Abstract
Background and Objectives
ABO blood group may affect risk of SARS‐CoV‐2 infection and/or severity of COVID‐19. We sought to determine whether IgG, IgA and neutralizing antibody (nAb) to SARS‐CoV‐2 vary by ABO blood group.
Materials and Methods
Among eligible convalescent plasma donors, ABO blood group was determined via agglutination of reagent A1 and B cells, IgA and IgG were quantified using the Euroimmun anti‐SARS‐CoV‐2 ELISA, and nAb titres were quantified using a microneutralization assay. Differences in titre distribution were examined by ABO blood group using non‐parametric Kruskal–Wallis tests. Adjusted prevalence ratios (aPR) of high nAb titre (≥1:160) were estimated by blood group using multivariable modified Poisson regression models that adjusted for age, sex, hospitalization status and time since SARS‐CoV‐2 diagnosis.
Results
Of the 202 potential donors, 65 (32%) were blood group A, 39 (19%) were group B, 13 (6%) were group AB, and 85 (42%) were group O. Distribution of nAb titres significantly differed by ABO blood group, whereas there were no significant differences in anti‐spike IgA or anti‐spike IgG titres by ABO blood group. There were significantly more individuals with high nAb titre (≥1:160) among those with blood group B, compared with group O (aPR = 1·9 [95%CI = 1·1–3·3], P = 0·029). Fewer individuals had a high nAb titre among those with blood group A, compared with group B (aPR = 0·6 [95%CI = 0·4‐1·0], P = 0·053).
Conclusion
Eligible CCP donors with blood group B may have relatively higher neutralizing antibody titres. Additional studies evaluating ABO blood groups and antibody titres that incorporate COVID‐19 severity are needed.
Keywords: ABO group, convalescent plasma, COVID‐19, SARS‐CoV‐2, titre
Introduction
Since its first description in China in December 2019, over 66 million cases of severe acute respiratory syndrome‐associated coronavirus 2 (SARS‐CoV‐2) infection – the cause of coronavirus‐19 disease (COVID‐19) – have been reported, spanning 191 countries or territories and accounting for over 1·5 million deaths [1]. Over a fifth (>14 million) of those cases have been reported in the United States.
Amid established sociodemographic (e.g. older age, male sex, racial and ethnic minorities) and clinical (diabetes, hypertension, smoking) risk factors for COVID‐19 severity, the possibility of an association with ABO blood group was raised early in the pandemic. Specifically, blood group A individuals were suggested to be at greater risk of infection, while blood group O was observed to be protective [2, 3, 4, 5]. Nonetheless, while over‐representation of group A (and under‐representation of group O) has been observed among COVID‐19 cases, the findings have been mixed with respect to clinical outcomes [6, 7, 8, 9].
Blood groups have long been suspected as playing a part in the pathogenesis of infectious diseases, spanning malaria to parvovirus B19 [10]. An association between SARS‐CoV‐2 risk (i.e. susceptibility and/or disease severity) and ABO blood group is plausible, yet remains uncertain. Prior to the COVID‐19 pandemic, a retrospective study of healthcare workers in Hong Kong reported group O participants to be less likely to become infected with SARS‐CoV – a closely related virus to SARS‐CoV‐2 – than non‐O participants [11]. A subsequent study showed that anti‐A isoagglutinins were capable of inhibiting the S protein/angiotensin‐converting enzyme 2 interaction suggesting a role blocking the virus from its receptor [12]. It has also been suggested that antibody subclass may be important with protection ascribed to anti‐A IgG rather than IgM [13]. Of note, anti‐A IgG is more common in group O individuals as compared to those of other non‐A groups. In short, anti‐A – rather than blood group itself – may be the central factor [14].
Given the plausibility of a role in immunopathogenesis of COVID‐19, we sought to assess the association between ABO blood group and SARS‐CoV‐2 antibody titres (IgA, IgG and neutralizing antibodies [nAbs]) in eligible COVID‐19 convalescent plasma (CCP) donors. Of note, transfusion of plasma from convalescent individuals (i.e. 'CCP') has emerged as a leading therapy for COVID‐19 [15, 16, 17, 18, 19, 20]. If there is a difference in titre by ABO type, it could potentially be exploited through preferential recruitment of CCP donors of certain types and/or selective use for manufacture of hyperimmune globulin. This offered another rationale for pursuing this study.
