Summary
The COVID‐19 pandemic has significantly changed the landscape of kidney transplantation in the United States and worldwide. In addition to adversely impacting allograft and patient survival in postkidney transplant recipients, the current pandemic has affected all aspects of transplant care, including transplant referrals and listing, organ donation rates, organ procurement and shipping, and waitlist mortality. Critical decisions were made during this period by transplant centers and individual transplant physicians taking into consideration patient safety and resource utilization. As countries have begun administering the COVID vaccines, new and important considerations pertinent to our transplant population have arisen. This comprehensive review focuses on the impact of COVID‐19 on kidney transplantation rates, mortality, policy decisions, and the clinical management of transplanted patients infected with COVID‐19.
Keywords: COVID‐19, kidney transplantation, mortality, wait list
Introduction
The 2019 novel coronavirus disease (COVID‐19) pandemic has transformed lives throughout the globe and affected the medical community in immeasurable ways. The field of kidney transplantation experienced disruptions to the established models of care with transplant organizations and centers rapidly attempting to adjust their policies in response to the pandemic. Initially, almost universally, kidney transplantation was halted. This response was based on multiple factors, but was mainly an attempt to minimize the risk of transmission of a life‐threatening, novel, and poorly understood infectious disease to an immunosuppressed population. Perhaps more pressingly, it was a response to the anticipated COVID‐19‐related surge in hospital admissions and strain on the healthcare system that prevented the safe performance of transplant surgeries. Since March 2020, we have come a long way in understanding the impact of these early practice changes on the field of kidney transplantation and the effect of therapeutic strategies on the mortality of kidney transplant recipients.
This narrative review highlights how COVID‐19 has changed the landscape of kidney transplantation from the pretransplant through the post‐transplant period, and discusses new insights into the care of kidney transplant recipients infected with COVID‐19, including our current state of knowledge on COVID vaccination in organ transplantation.
Transplant listing and waitlist mortality
Both patients undergoing workup to be listed for kidney transplant and those who were already waitlisted encountered hardships associated with the pandemic and the accompanying changes to transplant policy. An analysis of waitlist registration and mortality between March and April of 2020 using data from Scientific Registry of Transplant Recipients of the United States (US) uncovered a weekly decline in the numbers of waitlisted patients [1]. Centers in areas with lower COVID‐19 burden had a 9% decrease in listings whereas those in areas with high COVID‐19 burden had a 41% decrease in listings as compared to the same time period in 2019 [2]. United Network for Organ Sharing (UNOS) registry data showed an overall 25% decrease in waitlist additions in April 30, 2020, when compared to prepandemic months January and February 2020 [3]. Further, the number of waitlisted patients who were inactivated more than doubled as compared to the prepandemic era [1]. By the end of April 2020, as much as 40% of the US kidney transplant wait list was in inactive status [1]. The initial increase in inactivated patients could have been due to a necessary diversion of healthcare resources during a time of limited hospital beds, ventilators, and operating rooms. In the United Kingdom (UK), as a result of decreased transplantations, the National Health System projects that the number of waitlisted patients will increase by almost 30% in the 6 months following the pandemic. This is particularly concerning as it is expected to strain dialysis infrastructure in the country [4].
A study from a large transplant center in the United States found that COVID‐19‐infected waitlisted patients were significantly more likely to require hospitalization and more likely to die as compared to transplanted patients infected with COVID‐19 [5]. In the United States, high COVID‐19 burden areas witnessed a greater than 2.2 times increased waitlist mortality as compared to prepandemic mortality [1]. Kidney transplant waitlist deaths increased by 43%, the largest across each group of solid organ transplant waitlisted patients [3]. To put this in perspective, lung transplant waitlisted patients had a 12% increase in the number of deaths, liver transplant waitlisted deaths increased by 8%, and heart transplant waitlisted patients had a 36% increase in mortality [3]. In the UK, 10% of waitlisted patients who acquired COVID‐19 died [6]. In France, as many as 42% of waitlisted deaths in March and April 2020 were due to COVID‐19 [7]. Whether by infection or perhaps changes in practice and surveillance, COVID‐19 was directly and indirectly responsible for 73% excess deaths in this population as compared to waitlisted patients in previous years [7].
In addition, waitlisted patients were more likely to acquire COVID‐19 as compared to organ transplant recipients [6]. Therefore, the risks of not listing patients, or keeping them on the wait list as compared to the risks of transplanting them during the ongoing pandemic should be calculated very carefully. A personalized strategy tailored to each patient is necessary, and informed consent where waitlisted patients are allowed thorough consideration of their situation is imperative as this pandemic continues to loom.
