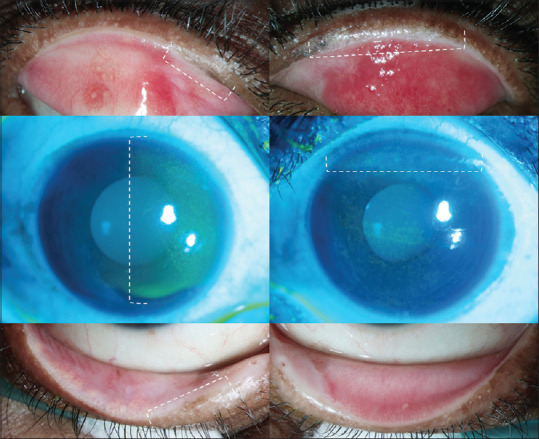
Upper lid margins, corneas and lower lid margins in the right and left eyes of the same patient depicts the corneal staining pattern consistent with lid-related keratopathy and with non lid-related keratopathy
3) The following structures should be examined in great detail: lid position, trichiatic or distichiatic lashes, Meibomian glands, puncta, tarsal conjunctiva (for fibrosis or keratinization), fornices, bulbar conjunctiva (for fibrosis, keratinization), corneal scars and corneal vascularization. The wetness of each eye should be tested with a Schirmer‘s strip.
An Ideal case for lid-margin MMG [Fig. 2] is when the eye has posterior migration of MCJ with LMK with early lid-related keratopathy with some wetness of the ocular surface, ideally a Schirmer‘s score >5 mm (at 5 min, without anesthesia). The outcomes are the best in this category since performing only a MMG in these eyes may be able to halt the keratopathy and prevent further deterioration of the ocular surface. One caveat here is that prophylactic MMG should be avoided in eyes where LMK has started but has not yet caused lid-related keratopathy. Although rare in SJS/TEN, such cases should be followed-up and MMG should be advised only when keratopathy starts. The areas of superior and inferior limbus where the lid comes in contact with cornea should be carefully examined for any signs of vascularization along with lid-related keratopathy. Also, it is essential to differentiate an irregular lid margin with atrophied meibomian glands from LMK since the former does not require MMG.
Donor area
The donor area should be carefully examined preoperatively. Mucosal tissue can be harvested from the upper or lower lip. The oral mucosa should be examined for active ulceration, which is rare in the chronic phase of SJS/TEN. MMG‘s can be performed even as early as 2 months postacute SJS/TEN if there is no ongoing ulceration in the oral mucosa. The posterior MCJ migration should be examined in the lips, to not include this junction while harvesting oral mucosa. Also, if the patient is a chronic user of smokeless tobacco products (guthka), chronic dysplastic changes on the oral mucosa may be seen. The affected areas should be avoided. The use of preoperative prophylactic antiseptic mouthwash a few days prior to harvesting oral donor mucosal graft has been reported in the literature;[11,23] however, the authors do not practice this.
Preoperative counseling
Usually patients with LMK are chronic sufferers, who have recovered from the near-death experience of SJS/TEN and their emotional balance is quite fragile. They frequently have many queries and may ask for a guaranteed outcome. This is out of their desperation to seek a way out of their misery and not to test the competence of the consulting ophthalmologist. Patients may need multiple counseling sessions before they understand both the benefits and the limitations of the surgery.
Anesthesia
Lid-margin MMG should always be performed under general anesthesia (GA). For adults, where GA is a contra-indication, surgery can be performed under local peribulbar anesthesia, however beginners should refrain from attempting this surgery under local anesthesia. Lid-margin MMG is a time-consuming surgery, especially for beginners, and GA offers better patient comfort during the duration of the surgery. In patients undergoing MMG, the following considerations are necessary for an anesthesiologist:
Patients may have sequelae of SJS/TEN following drug reactions to unknown medications. Known drug allergies should be noted and these drugs avoided. Some patients may have underlying systemic conditions such as epilepsy, auto-immune diseases, retroviral disease, medications for which predisposed them to SJS. Necessary precautions should be taken in such patients. Patients with a recent history of steroid therapy (administered in the last 6 months) will need peri-operative supplements of steroids.
Slight head-up tilt of the table (10 degrees) could assist in reducing blood flow to the head region reducing intra-operative bleeding.
Since surgery involves harvesting labial mucosa, a throat pack and cuffed tube (Ring-Adair-Elwyn tubes––south bend) should be secured to one side of the oral cavity to allow exposure of the operative field for the surgeon. [Fig. 3a and b]
The dissection of the scarred tissue of eyelid margin and tarsal conjunctiva causes oozing of blood which continuously disrupts the operative field for the surgeon. Maintaining a mean arterial blood pressure of around 60 mmHg and using analgesia (narcotic medications plus paracetamol) helps the surgeon. Other non-steroidal anti-inflammatory drugs are avoided due to possible drug reactions. Special precautions are required to avoid excessive bleeding in patients with retroviral disease.
Either Atracurium or vecuronium are good choices as muscle relaxants.
Fine-tuning of the blood pressure control can be achieved with Isoflurane 0.8% to 3%. The use of small incremental doses of dexmedetomidine or Labetalol is useful to control tachycardia.
Figure 3.
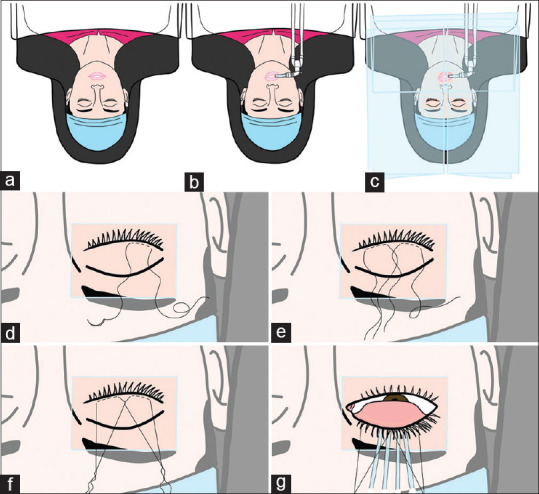
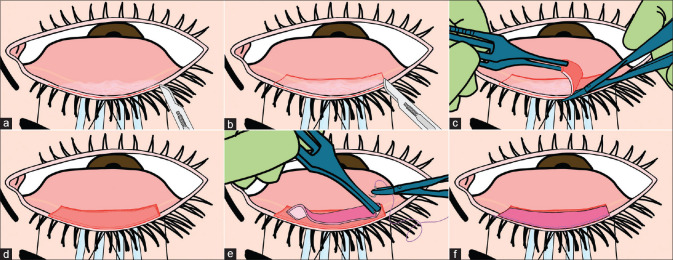
Preoperative preparation prior to lid-margin MMG. (a and b) Patient in supine position with cuffed tube for intubation secured to the right side of oral cavity. (c) Three-plastic drape technique used when operating bilaterally. (d) First stay suture (4-0 silk) passed laterally on the skin of upper lid 3-4 mm behind lash line. (e) Both stay sutures crossed over each other centrally. (f) Cantilever suspension with the ends tied and sutures stretched. (g) Lid flipped with four sterile cotton swabs with the lid held taut by suspension sutures anchored with artery forceps at the ends to the drape
For patients under local anesthesia, the following precautions are necessary:
Surgery for more than one lid should not be planned at the same sitting.