Materials and methods
Study participants
The study population comprised individuals who were deemed to be eligible to donate COVID‐19 convalescent plasma at the beginning of the pandemic in the United States (i.e. prior to FDA requirements for specific titres). Individuals aged at least 18 years who had a history of COVID‐19 as confirmed by a positive molecular test for SARS‐CoV‐2 were eligible to participate in the study. Recruitment was undertaken using a combination of self‐identification (in response to advertising or media postings) and referral from healthcare providers. Initial screening was conducted telephonically: individuals were informed that they needed to satisfy standard eligibility criteria for blood donation. Notable exclusion criteria included pregnancy in the preceding six weeks and/or an established diagnosis or risk factors for transfusion‐transmitted infections (notably HIV, hepatitis B virus or hepatitis C virus). Those who passed the initial telephonic screening were invited to participate in the study. Basic demographic information (age, sex, hospitalization with COVID‐19) was collected, and the original diagnosis of SARS‐CoV‐2 infection was confirmed by medical chart review or sharing of source documentation. Enrolment was performed under full informed consent after which ~25 ml of whole blood was collected in ACD tubes. The samples were separated into plasma and peripheral blood mononuclear cells within 12 h of collection. The plasma samples were immediately frozen at −80°C. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board prior to initiation.
ABO testing
Manual reverse group testing (i.e. determination of group A and group B antibodies) of the subjects’ samples was performed in accordance with the AABB (formerly American Association of Blood Banks) procedure for manual, tube‐based testing with A1 and B cells [21]. Agglutination reactivity was graded from 0 to 4+. Agglutination reactions of weak to 2 + were verified by repeat testing, of which all samples confirmed the same ABO blood group.
IgG, IgA and nAb titres
IgA, IgG and nAbs were quantified as previously described [22]. Briefly, Euroimmun anti‐SARS‐CoV‐2 IgG and IgA ELISAs (Mountain Lakes, NJ) for the S1 domain of spike protein were utilized per the manufacturer’s instructions. The optical density (OD) of the sample divided by calibrator provided arbitrary unit ratio (A.U.) that ≥1·1 were considered positive and ≥0·8 to <1·1 were considered indeterminate. Continuous AU values were interpreted as anti‐SARS‐CoV‐2 IgG and IgA titre levels. Quantification of nAb titres against 100 fifty per cent tissue culture infectious doses (TCID50) was performed using a microneutralization (NT) assay. nAb area under the curve (AUC) values were estimated using the exact number of wells protected from infection at every plasma dilution; samples that had no NT activity were assigned an arbitrary value of one‐half of the lowest nAb AUC. The overall distribution of nAb AUC values in this sample was a median of 60 (interquartile range [IQR]: 10, 150). For analytic purposes and consistency with our previous analyses using early recommendations by the FDA, nAb AUC values ≥160 were considered to indicate high neutralization potency [23, 24].
Statistical analysis
Characteristics of the study population were examined overall and stratified by ABO blood group using descriptive statistics. The primary study outcome was nAb titres to SARS‐CoV‐2 (AUC value); IgA and IgG antibody levels to the spike‐1 protein of SARS‐CoV‐2 (A.U.) were examined as secondary outcomes. Continuous titre outcome measures were log2‐transformed to approximate normal distributions. Differences in the distribution of titre outcome measures were examined by ABO blood group using global non‐parametric Kruskal–Wallis tests.
The association of ABO blood group with nAb titres was further examined using univariable and multivariable ordinary least‐squares linear regression. To assess whether ABO blood group is an independent correlate of nAb titres, the primary multivariable model included all covariates that have previously been shown to be determinants of SARS‐CoV‐2 antibody responses: age, sex, hospitalization status and time since first PCR‐positive test date for SARS‐CoV‐2 [22]. To further assess the primary outcome, the association between blood type and a high neutralizing antibody titre AUC value (≥160) was also examined, similar to previous investigations [24].For this analysis, prevalence ratios and adjusted prevalence ratios (aPR) were estimated by univariable and multivariable Poisson regression models with robust variance. The multivariable Poisson model included all covariates previously described.