Donor testing
Testing donors for COVID‐19 has become a cornerstone of kidney transplantation practices that ensures the safety of recipients, living donors, organ procurement teams, and transplant teams caring for the donor/recipient peri‐ and postdonation. In the first few months of the pandemic, donor screening swabs were deployed in organ procurement organizations (OPOs) within Australia, Canada, Italy, Spain, Switzerland, the Netherlands, France, and South Korea [4, 8, 9, 10]. In several countries, reverse transcription–polymerase chain reaction (RT‐PCR) on bronchoalveolar lavage or on distal tracheal aspirate is required for donor testing because the sensitivity of the nasopharyngeal swab is suboptimal [11, 12]. Furthermore, some transplant societies even recommended performing CT scans in donors suspected of having COVID‐19 whose COVID testing was negative [9, 10]. In countries where testing kits were limited, transplant societies recommended universal screening of donors by questionnaires for COVID‐19 symptoms or exposure [13]. Even though there is a theoretical risk of COVID‐19 transmission via organ transplantation, to date there have been no case reports of COVID‐19 transmission from organ donors to recipients. Whether that is due to a very careful selection of organs or due to very low risk of transmission via organs is unknown.
Donor supply
As kidney transplantation rates declined, so did organ procurement rates. The UK reported a 39% decrease in referrals of organs to OPOs [14]. France reported a 16% decrease in all deceased organ procurement rates as compared to previous years [10]. Part of these declines were due to procurement challenges faced by transplant programs and organizations, such as insufficient testing capabilities and delayed COVID‐19 test results, limited operating room availabilities, and mandates for the avoidance of nonessential clinical activities to preserve personnel and protective equipment. Further, transfers of recovered organs from location of recovery to recipient were limited in various ways, such as through cancelations of commercial flights cancelations during the pandemic [15]. In the United States, commercial flight numbers decreased by 39% during the month of April 2020, including a 65% decrease in morning and afternoon flights, and a 71% decrease in evening flights [2]. This resulted in some OPO Pairs losing all direct flight options connecting them [2]. This also meant an increase in median “next flight wait times” from 1.5 hours to 4.9 hours. The wait time between one pair of OPOs increased significantly from 1.1 hours to 7 days [2].
In Austria, despite organ procurement decline from mid‐March to mid‐April 2020, numbers rebounded in May and June to numbers comparable to the pre‐COVID era [16]. This was mainly due to early effective COVID‐19 mitigation efforts which were undertaken in Austria, which prevented intensive care units from getting overwhelmed with COVID‐positive patients. Segregation of COVID‐positive and negative patients in the hospital also allowed the transplant units to remain COVID‐free, which permitted early re‐start of transplant programs. We suspect similar trends may be seen in other countries, but none have been published yet. Interestingly, during the peak of the pandemic in the UK, 74% of families approached about potential organ donation were still interested in donation despite not being allowed to see their ill family members due to hospital restrictions [14].
Ongoing barriers to living‐donor transplantation include center concerns for donor and recipient safety, fewer donor inquiries, staff limitations, and government restrictions. With more than 42,000,000 people worldwide who have recovered from COVID‐19 to date, concern about limited data regarding the safety of organ donation from donors with previous infection may also be a contributing factor to reducing the donor pool. Recent American Society of Transplantation guidance note that “given the renal dysfunction associated with SARS‐CoV‐2 infection and unclear long‐term implications thereof, additional evaluation may be required when considering kidney transplantation from living donors with previous COVID‐19” [17].
Transplantation rates
In the early stages of the pandemic, data regarding the safety of kidney transplantation were lacking. In addition, some jurisdictions deemed kidney transplantation nonessential procedures, thus effectively prohibiting these surgeries temporarily. Therefore, 72% of programs in the United States suspended live donor transplantation [18], and deceased donor transplantation rates dropped by 76% [1]. Overall kidney transplantation rates were down by almost 50% [3, 19]. Italy reported a 25% decrease in transplantation rates with living kidney transplants being particularly affected [20]. In the UK, kidney transplantation rates were down by 68% [14], and France experienced a very significant > 90% decline in kidney transplantation rates [19]. By October 2020, Australia had observed a 27% decrease in transplantation rates as compared to 2019 [21]. Other countries including Canada, the Netherlands, Spain, Switzerland, Austria, and Japan halted living and deceased donor transplantation initially, except for select cases such as patients who were highly sensitized or had no dialysis access [8, 16, 22].