Surgery under local anesthesia should not be performed in chronic mouth- breathers as this creates complications intra-operatively. A throat pack cannot be used in local anesthesia. If the patient continues to breathe through the mouth, they may aspirate blood.
Due to the long duration of surgery and reduced temperature in the operating room, the nasal mucosa may get congested. The patient may find it difficult to breathe which is compounded by drapes covering the face. Hence, nasal decongestants should be used before surgery.
Surgery under local anesthesia should be avoided in very apprehensive patients since co-operation could be a limiting factor.
Bleeding and salivary secretions must be sucked continuously with low-pressure suction or mopped using wet cotton gauze.[24]
Preoperative vasoconstriction locally
The authors use two to three applications of brimonidine tartrate 0.15% and phenylephrine 5% eye drops alternatively for 5–10 min in the eye/eyes to be operated before shifting the patient to the operating room. The use of preoperative prophylactic brimonidine tartrate 0.15% has been reported prior to pterygium surgery,[25] and strabismus surgery.[26,27] The authors believe using these drops preoperatively in the eye before excision of keratinized lid margin may reduce intraoperative bleeding during dissection and excision of tarsal conjunctival epithelium adjacent to the keratinized lid margin. The lip mucosa is infiltrated with 5 mL of 2% Lidocaine mixed with adrenaline of 5 cc of Lignocaine with adrenaline (1:200 000) using a 26-gauge needle. Infiltration is administered superficially just under the area of the mucosa that is to be harvested.
Draping
The eye to be operated and the mouth should be cleaned with 5% betadine solution and draped, then a sufficient opening is made in the drapes to expose the area to be operated. A good betadine preparation of the oral mucosa with 5% or 10% solution is adequate for cleaning the oral mucosa. If the authors operate on both eyes of the same patient at the same setting, then the authors use a 3-plastic drape technique where one plastic-drape is draped on one eye, this is kept folded until this eye is operated on, the second plastic-drape is draped on the second eye and a separate third plastic-drape is used for the mouth. Fig. 3c shows the 3-plastic drape technique that the authors use.
Surgical technique
The surgical techniques for MMG and harvesting of oral mucosa have been reported previously.[10,11,12,13,28] The authors have adapted certain steps and modified certain techniques. In previous studies, eyes with chronic sequelae of SJS/TEN with lid margin keratinization with concomitant cicatricial entropion/distichiasis/trichiasis underwent MMG combined with other techniques to address combined pathologies.[10,12] In these eyes, a split of the anterior lamella was performed to address the concomitant conditions, followed by excision of keratinized lid margin, followed by recession of the anterior lamella. However, the authors, in this review, describe a technique of MMG to solely address the lid margin keratinization component in these eyes. The technique followed by the authors is described below.
Step-by-Step Surgical technique
Preparation of labial mucosa: Two stay sutures (4-0 silk) are passed 3-4 mm behind MCJ at the lip margin through the skin, these are done to evert the lip during dissection. Next, the local anesthetic agent should be infiltrated in the oral mucosa. This gives us two advantages, better hemostasis while dissecting the mucosa later and also better separation of the plane for dissection.
Preparation of lids: Two stay sutures (4-0 silk) each are passed through the lid margins, 3-4 mm behind the lash line through the skin. The lid sutures can then be crossed centrally. This provides a cantilever suspension for better exposure of the eye. With sterile cotton swabs placed under the crossing sutures, the lid can be kept taut, preventing excessive bleeding by providing pressure underneath the lids while dissecting the keratinized lid margins. [Fig. 3d-g] shows suspension traction sutures with self-retaining counter-pressure buds that the authors use.
Dissection of the keratinized lid margin: The anterior horizontal incision is placed at the gray line, with a no. 15 blade mounted on a Bard-Parker handle.[10,29] When the gray line is not discernible, the incision should be placed just posterior to the lash line. It is better to err and place an incision closer to the lash line than posteriorly behind the gray line since this can cause a posterior graft which fails to address the primary pathology. The incision is extended along the entire lid margin sparing a total length of 4-5 mm at the medial and the lateral ends (2-2.5 mm on either side). Two vertical posterior extensions of 4-5 mm are made at both ends of the horizontal incision, and these are joined by the posterior horizontal incision on the tarsal conjunctiva [Fig. 4a and b]. All the incisions are superficial with the intention of cleaving the epithelium without damaging the tarsus. The strip of epithelium, including the keratinized patches, within the incised area is then dissected off the tarsus with a Vannas‘ scissors and Pierse-Hoskin‘s forceps by starting at one of the corners [Fig. 4c]. Since the conjunctiva is tightly adherent to the tarsal plate in the upper eyelid, piece-mealing of the epithelial strip is common. Active counter-pressure on the self-retaining cotton buds and the use of suction cannula can help maintain a relatively bloodless field during dissection. After the dissection is completed, one should carefully inspect this area again to ensure that there are no residual patches of normal/keratinized epithelium on the tarsal conjunctival bed, since this could hinder the uptake of MMG causing graft necrosis. The size of the dissected bed should be overall 18-20 mm horizontally and 4-5 mm vertically in one eyelid [Fig. 4d]. The same steps are repeated for each eyelid being operated. The eye is then closed, to avoid exposure, and attention is shifted to the labial mucosa. It is important to keep the cornea lubricated throughout surgery by covering it with dispersive viscoelastic. One should avoid an inadvertent epithelial defect in an already compromised ocular surface at all costs.