In our conceptual framework, race/ethnicity was not considered as a potential covariate since it is a social construct and we are unaware of existing evidence that indicates it is a determinant of SARS‐CoV‐2 antibody responses. However, since blood type is linked to race/ethnicity, a sensitivity analysis was performed that included adjustment for race/ethnicity (White vs. all other races due to sparse data). A likelihood‐ratio test was used to assess whether inclusion of this race/ethnicity variable improved model fit to the data as compared to the multivariable linear regression model used in the primary analysis. The Akaike’s information criterion (AIC) was also compared between the multivariable models. Since the majority of the sample population was White, a separate sensitivity analysis was also performed restricted to White donors. Finally, since this sample is selected on those who recovered from COVID‐19 and hospitalization status may potentially be influenced by ABO type, a sensitivity analysis was conducted restricted to donors who were known to not have a history of hospitalization due to COVID‐19 (i.e. mild/moderate cases).
All P values are two‐sided. Analyses were performed in Stata/MP, version 15.1 (StataCorp, College Station, TX, USA).
Results
A total of 202 unique study participants were evaluated (Table 1). Overall, at the time of sample collection, the median number of days since PCR + nasal pharyngeal swab was 46 days (interquartile range [IQR], 39–56 days). The median age was 43 years (IQR: 32–56), and 53% were male; 76% were White. A total of 15 (7%) reported prior hospitalization for COVID‐19 (Table 1). Of the study population, 85 (42%) were blood group O, 65 (32%) were blood group A, 39 (19%) were blood group B, and 13 (6%) were blood group AB. Table 1 also provides sociodemographic and clinical characteristics of the study population stratified by ABO blood group. Of note, the median age was 48 (IQR: 34–57) years among donors with blood group A, 47 (IQR: 36–63) years among donors with blood group B, 43 (32–58) years among donors with blood group AB and 39 (IQR = 30–50) years among donors with blood group O.
Table 1.
Characteristics of the study population overall and stratified by ABO blood group
| Characteristic | Overall (n = 202) | Blood group | |||
|---|---|---|---|---|---|
| A (n = 65) | B (n = 39) | AB (n = 13) | O (n = 85) | ||
| Median age (IQR), years | 43 (32–56) | 48 (34–57) | 47 (36–63) | 43 (32–58) | 39 (30–50) |
| Age group, years | |||||
| 18–29 | 40 (20%) | 11 (17%) | 5 (13%) | 3 (23%) | 21 (25%) |
| 30–39 | 42 (21%) | 10 (15%) | 7 (18%) | 3 (23%) | 22 (26%) |
| 40–49 | 43 (21%) | 13 (20%) | 11 (28%) | 1 (8%) | 18 (21%) |
| 50–59 | 40 (20%) | 17 (26%) | 4 (10%) | 4 (31%) | 15 (18%) |
| ≥60 | 37 (18%) | 14 (22%) | 12 (31%) | 2 (15%) | 9 (11%) |
| Sex | |||||
| Female | 94 (47%) | 28 (43%) | 19 (49%) | 6 (46%) | 41 (48%) |
| Male | 108 (53%) | 37 (57%) | 20 (51%) | 7 (54%) | 44 (52%) |
| Race/ethnicity | |||||
| White | 154 (76%) | 52 (80%) | 26 (67%) | 11 (85%) | 65 (76%) |
| Black | 9 (4%) | 0 (0%) | 3 (8%) | 0 (0%) | 6 (7%) |
| Hispanic | 8 (4%) | 2 (3%) | 0 (0%) | 0 (0%) | 6 (7%) |