Other countries adopted a radically different approach. Denmark deemed transplants as lifesaving surgeries and did not restrict them, though living‐donor transplants were rescheduled [8]. Germany, which had relatively good control of the virus, continued to have robust rates of kidney transplantation that were unchanged from previous years [23].
Since May, most US centers have resumed kidney transplantation [1], though living‐donor transplants continue to be excessively affected by the pandemic. Based on the U.S. Organ Procurement and Transplantation Network, 24% fewer living‐donor transplantation have been performed between January and November 2020 as compared to the same period in 2019. We are still waiting on the most recent data from around the world to assess whether the new spikes and resultant lockdowns later in 2020 might have further affected transplantation rates.
Kidney transplant recipient survival after COVID‐19
Worldwide reports have consistently shown that hospitalized kidney transplant recipients infected with COVID‐19 had high mortality rates approaching 20‐30% [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. In Italy, mortality rates of transplant recipients infected with SARS‐CoV‐19 reached 33%, and were mainly attributed to respiratory failure [35]. An initial report from the French registry reported a mortality of up to 30% [37]. This was followed by the French IMPORTANT study which compared the impact of COVID‐19 on mortality by comparing the mortality of kidney transplant recipients during the pandemic with previous years [7]. The authors noted that the absolute number of deaths in April 2020 was more than twofold higher than the number of deaths in recipients in April 2018 or April 2019 [7]. Between March and June 2020, 44% of kidney recipient deaths were attributed to COVID‐19, and as a result, COVID‐19 was overwhelmingly responsible for the excess mortality in this population as compared to previous years [7]. The European Renal Association COVID‐19 database, which includes data from 26 European and Northern Mediterranean countries, found a mortality rate of 21% in kidney transplant recipients presenting with COVID‐19 between February 1, 2020, and May 1, 2020; albeit this figure was even higher, reaching 45% in those admitted to intensive care units [36]. However, the mortality rate was much lower at 3% in COVID‐19‐infected kidney transplant recipients who did not require hospitalization [36]. Nevertheless, this rate exceeded that of the general population by a significant margin.
Risk factors for mortality of kidney transplant recipients infected with COVID‐19 are generally similar to the risk factors observed in the general population. These included older age, higher degree of frailty, obesity, pre‐existing lung disease, and the presence of multiple comorbidities [29, 30, 32, 36, 38]. Unsurprisingly, worsening kidney (allograft) function was a risk factor as well [36].
It is important to note that published studies have included all kidney transplant recipients infected with COVID‐19 without distinction in duration of transplantation. To our knowledge, only one large study from France has evaluated this. Recipients in their first year post‐transplantation were found to have a higher mortality as compared to those who were more than one year out from transplant [7]. The agent used for induction immunosuppression did not affect mortality [7].
Clinical management of the transplant recipient with COVID‐19
Since the first cases of COVID‐19 reported out of Wuhan, China, one of the main priorities of the medical community has been to identify the best therapeutic strategy to improve the outcomes of patients infected with COVID‐19. Several randomized controlled trials have been executed to determine which drugs are beneficial in this population. Unfortunately, none of these trials have included patients with kidney transplants. Therefore, when it comes to the COVID‐19 care in this population, the transplant community is still largely relying on best clinical judgment and limited evidence that includes case reports with a small number of patients that lack comparison groups.
In general, kidney transplant recipients infected with COVID‐19 present with the same signs and symptoms as those of the general population. These include fever, cough, respiratory failure, fatigue, myalgias, and gastrointestinal symptoms [39]. Management of kidney transplant recipients has included antimetabolite withdrawal in most patients, and calcineurin inhibitor (CNI) and/or mTOR inhibitor (mTORi) withdrawal in a smaller number of patients [25, 28, 31, 36, 37, 39, 40]. These practices of antimetabolite and/or CNI/mTORi withdrawal were universal, implemented by transplant centers in most countries. A few centers discontinued all maintenance immunosuppression and replaced it with methylprednisolone [31, 41]. Patients who were on Belatacept had their doses postponed [37]. A European initiative, promoted by ERA‐EDTA and the DESCARTES working group, put forth recommendations on the management of immunosuppression in kidney transplant recipients who are more than 3 months out from transplant [42]. Despite reductions and changes to baseline immunosuppression, no cases of patients developing allograft rejection have been reported.