Harvesting the labial mucosa: The labial mucosa should be everted with traction sutures and marked. The area to be harvested should be at least 1-2 mm away from the MCJ anteriorly, and at least 3 mm away from the frenulum posteriorly. The mucosa can be marked with a caliper and skin-marker, and the graft is usually slightly oversized so that it can be later fit to size. The edges of the graft should be marked in a biconvex fashion, with edges tapering towards each other. The margins of the marked area are incised superficially with a no. 15 blade mounted on a Bard-Parker handle. One corner of the graft is grasped with the forceps and superficial dissection can be started by insinuating a round-tipped conjunctival spring scissors. The dissection is carried out between the lamina propria of the oral mucosa and the underlying connective tissue which includes the minor salivary glands and deeper muscle. A gauze piece should be kept in the oral area so that blood does not trickle into the oral cavity. Suction is best avoided near the graft during dissection, to avoid the graft being drawn into the suction tubing. If bleeding is noted from the oral mucosal bed, one can try applying pressure with a gauze piece soaked in a diluted adrenaline solution. Alternatively, the bleeders can be cauterized. This should not be excessive to avoid thermal nerve damage.[24] The donor area is then sutured closed with interrupted or continuous interlocking 6-0 polyglactin sutures. Alternately the donor area can be left open to heal by secondary intention. The harvested graft is transferred to a bowl of balanced salt solution or Ringer‘s lactate solution, while the oral wound is closed. To ensure rapid healing and to avoid excessive scarring, the graft for each eye is obtained from each lip. In adults, grafts for all four lids can be obtained from the lower lip alone.[11] However, the amount of complications at the donor site, especially lower lip paresthesia has been reported to be higher when large amounts of mucosa have been harvested from one lip, especially the lower lip.[30,31,32,33] Hence, the authors prefer to harvest labial mucosa from both the upper and lower lips if surgery is to be performed for all four lids to avoid harvesting a large amount of mucosa from one lip. It is also important to make sure the donor site is not skewed to one side of the lip, which can be done by aligning to the mid-line landmarks such as the central incisor teeth.
Trimming of the labial mucosal graft: The mucosal graft is thinned before transplantation to facilitate revascularization and reduce the metabolic demand of the grafted tissue.[9] The graft is placed epithelial side down on the surgical drape over the patient‘s glabella because this is a firm and flat surface to work on. The edge of the graft is held with the Pierse-Hoskins forceps and the graft is thinned down by removing the excess fat and the salivary lobules from the stromal side with Westcott scissors. The graft should be kept wet throughout this dissection. Care should be taken to avoid button-holing by holding the Hoskins forceps with the left hand to stretch the tissue so that there is no folding of the tissue within the spring scissors. The middle part of the blades of the scissors should be used instead of the tips to cut. The endpoint of this trimming is when the graft is translucent and the violet/blue markings of the skin marker on the epithelial side are visible. The dictum here is: remove red (muscle or clots), remove yellow (fat globules and glands), stop at white and blue. After the cleaning up, the graft is divided into the required number of strips.
Suturing the labial mucosal graft to the lid: Each strip of the mucosal graft is then placed on the recipient bed and cut to size. Suturing is started at one end with 7-0 polyglactin sutures. One anchoring suture is placed at one end and suturing is continued towards the other edge [Fig. 4e]. Continuous interlocking sutures are preferable. During the suturing, the graft is stretched continually with the Hoskin‘s forceps in the left hand, so that the tissue does not fall short length-wise. Each suture should be first passed through the mobile graft, through the superficial tarsus and then through the skin at the excised lid margin. The needle should be directed such that it exits the skin anterior to the lashes, thus avoiding the lashes getting trapped into the sutures. The suturing need not be tight; it needs to be sufficiently tight to avoid displacement of the graft for the next 4–5 days till it gets revascularized. Excessively tight sutures can cause a bulky MMG due to pouting of tissue in between sutures, this may be a permanent complication.
Ensuring the graft is not larger than the de-epithelized bed: After the suturing is completed, the raw epithelized bed should be inspected. The area of this bed should be larger than the area occupied by the graft [Fig. 4f]. If the graft is larger than the bed, the conjunctival epithelium can grow underneath the graft, thus lifting the mucosa off the tarsal bed and delay the uptake/reperfusion of the graft causing graft necrosis. It is important to have a visible gap of about 0.5 mm between the posterior margin of the mucosal graft and the anterior edge of the excised tarsal conjunctiva. If there is edge-to-edge apposition, the mucosal graft will over-ride the tarsal conjunctiva, when the lid is reverted to its anatomical position.
Fixing the posterior edge of the graft with fibrin glue: Fibrin glue (TISSEEL kit from Baxter AG, Vienna, Austria) can be injected on the underside of the graft after holding the posterior edges of the MMG with Hoskins forceps. The posterior edge of the graft is not sutured to prevent irritation and corneal abrasions until the sutures are removed. The posterior aspect of the graft can also be sutured with 8-0 polyglactin sutures if there is no access to fibrin glue, however, this could cause corneal epithelial abrasions and hence the necessity to place a bandage contact lens (BCL) at the end of surgery and close monitoring postoperatively. Using fibrin glue also reduces the duration of the surgery. If there is no access to fibrin glue, massaging the MMG to the underlying tarsus with a blunt instrument such as a muscle hook/iris repositor will suffice. The graft needs to be sufficiently trimmed and thin for it to appose to the underlying tarsus without fibrin glue or sutures. For surgeons early in the learning curve for this surgery, it is preferable to appose the posterior edge of the graft with fibrin glue or sutures. After the graft is completely in place, the graft area and bed should be inspected to ensure that a strip of de-epithelized area on the bed at the posterior edge of the graft is present and the graft is not flush to the tarsal conjunctival epithelium. A BCL can be placed on the cornea at the end of the surgery in all cases. The authors believe that a BCL significantly reduces the postoperative discomfort that patients face after surgery, especially since patients might also have concomitant dry eye. The BCL also protects the delicate corneal epithelium in these eyes with significant ocular surface problems, and is a safe and inexpensive option.
Figure 4.