| Asian | 22 (11%) | 8 (12%) | 7 (18%) | 1 (8%) | 6 (7%) |
| Mixed/Other/Unknown | 9 (4%) | 3 (5%) | 3 (8%) | 1 (8%) | 2 (2%) |
| Hospitalized (severity) | |||||
| No | 185 (92%) | 60 (92%) | 37 (95%) | 12 (92%) | 76 (89%) |
| Yes | 15 (7%) | 4 (6%) | 2 (5%) | 0 (0%) | 9 (11%) |
| Unknown | 2 (1%) | 1 (2%) | 0 (0%) | 1 (8%) | 0 (0%) |
| Median days since PCR + (IQR) | 46 (39–56) | 43 (39–51) | 43 (35–56) | 46 (39–55) | 49 (42–59) |
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
There were no significant differences in the distribution of anti‐SARS‐CoV‐2 IgG and IgA levels by ABO blood groups (Fig. 1a,b). In contrast, the distribution of nAb titre AUC values varied significantly by ABO blood group (Kruskal–Wallis, P = 0·018; Fig. 1c). The mean nAb log2(AUC) value was 5·5 (standard deviation [SD] = 2·4) among donors with blood group A, 6.4 (SD = 2·4) among donors with blood group B, 4·4 (SD = 2·1) among donors with blood group AB and 5·3 (SD = 2·2) among donors with blood group O. In multivariable linear regression analysis, donors with blood group B had significantly higher nAb titres (log2[AUC]) than donors with blood group O (adjusted β = 0·9 [95% CI: 0·1, 1·8], P = 0·026) (Table 2). Donors with blood group A (adjusted β = −0·9 [95% CI: −1·8, −0·1], P = 0·031) and blood group AB (adjusted β = −2·0 [95% CI: −3·4, −0·6], P = 0·005) had significantly lower nAb titres than donors with blood group B.
Fig. 1.
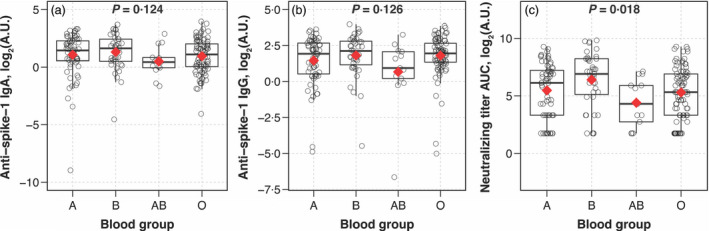
Distribution of IgA, IgG and neutralizing antibody titres to SARS‐CoV‐2 by ABO blood group in eligible convalescent plasma donors. Box‐and‐whisker plots were used to depict the median (thick horizontal line), interquartile ranges and upper/lower extreme limits. The red diamond depicts the arithmetic mean. Circles depict the individual data points. P values were determined from non‐parametric Kruskal–Wallis tests.
Table 2.
Association of ABO blood group with neutralizing antibody titres to SARS‐CoV‐2 in eligible convalescent plasma donors
| Blood group (vs. reference blood group) | SARS‐CoV‐2 neutralizing titre AUC, log2(arbitrary units) | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| β (95% CI) a | P value | β (95% CI) b | P value | |
| A vs. O | 0·2 (−0·6, 0·9) | 0·637 | 0·0 (−0·7, 0·7) | 0·994 |
| B vs. O | 1·1 (0·2, 2·0) | 0·014 | 0·9 (0·1, 1·8) | 0·026 |
| AB vs. O | ‐0·9 (−2·2, 0·5) | 0·201 | ‐1·0 (−2·3, 0·3) | 0·116 |
| A vs. B | ‐0·9 (−1·9, −0·0) | 0·048 | ‐0·9 (−1·8, −0·1) | 0·031 |
| AB vs. B | ‐2·0 (−3·5, −0·5) | 0·008 | ‐2·0 (−3·4, −0·6) | 0·005 |
| A vs. AB | 1·1 (−0·3, 2·5) | 0·131 | 1·0 (−0·3, 2·4) | 0·120 |
| Blood group (vs. reference blood group) | SARS‐CoV‐2 neutralizing titre AUC ≥ 160 arbitrary units | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| Crude PR (95% CI) c | P value | Adjusted PR (95% CI) d | P value | |
| A vs. O | 1·2 (0·7, 2·2) | 0·500 | 1·1 (0·6, 2·0) | 0·684 |
| B vs. O | 2·2 (1·2, 3·8) | 0·006 | 1·9 (1·1, 3·3) | 0·029 |
| AB vs. O e | ‐ | ‐ | ‐ | ‐ |
| A vs. B | 0·6 (0·3, 1·0) | 0·044 | 0·6 (0·4, 1·0) | 0·053 |
| AB vs. B e | ‐ | ‐ | ‐ | ‐ |
| A vs. AB e | ‐ | ‐ | ‐ | ‐ |
Abbreviations: AUC, area under the curve; CI, confidence interval; PR, prevalence ratio.