Hydroxychloroquine and azithromycin were used ubiquitously in the early months of the pandemic. However, this practice has been abandoned given the overwhelming evidence failing to support any benefit and in some instances indicating harm [43]. In Italy, antivirals such as lopinavir, ritonavir, and darunavir were being given, though these drugs ultimately did not prove effective in treating COVID‐19 [41]. Of note, these antivirals interact with CNIs and therefore patients who received them may have had detectable CNI levels for several days after CNIs were withdrawn. Remdesivir is an inhibitor of the viral RNA‐dependent, RNA polymerase that started being used early in the pandemic and continues to be widely used to treat COVID‐19 patients. Remdesivir does not improve mortality but shortens the time to recovery in hospitalized patients.[44] Unfortunately, no studies have clearly addressed the use of Remdesivir in kidney transplant recipients, and so we are unable to draw any conclusions on the safety and benefit of these treatments in this population. Nevertheless, many centers in the United States are using it for hospitalized transplant patients with COVID‐19 pneumonia.
The pathophysiology of COVID‐19 and the associated cytokine storm prompted the use immunomodulatory therapies that target different pathways in the inflammatory cascade. Tocilizumab, a recombinant humanized anti‐interleukin‐6 (IL‐6) receptor monoclonal antibody, is used in the treatment of chronic antibody‐mediated rejection [45], conditions like rheumatoid arthritis [46], as well as treatment of severe cytokine release syndrome induced by chimeric antigen receptor T cells [47]. Due to potential benefits against the cytokine storm seen in severe cases of COVID‐19, Tocilizumab has been used since the start of the pandemic to treat kidney transplant recipients with COVID‐19 [25, 40, 41, 45]. Over the past few months, more data have been accumulated on the use of this agent, as summarized in Table 1. While Tocilizumab is generally safe and well tolerated in most studies, no significant improvement in need for mechanical ventilation, hospital discharge, or mortality has been noted [25, 29, 40, 41, 45, 48, 49, 50].
Table 1.
Summary of studies assessing the effect of Tocilizumab in kidney transplant patients with COVID‐19*
| Study | Country | Type of study | Population | Outcomes |
|---|---|---|---|---|
| Pereira et al. [48] | US | Retrospective matched cohort; single center |
|
|
|
|
|||
| Perez‐Saez et al. [29] | Spain | Retrospective cohort; multicenter |
|
|
|
|
|||
| Cravedi et al. [25] TANGO study | US, Italy, Spain | Retrospective cohort; multicenter |
|
|
| Trujillo et al. [49] | Spain | Retrospective; single center |
|
|
| Alberici et al. [50] | Italy | Case series |
|
|
| Bossini et al. [35]† | Italy | Retrospective, multicenter |
|
|
CRP, C‐reactive protein; KTR, kidney transplant recipient; RRT, renal replacement therapy; SOT, solid organ transplant.
The table only includes studies of 10 or more patients.
Includes patients from study by Alberici et al. [50].
Corticosteroids are the most widely used immunomodulatory agents, ubiquitously used in kidney transplant recipients to prevent and treat rejection. The RECOVERY trial, which randomized COVID‐19‐infected patients to dexamethasone vs. usual care, showed a significant mortality benefit in those who received steroids as compared to those who received usual care. The benefit was significant at 36% in those with severe COVID‐19 requiring mechanical ventilation and 18% in those requiring oxygen [51]. Even though transplant patients infected with COVID‐19 were not included in randomized trials assessing the mortality benefits of high dose steroids, care has been extrapolated from trials such as the RECOVERY trial. It is now standard of care to use high dose steroids in severely ill transplant patients hospitalized with COVID‐19 pneumonia (Fig. 1).
Figure 1.