Illustrations describing the surgical steps of lid margin mucous membrane grafting. (a) Everted and properly exposed keratinized lid margin of the upper lid. (b) Marking of a rectangular area including the keratinized lid margin and 4 mm of tarsal conjunctiva excluding 4-5 mm at medial and lateral ends. (c) Dissection of entire keratinized margin with tarsal conjunctiva off the tarsus by starting at one of the vertical edges. (d) The dissected bed is usually sized 18-20 mm horizontally and 4-5 mm vertically. (e) Suturing of labial mucosal graft from one end with 7 0 polyglactin sutures. (f) After completion of suturing, area of the bed should be larger than the area occupied by graft. A more detailed description of the surgical technique can be found at https://www.youtube.com/watch?v=SzCu-LbVlhs
Postoperative course
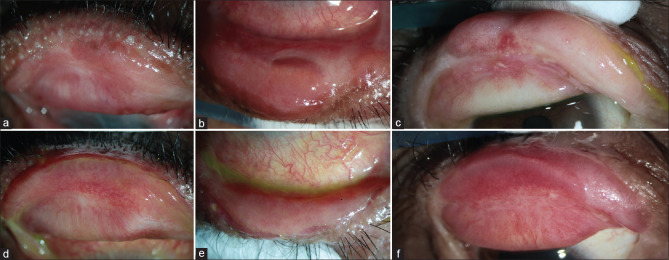
The ideal anatomical outcome post lid-margin MMG and the postoperative appearance of the MMG‘s from day 1 until day 18 is depicted in Fig. 5. Typically, the graft appears pale with varying degrees of underlying dark red to black patches [Fig. 5b] for the first couple of days, because of the blood clots in the interface and should not be mistaken as graft necrosis. Small pinpoint dilated capillaries can be observed by 3–5 days [Fig. 5c], and the graft appears bright red by the end of the first week [Fig. 5d]. Sutures can be safely removed without any risk of bleeding or dislodging the graft after 5–7 days. When the graft is uniformly reperfused and dilated vessels return to their normal caliber, the graft appears uniformly pink, usually after 10–14 days [Fig. 5e]. Scleral contact lens use can be resumed after the sutures are out. Table 1 mentions the entire postoperative regimen that is followed by the authors until postoperative 2 weeks. Regarding the use of topical steroids post-MMG, in the study by Iyer et al., postoperative topical steroids were not used,[11] while Fu et al. recommended the use of topical steroids which were tapered over a period of 1–2 months postoperatively.[12] The authors believe that topical steroids are not required routinely in all eyes post-MMG; however, the authors recommend the use of topical steroids in inflamed eyes with vascularized corneas.
Figure 5.
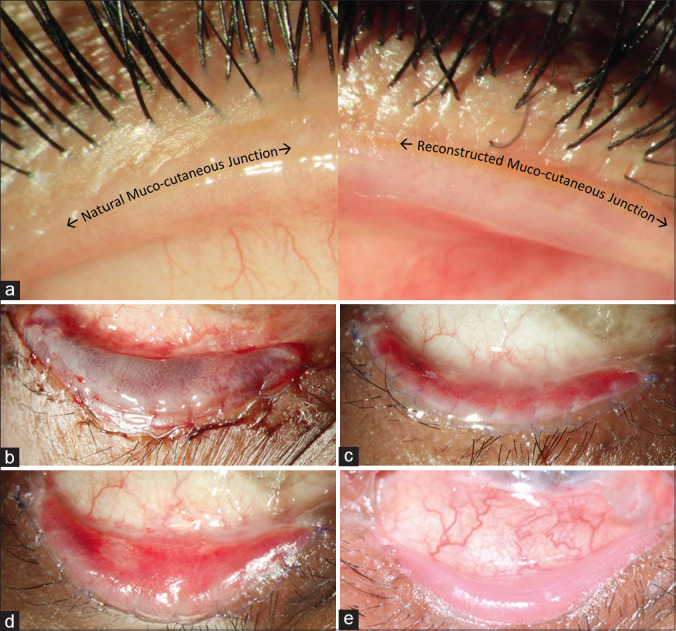
Ideal anatomical outcomes and postoperative appearance of lid-margin MMG. (a) Normal anatomy of the mucocutaneous junction (MCJ) on the left and reconstructed MCJ post MMG on the right. (b-e) Postoperative appearance of the MMG in the lower eyelid of the same eye in a patient with chronic sequelae of SJS. (b) first postop day appearance – petechial hemorrhages seen in the mucosal graft. (c) postoperative day 5 – reddish-pink appearance; (d) postoperative day 8 – vascularized graft. (e) postoperative day 18 - healthy pink labial mucosal graft after suture removal
Table 1.
Postoperative regimen post lid-margin mucous membrane grafts
| Duration after surgery | Operated eye | Oral mucosa | Systemic |
|---|---|---|---|
| Immediately after surgery on the same day | Eye is patched No topical medications |
Anesthetic lip gel (Choline salicylate) before meals Chlorhexidine mouthwash after meals (to spit after mouthwash) |
Tab Paracetamol 650 mg SOS (to ensure the patient is not allergic to Paracetamol) Inj. Hydrocortisone 3-5 mg/ kg divided in 2 doses (not necessary for all patients, mainly given if expected tissue edema, to consult anesthetist before administering to avoid double dosage) |
| Postop Day 1 | Topical steroid-antibiotic ointment 2 times/day until the sutures and bandage contact lens (BCL) are removed Lubricants to be continued, in cases of severe dry eye, same dose as preoperative For patients who require a higher intensity of topical steroids, especially for those with vascularized corneas, instead of a combination, topical steroids and topical antibiotics can be given separately. |
To continue the above regimen for the oral mucosa for 2 weeks | Oral systemic steroids in tapering doses for the first 3-4 weeks (in children <8 years old) |
| At postop 2 weeks | Remove sutures and bandage contact lens (BCL) Topical steroid-antibiotic ointment to be continued at night time Topical antibiotics can be stopped after the BCL is removed Sutures can be removed anytime from postop 5 days until 2 weeks Topical steroids (if started postoperatively) to be tapered over the next 6 weeks Continue topical lubricants |
Examine oral mucosa and stop the oral anesthetic gel and mouthwash |
Mechanism of Action
In eyes with LMK sequelae, MMG helps in many ways. It provides a smooth lid margin thus preventing further damage to the ocular surface, especially the limbal epithelial stem cells.[34,35] If performed within the critical window of opportunity, not only can it maintain corneal clarity and vision but also help in ameliorating corneal vascularization and scarring.[10,11,12,14,15,16,28,36] It reduces irritation and photophobia, improves patient comfort.[10,11,12] It acts as a barrier to prevent posterior migration of MCJ and further keratinization. It enables the patient to use scleral lenses, this is especially relevant in the pediatric age group.[15,16] It also improves the milieu of the ocular surface for future interventions such as allogeneic SLET[37] and keratoprosthesis.