Bold values correspond to statistically significant findings.
β represents the absolute difference in log2 SARS‐CoV‐2 nAb AUC value with the reference group as estimated by univariable linear regression.
β represents the absolute difference in log2 SARS‐CoV‐2 nAb AUC value with the reference group after adjusting for blood group, age, sex, hospitalization status and time since first PCR + test for SARS‐CoV‐2 infection, as estimated by multivariable linear regression.
Crude prevalence ratios for SARS‐CoV‐2 nAb AUC ≥ 160 were estimated from univariable modified Poisson regression models with robust variance.
Adjusted prevalence ratios for SARS‐CoV‐2 nAb AUC ≥ 160 were estimated from multivariable Poisson regression models with robust variance. The multivariable model included adjustment for age, sex, hospitalization status and time since first PCR + test for SARS‐CoV‐2 infection.
Group AB had no observations with SARS‐CoV‐2 nAb AUC ≥ 160; thus, estimates were not calculated.
The prevalence of high nAb titres (AUC ≥ 160) was 25% (16/65) among donors with blood group A, 44% (17/39) among donors with blood group B, 0% (0/13) among donors with blood group AB and 20% (17/85) among donors with blood group O. Notably, donors with blood group B were significantly more likely to have high nAb titres than donors with blood group O (aPR = 1·9 [95% CI = 1·1, 3·3], P = 0·029) (Table 2). In addition, fewer donors had a high nAb titre among those with blood group A, compared to those with group B (aPR = 0·6 [95%CI = 0·4, 1·0], P = 0·053).
Effect estimates for other covariates in the primary linear regression and modified Poisson regression models are shown in Table S1. Multivariable regression results were insensitive to adjustment for race/ethnicity (Table S2). Inclusion of race/ethnicity in the multivariable linear regression model did not significantly improve model fit to the data based on a likelihood‐ratio test (likelihood‐ratio χ2 = 2·47, P = 0·116). This was further supported by minimal change in the AIC value between the linear regression models (primary analysis multivariable model: AIC = 874·6 vs. sensitivity analysis multivariable model with race/ethnicity: AIC = 874·2). When the analysis was restricted to White donors, the comparison of nAb levels between donors with blood group A vs. B was significantly attenuated (Table S3). However, all other results in the White donor sample remained in the same direction of association as the primary analysis. Associations observed in the analysis restricted to donors who did not have a history of hospitalization were consistent with those observed in the primary analysis (Table S4 ).
Discussion
The ABO blood group of an individual has been suggested to impact the risk of susceptibility to SARS‐CoV‐2 infection and/or disease severity in COVID‐19. Using a sampling of convalescent individuals, we sought to determine whether there were differences in antibodies against SARS‐CoV‐2 by ABO type. Although significant differences in anti‐spike IgA or anti‐spike IgG by ABO blood group were not detected, those individuals of blood group B had higher nAb titres, especially when compared to those who were group AB and group O. There were also significantly more individuals with blood group B than blood group O with high nAb titres (≥1:160).
A growing number of studies suggest that ABO type plays a role in the pathogenesis of COVID‐19 [2, 4, 5, 6, 25, 26]. A large genome‐wide association analysis of patients with COVID‐19‐induced respiratory failure identified genetic susceptibility at the ABO blood group locus, offering a biological basis for an association with ABO type [27]. In the same study, group A was associated with a significantly higher risk of COVID‐19, while group O was observed to have lower risk. This finding has been reported in other studies [2, 4, 6, 27]. Further, a lower prevalence of nAbs against SARS‐CoV‐2 was reported among group O individuals (compared with donors of other types) in a large cross‐sectional sample of asymptomatic French blood donors [9]. This suggested an effect on susceptibility to infection (i.e. relative protection in the case of group O individuals), and not only clinical outcomes.