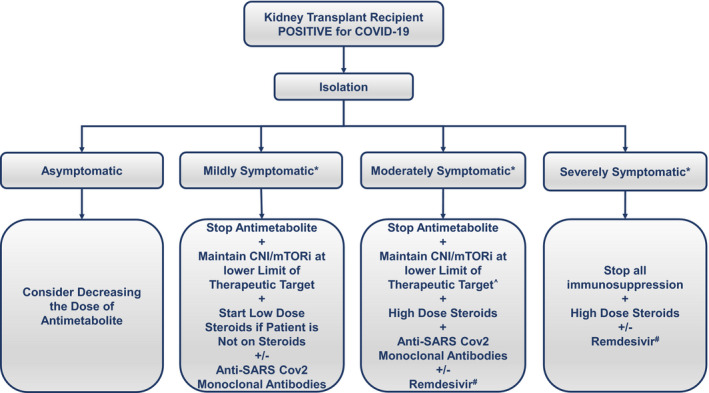
Suggested algorithm for the management of the COVID‐19‐positive kidney transplant recipient. *Symptoms of upper respiratory tract infection, gastrointestinal symptoms, and loss of taste or smell could be present in all patients. Mildly symptomatic is defined as a patient who has oxygen saturation > 95%, has no tachypnea, and has no evidence of COVID‐19 on imaging. Moderately symptomatic is defined as a patient who has evidence of COVID‐19 on imaging, but has an oxygen saturation > 94% and a respiratory rate < 30. Severely symptomatic is defined as a patient who has a low oxygen saturation < 94% or a respiratory rate > 30 despite supplemental oxygen, or respiratory failure requiring mechanical ventilation. ^Physicians can consider stopping CNI/mTORi in select patients who are at low risk for rejection. #There is significant variation between individual countries in Remdesivir indications.
Imbalances in the Janus kinase (JAK) and signal transducer and activator of transcription protein (STAT) pathway have been implicated in the COVID‐19 inflammatory state. A multicenter trial of 1033 patients with moderate to severe COVID‐19 infection randomized to Baricitinib, an oral JAK inhibitor, and Remdesivir or Remdesivir alone showed that patients who received the combination therapy had a median 1 day shorter time to recovery and lower adverse events as compared to Remdesivir alone[52]. JAK inhibitors have not been studied in kidney transplant recipients with COVID‐19, but previous studies using JAK inhibitors as substitutes for other immunosuppressants in kidney transplant recipients have shown promising results in preventing rejection [53].
In addition to immunomodulatory therapies, one important strategy to combat the virus has been the use antibodies to neutralize the coronavirus until the patient can mount an innate humoral immune response. However, convalescent plasma use in nontransplant patients with severe COVID‐19 did not improve survival [54]. One case report of 2 kidney transplant patients treated with convalescent plasma indicated that the treatment was safe; and both patients survived, albeit one of them required hemodialysis during hospitalization and remained on hemodialysis at discharge [55]. Subsequently, efforts have shifted to the development of monoclonal antibodies that specifically target the spike protein of SARS‐CoV‐2 [56, 57]. The US Foods and Drug Administration (FDA) has given emergency use authorization for the use of monoclonal antibodies in treatment of patients with COVID‐19 who are at risk of progressing to severe disease. Our center and others are administering monoclonal antibodies to transplant recipients with COVID‐19.
COVID‐19 vaccination in transplanted patients
In the absence of a definitive cure for COVID‐19, all eyes are on vaccine development and distribution. In nontransplant patients with COVID‐19, serologic studies have indicated that immunoglobulin (Ig)M levels rise within 5–10 days of infection onset. IgG response typically follows with most patients developing an IgG response within 12–14 days of symptom onset [58]. Follow‐up studies indicate that these responses last for at least 5 months following infection and can confer immunity against recurrent COVID‐19 infections [59]. Very few studies have evaluated the serologic response of transplant recipients to COVID‐19. SARS‐CoV‐2 IgG serology turned positive in 7 kidney transplant patients who were hospitalized with COVID‐19 with seroconversion occurring between 4 and 38 days after infection [60]. Interestingly, one patient who was less than 6 months from transplantation was able to seroconvert. In a different study, 855 consecutive kidney transplant recipients had their sera tested for the presence of nucleocapsid protein (NP) antibodies [61]; Of those 855, 33 had been previously diagnosed with COVID‐19 as confirmed by RT‐PCR. 66.6% of those who had proven infection had evidence of IgG at a median time of testing of 36 days following their diagnosis [61]. Therefore, despite paucity of data, there is evidence supporting satisfactory seroconversion rates in kidney transplant recipients.
As countries have begun administering the COVID vaccines, transplant patients are faced with questions about the efficacy and safety of these vaccines. There are several SARS‐CoV2 vaccines under development, but two mRNA‐based vaccines, mRNA‐1273 [Moderna] [62] and BNT162b2 [Pfizer and BioNTech] [63], have completed phase 3 trials demonstrating greater than 94.1% and 95% efficacy at preventing COVID infection. The mRNA vaccines have so far shown a favorable safety profile with most reactions being limited to viral‐like symptoms for a few days after vaccine administration. No vaccine phase 3 trial has included transplant recipients; therefore, the efficacy of vaccines in transplant patients has yet to be demonstrated. Based on data from other vaccines in the transplant population, we expect that seroconversion rates will be lower than those of immunocompetent individuals and that immunity will wean faster particularly in patients who require B‐cell depleting therapy.