Gurumurthy et al. showed in their study that post MMG, levels of pro-inflammatory cytokines on the ocular surface declined with a concomitant improvement in anti-inflammatory cytokines.[38] Whether performing MMG reduces the component of dry eye in these eyes is disputed. A previous study by Iyer et al. pointed to a reduction in dry eye post-MMG performed for LMK in SJS with improvement of Schirmer scores and attributed this to the presence of goblet cells in the mucosal graft.[11] However, there is little evidence to support the presence of goblet cells in the oral/lip mucosa.[9] While it is true that wetting does improve occasionally, the reasons for these are unknown. It should neither be expected nor promised to the patient as the goal of surgery.
Clinical Efficacy and Complications
Clinical efficacy
Outcomes of MMG are difficult to quantify objectively. The most important function of the MMG is to bring a halt to the deterioration of the ocular surface. The most important outcome post-MMG is anatomical where the keratinization may recur but stops abruptly at the edge of the MMG. Functional outcomes post-MMG are mainly in the form of maintenance or improvement of BCVA [Fig. 6]. Other subjective outcomes include improvement in symptoms and signs, these are further elucidated in Table 2. Table 2 also shows the outcomes in the previously published literature of MMG in eyes with different etiologies, the most common being SJS/TEN.[10,12,14,16]
Figure 6.
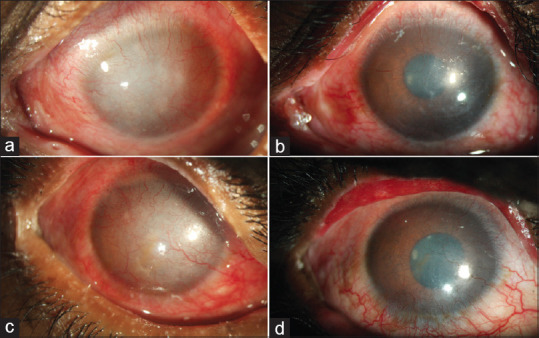
Functional outcomes post-MMG. Severe ocular surface inflammation, corneal epithelial haze, irregularity, and superficial vascularization due to lid margin keratinization in the left (a) and right (c) eyes of a 25-year old man with a 12-year history of ocular symptoms after SJS. Post-MMG surgery, the ocular surface and cornea show dramatic recovery with remarkable improvement in corneal clarity in both eyes (b, d) leading to significantly improved uncorrected and scleral lens corrected visual acuity
Table 2.
Literature review of studies where lid-margin mucous membrane graft (MMG) was performed in eyes with lid-related keratopathy, with graft harvested from the labial mucosa (largest studies from single centers included)
| Author | Country | Year | Number of patients/number of eyes | Overall Indications (number of eyes) | Follow-up post MMG | Outcomes in eyes with SJS/TEN | Complications/Repeat MMG required | ||
|---|---|---|---|---|---|---|---|---|---|
| Anatomical changes in the lids and cornea | Functional improvement in terms of visual acuity (VA) | Functional improvement in terms of improvement in symptoms and signs | |||||||
| McCord et al.[10] | U.S.A | 1983 | 17 eyes of 17 patients (16 adult, 1 pediatric) | SJS/TEN (8), MMP (4), Trachoma (1), CB (1), Miscellaneous causes (3) | 24 months | Details NA | Improvement in VA noted (further details NA) | Symptomatic relief of pain, irritation, decrease in redness and reflex tearing in 7/8 eyes | Details NA |
| Fu et al.[12] | U.S.A | 2011 | 22 eyes of 19 patients (14 adult, 5 pediatric) | SJS/TEN (12), MMP (4), CB (3), Postsurgical scarring (3) | 16.2±7 months (mean, range 6-29 months) | Keratinization corrected in all 12 eyes, Distichiasis corrected in 5/7 eyes, Trichiasis corrected in 3/5 eyes, Incomplete closure corrected in 4/5 eyes; PED resolved in 3/3 eyes, SPK’s resolved in ½ eyes, corneal vascularization resolved in 1/1 eye | Improvement of VA in 8/12 eyes | Foreign body sensation reduced in 6/8 eyes, Pain reduced in 2/2 eyes, Photophobia reduced in 6/7 eyes, Discharge reduced in 3/3 eyes, Burning reduced in 3/3 eyes | Dislodgement of graft in one eyelid, corrected |
| Iyer et al.[14] | India | 2016 | 393 eyes of 230 patients | SJS/TEN | 3 months of minimum follow-up | Improvement of VA in 129/393 eyes | Corneal fluorescein staining improved in 130/393 eyes; Schirmer I test improved in 111/393 eyes. | Recurrence of keratinization along the edges of the grafted mucosa causing symptoms and/or corneal staining in 33 eyes (8.4%) over follow-up. Revision MMG performed. | |
| Shanbhag et al.[16] | India | 2020 | 100 eyes (81 adult, 19 pediatric) | SJS/TEN | 60 months of median follow-up | Details NA | Improvement of VA noted; median VA improvement from 20/100 to 20/30 in 19 eyes (pediatric); median VA improvement from 20/100 to 20/60 in 81 eyes (adult) | Details NA | Details NA |
SJS=Stevens-Johnson syndrome; TEN=Toxic epidermal necrolysis; MMP=Mucous membrane pemphigoid; CB=Chemical burns; NA=Not available; SPK=Superficial punctate keratitis; PED=Persistent epithelial defect; [We did not include studies where mucous membrane grafts were performed solely for specific etiologies such as studies with the only etiology being cicatricial entropion or advanced vernal palpebral keratoconjunctivitis)]
Complications
None of the previous studies have reported major intra-operative or postoperative complications post-MMG performed for LMK in SJS, in the graft or the donor area.[10,11,12,15,16] However, Shore et al., in their study where MMG was performed for cicatricial entropion post MMP after adequate systemic immunosuppression reported complications that are similar to complications we have listed.[29] The intra-operative complications, mechanism, prevention, and management are mentioned in Table 3. The postoperative complications, mechanism, prevention, and management are mentioned in Table 4. Clinical preoperative and postoperative images of repeat MMG in eyes with inappropriately performed or improperly positioned MMG which were referred to us for repeat surgery or scleral lenses are shown in Fig. 7. The complications at the donor site are generally self-limiting. The authors have not seen any long-term complications at the donor site in their patients. However, the complications at the donor site that have been reported in literature are scarring, retention cysts at suturing site, lip contracture, inversion of the vermillion, lower lip paresthesia due to damage to the mental nerve, persistent intra-oral discomfort, and altered salivary flow (temporary).[31,33,39] Lower lip paresthesia may occur when the donor site is allowed to heal secondarily as the nerves have to traverse a greater distance compared to donor sites which are primarily closed with sutures.[33] Infection of the graft is a very rare complication post-MMG.