Why blood group B individuals should have higher SARS‐CoV‐2 titres is unknown. While speculative, one possibility is cross‐reactivity between the virus and the B antigen, thus stimulating antibody production. Alternatively, the viral antigen may appear more foreign to individuals who are blood group B compared with blood group A or O. Further research is needed in this regard. Nonetheless, blood groups have long been recognized to interact with diverse viruses, parasites and bacteria, playing a role in both susceptibility to infection and severity of resulting disease [10].
While an association between ABO type and nAb titres was demonstrated in our study, this was not the case for ABO type and IgG or IgA against spike protein. The titres of nAbs have been shown to correlate well – albeit not perfectly – with those of antibodies against spike protein or receptor binding domain, as determined using clinical assays (i.e. ELISAs) [28, 29, 30, 31, 32]. Other factors (e.g. advanced age, male sex and hospitalization status) that are known to impact the antibody response to SARS‐CoV‐2 were controlled for in the study [22].
The kinetics of the humoral response to SARS‐CoV‐2 are still being learned [24, 33, 34, 35]. While speculative, there are many factors that could account for the conflicting finding across studies investigating the relationship between ABO group and COVID‐19. For one, there are differences in the populations that are being studied. While the racial distribution in our study population was representative of the U.S. blood donor population (i.e. over‐representation of White patients), the ABO blood group distribution was disproportionately skewed towards group B and AB individuals. Specifically, the expected frequencies by ABO type in a US donor population for groups O, A, B and AB are 45%, 40%, 11% and 4%, respectively [21]; by contrast, the observed frequencies in our study were 42%, 32%, 19% and 6%, respectively. Of note, a previous study reported over‐representation of group A and under‐representation of group O in White patients with COVID‐19, yet did not observe a difference in Black or Hispanic patients [6].
This study has limitations. For one, it was confined to a cross‐sectional convenience sample of eligible donors from the Baltimore/Washington DC metropolitan areas. The small sample size particularly for some blood types (e.g. AB) may have resulted in sparse data bias and prevented us from conducting additional stratified analyses, including by titre. Second, selection of prospective blood donors may also limit generalizability, as these donors are not representative of a general population, with respect to both race/ethnicity and health status [36]. Selection is also skewed towards COVID‐19 survivors who were sufficiently healthy to be recruited as convalescent plasma donors. By extension, the low proportion of subjects who had been hospitalized reflects individuals with a lower index of disease severity. In short, the study sample is unlikely to be representative of all patients with COVID‐19 and additional studies that address disease severity by ABO type are needed. Third, only plasma was available for testing; this precluded forward typing of the red cells; that is, the ABO group was presumed based on the reverse typing. Fourth, this study population had a higher‐than‐expected proportion of group B and AB patients. However, sensitivity analyses confirm the findings of the higher titres are not due to the racial/ethnic composition of the study population. Fifth, titering of ABO isoagglutinins was not undertaken. That could have provided further insight. This study also did not evaluate IgM; while one cannot rule out the possibility that IgM accounts for the observed differences with the neutralization assay, it is was deemed unlikely given the time since test positivity (i.e. a surrogate of the initial infection). When combined with incubation and duration of symptomatic disease, IgM was considered to be an unreliable marker whereby the majority of individuals were at least six weeks from initial diagnosis. Finally, there may be residual and unmeasured confounding from variables that have not been considered.
In conclusion, individuals with blood group B who survive COVID‐19 may potentially have higher nAb titres against SARS‐CoV‐2 compared with survivors of other blood groups, particularly blood group O. Further work is warranted in different populations to test generalizability of these results.