Another important consideration includes the safety of vaccination in the kidney transplant population. The mRNA vaccines encode for the SARS‐CoV2 spike protein. Since the vaccine does not introduce the virus, there are no concerns about inducing COVID‐19. A theoretical risk of rejection following vaccination and immune system activation has been considered with previous vaccinations. For example, some studies have raised concerns that the administration of the influenza vaccine post‐transplantation can cause immune upregulation and the development of de novo donor‐specific antibodies [64]. Nevertheless, follow‐up studies indicated that the vaccine does not increase the risk of rejection in the kidney transplant population [65] and is associated with lower allograft loss and death [66].
Despite the lack of efficacy or safety data, The American Society of Transplantation (AST) suggests that “the benefits of vaccination outweigh any theoretical risks especially in countries where SARS‐CoV‐2 transmission continues at a high level.” Additionally, The AST 2019 Guidelines explicitly state that "In the post‐transplant setting, inactivated vaccines can be administered starting at 3–6 months post‐transplant (strong; moderate) except influenza vaccine which can be given as early as 1‐month post‐transplant (strong; low)" [67]. Accordingly, it is very likely that transplant centers will recommend the COVID‐19 vaccine to recipients who have been transplanted for greater than 1 month. The American Society of Nephrology is additionally requesting that the Advisory Committee on Immunization Practices prioritize dialysis‐dependent patients for COVID vaccination. This will be a key recommendation given the kidney transplant waitlist mortality.
Conclusion
Kidney transplant recipients and waitlisted patients are immunologically vulnerable and at higher risk of mortality from COVID‐19 as compared to the general population. COVID‐19 has not only disturbed our transplant patients’ lives, it has additionally impacted transplantation policies, donation chains, and timely and safe transplant surgeries (Fig. 2). Our understanding of this virus and its treatment strategies is constantly evolving. As of today, we still lack vaccines and proven beneficial and safe antiviral therapies; therefore, COVID‐19 continues to threaten our patients and transplantation practices and programs. Due to lack of rigorous randomized controlled trials in transplant patients at this time, current practices are based on expert opinion and individual transplant center experience. Most studies include small numbers of patients with short‐term data. Long‐term consequences of this pandemic in general and this virus in particular are yet to be determined. Recommendations will doubtless continue to evolve as our knowledge and therapies expand. Conceivably, however, the silver lining of this pandemic has been the close national and international collaboration among physicians, transplantation centers, and patients to ensure that we are constantly striving to determine the best possible care, while mitigating the future negative consequences of the pandemic on the field of kidney transplantation.
Figure 2.
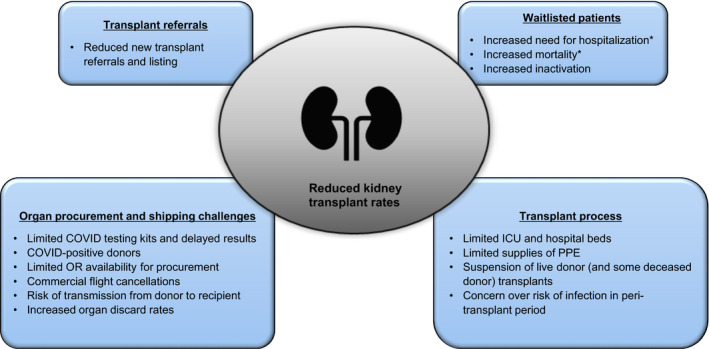
Factors affecting kidney transplant rates during the COVID‐19 pandemic. ICU: intensive care unit; PPE: personal protective equipment. *Due to COVID‐19 infection.
Funding
The authors have declared no funding.
Conflict of interest
The authors have declared no conflict of interest.