Table 3.
Intra-operative complications of lid-margin mucous membrane grafts
| Site | Complication | Mechanism | Prevention | Management |
|---|---|---|---|---|
| Donor site | Excessive bleeding | Occurs secondary to deeper dissection, injuring muscle. Could increase further after patient is out of anesthesia as patient is not in a hypotensive state anymore | Precautions during general anesthesia | Pressure with gauze; Use of light cautery Clear the oral cavity of the blood clots, Admit the patient, because gag reflex is absent for the first view hours and aspiration of blood could occur; suture the wound with 6-0 polyglactin |
| Graft related | Button-holing of the graft | During the step to thin the graft, excessive thinning could lead to inadvertent button-holing | While thinning the graft, keep the hinge of the scissors flat over the graft instead of the sharp blades | Suture the gap in the tissue with 8-0 polyglactin; if the button-hole is towards the central portion of the graft, can ensure that division of the graft into parts is through the button-hole |
| Under-sized graft horizontally (undersized graft vertically is not a problem unless the graft is <4 mm wide) [Fig. 7] | When the measurement of the raw bed is not done accurately; or miscalculation of the tissue required, ideal tissue size is 20 mm by 4-5 mm for each lid | Measure the raw de-epithelized bed on the lid margin, the oral mucosa should be marked and then excised accordingly | More tissue should be harvested from the oral mucosa and should be sutured to areas which need addressing of keratinization. Repeat MMG may be required in some eyes [Fig. 7] | |
| Operated eye | Inappropriate positioning of the graft with postoperative posterior MMG [Fig. 7] | Initial cut too posterior to the gray line | Initial cut should be at the gray line, and if gray line is not discernable, should be just posterior to the lash line | If recognized intra-operatively, can address this at the same sitting by incising the lid margin at the right position. If recognized later, may cause early recurrence of LMK, which may need early repeat MMG [Fig. 7] |
MMG=Mucous membrane graft; LMK=Lid margin keratinization
Table 4.
Postoperative complications post lid-margin MMG
| Complication | Mechanism | Prevention | Management |
|---|---|---|---|
| Displacement of graft (immediate postop) | a) Inadequate or excessive fibrin glue on the posterior aspect of MMG | Judiciously use fibrin glue, keep the graft sufficiently thin | May need to repeat surgical procedure immediately to attach the graft, could lead to graft necrosis if not handled on time |
| b) Excessively thick graft | |||
| Graft necrosis (immediate postop) | Graft larger than the de-epithelized bed; areas of the intact epithelium in the raw bed | Area of de-epithelized bed should be larger than the graft (intra-operatively if recognized, the graft can be trimmed down horizontally or one horizontal strip of the tarsal conjunctival epithelium can be excised) | Needs repeat MMG |
| Ectropion of the lower lid due to bulky MMG [Fig. 7] | Oversized and thick graft with residual fat, large MMG (increased vertical length) in the lower lid | The graft should be sufficiently thinned; even if excessive conjunctival epithelium excised in the lower lid | Since this is a cosmetic concern, may need debulking of the MMG or repeat MMG [Fig. 7] |
| Irregular and bumpy graft [Fig. 7] | Residual fat in the graft, tight suturing, extra stromal tissue in the graft | The graft should be sufficiently thinned with the removal of excessive stroma and fat | May need repeat MMG if the MMG is not performing its function adequately [Fig. 7] |
| Break-through trichiasis/distichiasis | Graft coverage of a lash follicle with subsequent lash growth, could occur at posterior edge of the graft or through the graft | Pretreat trichiatic/distichiatic lashes with electrolysis (especially in patients with extensive preoperative trichiasis/distichiasis) | If extensive, a spot treatment with cryotherapy (double freeze thaw). If extensive with a row of lashes, may need excision of MMG, cryotherapy and repeat MMG. If few in number, electrolysis can be performed |
| Entropion post MMG | During excision of the tarsal conjunctival epithelium, deeper dissection involving tarsal tissue | Keep the dissection superficial involving tarsal conjunctiva only | Entropion correction |
| Recurrent hordeolum/chalazion | Inflammation and blockage of meibomian gland openings otherwise or by the MMG | Express all meibomian glands intraoperatively with a blunt instrument, after keratinized lid margins excised | Regular warm compresses |
| Keratinization of the graft surface/Keratinization at the posterior edge of the graft | Inadequate removal of keratinized epithelium intra-operatively/due to a small and thin graft | Excise all the keratinized tarsal conjunctival epithelium, ensure adequate coverage of the graft over the entire de-epithelized area | Scleral contact lenses/repeat MMG with the removal of all the keratinized epithelium on the tarsus and the lid margin |
MMG=Mucous membrane graft
Figure 7.