Funding
This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) AI052733 and AI15207 (A.C.); NIAID R01AI120938, R01AI120938S1 and R01AI128779 (A.A.R.T); NIAID T32AI102623 (E.U.P.); the Division of Intramural Research, NIAID, NIH (O.L., A.R., T.Q.); National Heart Lung and Blood Institute 1K23HL151826‐01 (E.M.B) and R01HL059842 (A.C.), Bloomberg Philanthropies (A.C.); Department of Defense W911QY2090012 (D.S.).
Conflict of interests
EMB reports personal fees and non‐financial support from Terumo BCT, Grifols Diagnostics Solutions and Abbott Laboratories outside of the submitted work; EMB is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Any views or opinions that are expressed in this manuscript are those of the authors, based on his own scientific expertise and professional judgement; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of FDA, and also do not bind or otherwise obligate or commit either advisory committee or the agency to the views expressed.
Supporting information
Table S1. Full multivariable model results from primary analysis of associations with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors.
Table S2. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors adjusting for race/ethnicity.
Table S3. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors who identified as white.
Table S4. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors without a history of hospitalization due to COVID‐19.
Acknowledgements
We are grateful to all participants who enrolled in this study and donated plasma. We thank the National Institute of Infectious Diseases, Japan, for providing VeroE6TMPRSS2 cells and acknowledge the Centers for Disease Control and Prevention, BEI Resources, NIAID, NIH for SARS‐related coronavirus 2, Isolate USA‐WA1/2020, NR‐5228. EMB, EUP and AART were responsible for study conception, oversight, analysis and writing the first draft of the manuscript. CM and KL conducted antibody testing. RS and IB conducted donor screening. AP, RG, BJG, JLW, OL, SS, DS, EAG, ADD, TCQ, AC and AP provided input on laboratory testing and data analysis. All contributed to editing the manuscript.
References
- 1. JHU : Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering at Johns Hopkins. The Center for Systems Science and Engineering (CSSE) at JHU, December 5, 2020 2020. https://coronavirus.jhu.edu/map.html (Last accessed December 5 2020)
- 2. Wu Y, Feng Z, Li P, et al.: Relationship between ABO blood group distribution and clinical characteristics in patients with COVID‐19. Clin Chim Acta 2020; 509:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan Q, Zhang W, Li B, et al.: Association Between ABO Blood Group System and COVID‐19 Susceptibility in Wuhan. Front Cell Infect Microbiol 2020; 10:404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Wang X, Chen J, et al.: Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol 2020; 190:24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Yang Y, Huang H, et al.: Relationship between the ABO Blood Group and the COVID‐19 susceptibility. Clin Infect Dis 2020;ciaa1150. 10.1093/cid/ciaa1150. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leaf RK, Al‐Samkari H, Brenner SK, et al.: ABO phenotype and death in critically ill patients with COVID‐19. Br J Haematol 2020; 190:e204–e208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latz CA, DeCarlo C, Boitano L, et al.: Blood type and outcomes in patients with COVID‐19. Ann Hematol 2020; 99:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dzik S, Eliason K, Morris EB, et al.: COVID‐19 and ABO blood groups. Transfusion 2020; 60:1883–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallian P, Pastorino B, Morel P, et al.: Lower prevalence of antibodies neutralizing SARS‐CoV‐2 in group O French blood donors. Antiviral Res 2020; 181:104880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooling L: Blood groups in infection and host susceptibility. Clin Microbiol Rev 2015; 28:801–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Cheng G, Chui CH, et al.: ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005; 293:1450–1451 [DOI] [PubMed] [Google Scholar]
- 12. Guillon P, Clément M, Sébille V, et al.: Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology 2008; 18:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Focosi D: Anti‐A isohaemagglutinin titres and SARS‐CoV‐2 neutralization: implications for children and convalescent plasma selection. Br J Haematol 2020; 190:e148–e150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gérard C, Maggipinto G, Minon JM: COVID‐19 and ABO blood group: another viewpoint. Br J Haematol 2020; 190:e93–e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bloch EM, Shoham S, Casadevall A, et al.: Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest 2020; 130:2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Budhai A, Wu AA, Hall L, et al.: How did we rapidly implement a convalescent plasma program? Transfusion 2020; 60:1348–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Natarajan H, Crowley AR, Butler SE, et al.: SARS‐CoV‐2 antibody signatures robustly predict diverse antiviral functions relevant for convalescent plasma therapy. medRxiv 2020; 10.1101/2020.09.16.20196154 [DOI] [Google Scholar]