References
- 1. Boyarsky BJ, Durand CM, et al. Early national and center‐level changes to kidney transplantation in the United States during the COVID‐19 epidemic. Am J Transplant 2020; 20: 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strauss AT, Cartier D, Gunning BA, et al. Impact of the COVID‐19 pandemic on commercial airlines in the United States and implications for the kidney transplant community. Am J Transplant 2020; 20: 3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cholankeril G, Podboy A, Alshuwaykh OS, et al. Early Impact of COVID‐19 on Solid Organ Transplantation in the United States. Transplantation 2020; 104: 2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma V, Shaw A, Lowe M, et al. The impact of the COVID‐19 pandemic on renal transplantation in the UK. Clin Med (Lond) 2020; 20: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Craig‐Schapiro R, Salinas T, Lubetzky M, et al. COVID‐19 Outcomes in Patients Waitlisted for Kidney Transplantation and Kidney Transplant Recipients. Am J Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravanan R, Callaghan CJ, Mumford L, et al. SARS‐CoV‐2 infection and early mortality of wait‐listed and solid organ transplant recipients in England: a national cohort study. Am J Transplant 2020; 20: 3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thaunat O, Legeai C, Anglicheau D, et al. IMPact of the COVID‐19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int 2020; 98: 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn C, Amer H, Anglicheau D, et al. Global Transplantation COVID Report March 2020. Transplantation 2020; 104: 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Vries APJ, Alwayn IPJ, Hoek RAS, et al. Immediate impact of COVID‐19 on transplant activity in the Netherlands. Transpl Immunol. 2020; 61: 101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Legeai C, Malaquin G, Lamotte C, et al. Impact of Coronavirus disease 2019 on organ donation and transplantation in France. Transpl Int 2021; 34: 204–206. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in Different Types of Clinical Specimens. JAMA 2020; 323: 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS‐CoV‐2 Infection ‐ Challenges and Implications. N Engl J Med. 2020; 383: e38. [DOI] [PubMed] [Google Scholar]
- 13. Kumar D, Manuel O, Natori Y, et al. COVID‐19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant 2020; 20: 1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manara AR, Mumford L, Callaghan CJ, Ravanan R, Gardiner D. Donation and transplantation activity in the UK during the COVID‐19 lockdown. Lancet 2020; 396: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyarsky BJ, Ruck JM, Chiang TP, et al. Evolving Impact of COVID‐19 on Transplant Center Practices and Policies in the United States. Clin Transplant 2020; 34: 14086 [DOI] [PubMed] [Google Scholar]
- 16. Watschinger B, Watschinger C, Reindl‐Schwaighofer R, et al. Impact of Timely Public Health Measures on Kidney Transplantation in Austria during the SARS‐CoV‐2 Outbreak‐A Nationwide Analysis. J Clin Med 2020; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Society of Transplantation Recommendations and Guidance for Organ Testing 2020 [cited 2020]. Available from: https://www.myast.org/recommendations‐and‐guidance‐organ‐donor‐testing.
- 18. Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant 2020; 20: 1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loupy A, Aubert O, Reese PP, et al. Organ procurement and transplantation during the COVID‐19 pandemic. Lancet 2020; 395: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angelico R, Trapani S, Manzia TM, et al. The COVID‐19 outbreak in Italy: Initial implications for organ transplantation programs. Am J Transplant 2020; 20: 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chadban SJ, McDonald M, Wyburn K, et al. Significant impact of COVID‐19 on organ donation and transplantation in a low‐prevalence country: Australia. Kidney Int 2020; 98: 1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martino F, Plebani M, Ronco C. Kidney transplant programmes during the COVID‐19 pandemic. Lancet Respir Med. 2020; 8: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qu Z, Oedingen C, Bartling T, Schrem H, Krauth C. Organ procurement and transplantation in Germany during the COVID‐19 pandemic. Lancet 2020; 396: 1395. [DOI] [PubMed] [Google Scholar]
- 24. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and Kidney Transplantation. N Engl J Med 2020; 382: 2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cravedi P, Suraj SM, Azzi Y, et al. COVID‐19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant 2020; 20: 3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: A single‐center case series from Spain. Am J Transplant 2020; 20: 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nair V, Jandovitz N, Hirsch JS, et al. COVID‐19 in kidney transplant recipients. Am J Transplant 2020; 20: 1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen TY, Farghaly S, Cham S, et al. COVID‐19 pneumonia in kidney transplant recipients: Focus on immunosuppression management. Transpl Infect Dis 2020; 22: e13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pérez‐Sáez MJ, Blasco M, Redondo‐Pachón D, et al. Use of tocilizumab in kidney transplant recipients with COVID‐19. Am J Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aziz H, Lashkari N, Yoon YC, et al. Effects of