Clinical preoperative and postoperative images of repeat mucous membrane grafts (MMG) in eyes with inappropriately performed or improperly positioned MMG. (a-c) Preoperative lid MMG‘s performed elsewhere with a) Posteriorly placed upper lid MMG, partially retained and absent centrally; (b) Bulky lower lid MMG causing ectropion; (c) Bulky and irregular upper lid MMG decentered medially. (d-f) Postoperative lid MMG‘s after repeat MMG‘s were performed in the same eyes – central, appropriately positioned and thin MMG‘s
Summary
The authors have previously reported, that two-thirds of patients presented more than a year after acute SJS/TEN, 99% without prior AMT, with low vision or blindness in 60% of eyes.[15] Hence, the authors consider that every ophthalmologist/cornea specialist/oculoplasty specialist should learn how to identify sequelae such as LMK in the eyes of patients with SJS/TEN and also due to other rarer etiologies. Next, they should equip themselves with the correct armamentarium in the form of knowledge and skills for performing MMG‘s. This can be achieved by observing/attending surgical workshops organized by institutes/specialists who perform this technique routinely. This should further be supplemented by knowledge of scleral lenses and their indications in these eyes. If the treating ophthalmologist sees changes such as LMK and does not yet possess the skills for performing a MMG, they should ideally refer these cases at the earliest to a specialist trained at performing this surgery.
The non-surgical approach for LMK that has also shown good results is scleral lenses which work by preventing mechanical contact of the keratinized epithelium of the tarsal conjunctiva on the cornea.[15,16,40,41,42] Scleral lenses also maintain a reservoir of fluid intact between the lens and the cornea, which helps in keeping corneas in an otherwise compromised ocular surface, healthy. However, scleral lenses cannot be used throughout the day and when scleral lenses are not used, keratopathy can still progress secondary to keratinized epithelium abrasively rubbing on the cornea. In fact, it has been shown that although scleral lenses alone may not be as effective as MMG, particularly in children, these two modalities have a synergistic and complementary effect in cases of LMK.[15,16] While the MMG helps in improving symptoms and enhances patient comfort, scleral lenses improve the visual quality by compensating for the surface irregularity.
This review aimed to guide ophthalmologists who would like to perform this surgery to help their patients, but do not know where to begin. This review was intentionally written in a detailed and descriptive way to help ophthalmology residents, fellowship trainees, general ophthalmologists, cornea specialists, and oculoplasty specialists to understand the indications, surgical technique, and clinical outcomes of this technique based on the experience of the authors. However, all beginners who are interested in learning this technique should actively seek out help and pursue short clinical rotations at high-volume centers with experienced ocular surface surgeons. This will ensure the best possible treatment for their patients and thus go a long way in reducing the rate of blindness due to such dreadful corneal conditions.
Financial support and sponsorship
Hyderabad Eye Research Foundation (HERF)
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank Dr. Chirag Mittal (Centre for Sight, Delhi) for providing us with Fig. 1c, and Dr. Kiranmaye Turaga (L.V. Prasad Eye Institute, Visakhapatnam) for providing us with Fig. 1d for this review.
References
- 1.Knop E, Korb DR, Blackie CA, Knop N. The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Dev Ophthalmol. 2010;45:108–22. doi: 10.1159/000315024. [DOI] [PubMed] [Google Scholar]
- 2.Knop E, Knop N, Zhivov A, Kraak R, Korb DR, Blackie C, et al. The lid wiper and muco-cutaneous junction anatomy of the human eyelid margins: An in vivo confocal and histological study. J Anat. 2011;218:449–61. doi: 10.1111/j.1469-7580.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korb DR, Greiner JV, Herman JP, Hebert E, Finnemore VM, Exford JM, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. CLAO J. 2002;28:211–6. doi: 10.1097/01.ICL.0000029344.37847.5A. [DOI] [PubMed] [Google Scholar]
- 4.Efron N, Brennan NA, Morgan PB, Wilson T. Lid wiper epitheliopathy. Prog Retin Eye Res. 2016;53:140–74. doi: 10.1016/j.preteyeres.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Maumenee AE. Keratitis secondary to keratinization of the tarsal conjunctiva. Am J Ophthalmol. 1956;41:477–87. doi: 10.1016/0002-9394(56)91261-2. [DOI] [PubMed] [Google Scholar]
- 6.Srividya G, Angayarkanni N, Iyer G, Srinivasan B, Agarwal S. Altered retinoid metabolism gene expression in chronic Stevens-Johnson syndrome. Br J Ophthalmol. 2019;103:1015–23. doi: 10.1136/bjophthalmol-2018-312849. [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag SS, Basu S. Controversial role of retinoids in ocular surface disease. Br J Ophthalmol. 2019;103:1013–4. doi: 10.1136/bjophthalmol-2019-314241. [DOI] [PubMed] [Google Scholar]
- 8.Mai C, Bertelmann E. Oral mucosal grafts: Old technique in new light. Ophthalmic Res. 2013;50:91–8. doi: 10.1159/000351631. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg DA, Tham V, Hardin N, Antley C, Cohen AJ, Hunt K, et al. Eyelid mucous membrane grafts: A histologic study of hard palate, nasal turbinate, and buccal mucosal grafts. Ophthalmic Plast Reconstr Surg. 2007;23:211–6. doi: 10.1097/IOP.0b013e318050d2d1. [DOI] [PubMed] [Google Scholar]
- 10.McCord CD, Jr, Chen WP. Tarsal polishing and mucous membrane grafting for cicatricial entropion, trichiasis and epidermalization. Ophthalmic Surg. 1983;14:1021–5. [PubMed] [Google Scholar]
- 11.Iyer G, Pillai VS, Srinivasan B, Guruswami S, Padmanabhan P. Mucous membrane grafting for lid margin keratinization in Stevens-Johnson syndrome: Results. Cornea. 2010;29:146–51. doi: 10.1097/ICO.0b013e3181ae2691. [DOI] [PubMed] [Google Scholar]