- 18. Bloch EM: Convalescent plasma to treat COVID‐19. Blood 2020; 136:654–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bloch EM, Goel R, Montemayor C, et al.: Promoting access to COVID‐19 convalescent plasma in low‐ and middle‐income countries. Transfus Apher Sci 2020;102957. 10.1016/j.transci.2020.102957. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tobian AAR, Shaz BH: Earlier the better: convalescent plasma. Blood 2020; 136:652–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohn C, Delaney M, Johnson S, et al.: Technical Manual, 20th edn. Bethesda, MD: AABB;2020. [Google Scholar]
- 22. Klein SL, Pekosz A, Park HS, et al.: Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest 2020;130(11):6141‐6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. FDA : Recommendations for investigational COVID‐19 convalescent plasma. 2020. https://www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/recommendations‐investigational‐covid‐19‐convalescent‐plasma (Last accessed May 18 2020)
- 24. Benner SE, Patel EU, Laeyendecker O, et al.: SARS‐CoV‐2 antibody avidity responses in COVID‐19 patients and convalescent plasma donors. J Infect Dis 2020; 222(12):1974–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Göker H, Aladağ Karakulak E, Demiroğlu H, et al.: The effects of blood group types on the risk of COVID‐19 infection and its clinical outcome. Turk J Med Sci 2020; 50:679–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golinelli D, Boetto E, Maietti E, et al.: The association between ABO blood group and SARS‐CoV‐2 infection: A meta‐analysis. PLoS One 2020; 15:e0239508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellinghaus D, Degenhardt F, Bujanda L, et al.: Genomewide Association Study of Severe Covid‐19 with respiratory failure. N Engl J Med 2020; 383:1522–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okba NMA, Müller MA, Li W, et al.: Severe Acute Respiratory Syndrome coronavirus 2‐specific antibody responses in Coronavirus disease patients. Emerg Infect Dis 2020; 26:1478–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amanat F, Stadlbauer D, Strohmeier S, et al.: A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med 2020; 26:1033–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel E, Bloch EM, Clarke W, et al.: Comparative performance of five commercially available serologic assays to detect antibodies to SARS‐CoV‐2 and identify individuals with high neutralizing titers. J Clin Microbiol 2020; 10.1128/JCM.02257-20. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conklin SE, Martin K, Manabe YC, et al. Evaluation of serological SARS‐CoV‐2 lateral flow assays for rapid point of care testing. medRxiv 2020; 10.1128/JCM.02020-20. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luchsinger LL, Ransegnola B, Jin D, et al.: Serological Assays Estimate Highly Variable SARS‐CoV‐2 Neutralizing Antibody Activity in Recovered COVID‐19 Patients. medRxiv 2020;58(12):e02005‐20. 10.1128/JCM.02005-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long QX, Liu BZ, Deng HJ, et al.: Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med 2020; 26:845–848 [DOI] [PubMed] [Google Scholar]
- 34. Luo YR, Chakraborty I, Yun C, et al.: Kinetics of SARS‐CoV‐2 antibody avidity maturation and association with disease severity. Clin Infect Dis 2020; ciaa1389. 10.1093/cid/ciaa1389. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonny TS, Patel EU, Zhu X, et al.: Cytokine and chemokine levels in COVID‐19 convalescent plasma. Open Forum Infect Dis 2020; ofaa574. 10.1093/ofid/ofaa574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel EU, Bloch EM, Grabowski MK, et al.: Sociodemographic and behavioral characteristics associated with blood donation in the United States: a population‐based study. Transfusion 2019; 59:2899–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Full multivariable model results from primary analysis of associations with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors.
Table S2. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors adjusting for race/ethnicity.
Table S3. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors who identified as white.
Table S4. Sensitivity analysis: Association of ABO blood group with neutralizing antibody titers to SARS‐CoV‐2 in eligible convalescent plasma donors without a history of hospitalization due to COVID‐19.