- 12.Fu Y, Liu J, Tseng SC. Oral mucosal graft to correct lid margin pathologic features in cicatricial ocular surface diseases. Am J Ophthalmol. 2011;152:600–8 e1. doi: 10.1016/j.ajo.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Sharma N, Basu S, Iyer G, Ueta M, Sotozono C, et al. Stevens-Johnson syndrome: The role of an ophthalmologist. Surv Ophthalmol. 2016;61:369–99. doi: 10.1016/j.survophthal.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Iyer G, Srinivasan B, Agarwal S, Pillai VS, Ahuja A. Treatment modalities and clinical outcomes in ocular sequelae of Stevens-Johnson syndrome over 25 years--A paradigm shift. Cornea. 2016;35:46–50. doi: 10.1097/ICO.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: Long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17–28. doi: 10.1016/j.ajo.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Shanbhag SS, Shah S, Singh M, Bahuguna C, Donthineni PR, Basu S. Lid-related keratopathy in Stevens-Johnson syndrome: Natural course and impact of therapeutic interventions in children and adults. Am J Ophthalmol. 2020 doi: 10.1016/j.ajo.2020.07.006. doi: 10.1016/j.ajo. 2020.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Catt CJ, Hamilton GM, Fish J, Mireskandari K, Ali A. Ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Am J Ophthalmol. 2016;166:68–75. doi: 10.1016/j.ajo.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol. 2018;188:99–103. doi: 10.1016/j.ajo.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Macfaul PA, Bedford MA. Ocular complications after therapeutic irradiation. Br J Ophthalmol. 1970;54:237–47. doi: 10.1136/bjo.54.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karp LA, Streeten BW, Cogan DG. Radiation-induced atrophy of the Meibomian gland. Arch Ophthalmol. 1979;97:303–5. doi: 10.1001/archopht.1979.01020010155013. [DOI] [PubMed] [Google Scholar]
- 21.Shanbhag SS, Chanda S, Donthineni PR, Sane SS, Priyadarshini SR, Basu S. Clinical clues predictive of Stevens-Johnson syndrome as the cause of chronic cicatrising conjunctivitis. Br J Ophtalmol. 2020;104:1005–9. doi: 10.1136/bjophthalmol-2019-314928. [DOI] [PubMed] [Google Scholar]
- 22.Mondino BJ, Brown SI, Lempert S, Jenkins MS. The acute manifestations of ocular cicatricial pemphigoid: Diagnosis and treatment. Ophthalmology. 1979;86:543–55. doi: 10.1016/s0161-6420(79)35486-0. [DOI] [PubMed] [Google Scholar]
- 23.Barbagli G, Balò S, Montorsi F, Sansalone S, Lazzeri M. History and evolution of the use of oral mucosa for urethral reconstruction. Asian J Urol. 2017;4:96–101. doi: 10.1016/j.ajur.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goel A, Dalela D, Sinha RJ, Sankhwar SN. Harvesting buccal mucosa graft under local infiltration analgesia--mitigating need for general anesthesia. Urology. 2008;72:675–6. doi: 10.1016/j.urology.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Ucar F, Cetinkaya S. The results of preoperative topical brimonidine usage in pterygium surgery. J Ocul Pharmacol Ther. 2020;36:234–7. doi: 10.1089/jop.2019.0085. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Kim CY, Seong GJ, Han SH. Effect of prophylactic brimonidine instillation on bleeding during strabismus surgery in adults. Am J Ophthalmol. 2007;144:469–70. doi: 10.1016/j.ajo.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Kekunnaya R, Sachdeva V, Rao HL. Strabismus surgery hemostasis. Ophthalmology. 2012;119:649–50. doi: 10.1016/j.ophtha.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - A comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. 2016;14:168–88. doi: 10.1016/j.jtos.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Shore JW, Foster CS, Westfall CT, Rubin PAD. Results of buccal mucosal grafting for patients with medically controlled ocular cicatricial pemphigoid. Ophthalmology. 1992;99:383–95. doi: 10.1016/s0161-6420(92)31962-1. [DOI] [PubMed] [Google Scholar]
- 30.Neuhaus RW, Baylis HI, Shorr N. Complications at mucous membrane donor sites. Am J Ophthalmol. 1982;93:643–6. doi: 10.1016/s0002-9394(14)77381-7. [DOI] [PubMed] [Google Scholar]
- 31.Kamp S, Knoll T, Osman M, Hacker A, Michel MS, Alken P. Donor-site morbidity in buccal mucosa urethroplasty: Lower lip or inner cheek? BJU Int. 2005;96:619–23. doi: 10.1111/j.1464-410X.2005.05695.x. [DOI] [PubMed] [Google Scholar]
- 32.Grixti A, Malhotra R. Oral mucosa grafting in periorbital reconstruction. Orbit. 2018;37:411–28. doi: 10.1080/01676830.2018.1435693. [DOI] [PubMed] [Google Scholar]
- 33.Markiewicz MR, DeSantis JL, Margarone JE, 3rd, Pogrel MA, Chuang SK. Morbidity associated with oral mucosa harvest for urological reconstruction: An overview. J Oral Maxillofac Surg. 2008;66:739–44. doi: 10.1016/j.joms.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Di Pascuale MA, Espana EM, Liu DT, Kawakita T, Li W, Gao YY, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–12. doi: 10.1016/j.ophtha.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa Y, Ueta M, Fukuoka H, Inatomi T, Yokota I, Teramukai S, et al. Long-term progression of ocular surface disease in Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea. 2020;39:745–53. doi: 10.1097/ICO.0000000000002263. [DOI] [PubMed] [Google Scholar]
- 36.Iyer G, Srinivasan B, Agarwal S, Kamala Muralidharan S, Arumugam S. Comprehensive approach to ocular consequences of Stevens Johnson Syndrome-The aftermath of a systemic condition. Graefes Arch Clin Exp Ophthalmol. 2014;252:457–67. doi: 10.1007/s00417-014-2568-8. [DOI] [PubMed] [Google Scholar]
- 37.Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. 2019;67:1265–77. doi: 10.4103/ijo.IJO_117_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurumurthy S, Iyer G, Srinivasan B, Agarwal S, Angayarkanni N. Ocular surface cytokine profile in chronic Stevens-Johnson syndrome and its response to mucous membrane grafting for lid margin keratinisation. Br J Ophthalmol. 2018;102:169–76. doi: 10.1136/bjophthalmol-2017-310373. [DOI] [PubMed] [Google Scholar]
- 39.Jang TL, Erickson B, Medendorp A, Gonzalez CM. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urology. 2005;66:716–20. doi: 10.1016/j.urology.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 40.Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology. 2015;122:248–53. doi: 10.1016/j.ophtha.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Tougeron-Brousseau B, Delcampe A, Gueudry J, Vera L, Doan S, Hoang-Xuan T, et al. Vision-related function after scleral lens fitting in ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009;148:852–9.e2. doi: 10.1016/j.ajo.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Rao R, Jacobs DS, Saeed HN. Prosthetic replacement of the ocular surface ecosystem treatment for ocular surface disease in pediatric patients with Stevens-Johnson syndrome. Am J Ophthalmol. 2019;201:1–8. doi: 10.1016/j.ajo.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]