Coronavirus Disease 2019 on Solid Organ Transplantation. Transplant Proc. 2020; 5252, 2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, et al. Covid‐19 in transplant recipients: the spanish experience. Am J Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azzi Y, Parides M, Alani O, et al. COVID‐19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int 2020; 98: 1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bell S, Campbell J, McDonald J, et al. COVID‐19 in patients undergoing chronic kidney replacement therapy and kidney transplant recipients in Scotland: findings and experience from the Scottish renal registry. BMC Nephrol 2020; 21: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA‐EDTA Registry indicate a high mortality due to COVID‐19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS‐CoV‐2 infection: The Brescia Renal COVID task force experience. Am J Transplant 2020; 20: 3019–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID‐19‐related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to Covid‐19 in recipients of kidney transplants. Kidney Int 2020; 98: 1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Favà A, Cucchiari D, Montero N, et al. Clinical characteristics and risk factors for severe COVID‐19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am J Transplant 2020; 20: 3030–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lum E, Bunnapradist S, Multani A, et al. Spectrum of Coronavirus Disease 2019 Outcomes in Kidney Transplant Recipients: A Single‐Center Experience. Transplant Proc 2020; 52: 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi SG, Rogers AW, Saharia A, et al. Early Experience With COVID‐19 and Solid Organ Transplantation at a US High‐volume Transplant Center. Transplantation 2020; 104: 2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coates PT, Wong G, Drueke T, Rovin B, Ronco P. Early experience with COVID‐19 in kidney transplantation. Kidney Int 2020; 97: 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID‐19? An ERA‐EDTA DESCARTES expert opinion. Nephrol Dial Transplant 2020; 35: 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lentine KL, Lam NN, Caliskan Y, et al. Hydroxychloroquine and Maintenance Immunosuppression Use in Kidney Transplant Recipients: Analysis of Linked U.S. Registry and Claims Data. Clin Transplant 2020; 34: e14118 [DOI] [PubMed] [Google Scholar]
- 44. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid‐19 ‐ Final Report. N Engl J Med 2020; 383: 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lavacca A, Presta R, Gai C, et al. Early effects of first‐line treatment with anti‐interleukin‐6 receptor antibody tocilizumab for chronic active antibody‐mediated rejection in kidney transplantation. Clin Transplant 2020; 34: e13908. [DOI] [PubMed] [Google Scholar]
- 46. Smolen JS, Beaulieu A, Rubbert‐Roth A, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet 2008; 371: 987. [DOI] [PubMed] [Google Scholar]
- 47. Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell‐Induced Severe or Life‐Threatening Cytokine Release Syndrome. Oncologist 2018; 23: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pereira MR, Aversa MM, Farr MA, et al. Tocilizumab for severe COVID‐19 in solid organ transplant recipients: a matched cohort study. Am J Transplant 2020; 20: 3198–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trujillo H, Caravaca‐Fontán F, Sevillano Á, et al. Tocilizumab use in Kidney Transplant Patients with COVID‐19. Clin Transplant 2020; 34: e14072 [DOI] [PubMed] [Google Scholar]
- 50. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int 2020; 97: 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid‐19 ‐ Preliminary Report. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid‐19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baan CC, Kannegieter NM, Felipe CR, Tedesco Silva H Jr. Targeting JAK/STAT Signaling to Prevent Rejection After Kidney Transplantation: A Reappraisal. Transplantation 2016; 100: 1833. [DOI] [PubMed] [Google Scholar]
- 54. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A Randomized Trial of Convalescent Plasma in Covid‐19 Severe Pneumonia. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fung M, Nambiar A, Pandey S, et al. Treatment of Immunocompromised COVID‐19 patients with Convalescent Plasma. Transpl Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. An EUA for Bamlanivimab‐A Monoclonal Antibody for COVID‐19 . Jama. 2020.
- 57. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid‐19. N Engl J Med 2020; 384: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID‐19 pandemic response. Lancet Infect Dis 2020; 20: e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science 2020; 370: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID‐19: A case series from the United States. Am J Transplant. 2020; 20: 3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prendecki M, Clarke C, Gleeson S, et al. Detection of SARS‐CoV‐2 Antibodies in Kidney Transplant Recipients. J Am Soc Nephrol. 2020; 31: 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS‐CoV‐2 ‐ Preliminary Report. N Engl J Med 2020; 383: 1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid‐19 Vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fairhead T, Hendren E, Tinckam K, et al. Poor seroprotection but allosensitization after adjuvanted pandemic influenza H1N1 vaccine in kidney transplant recipients. Transpl Infect Dis 2012; 14: 575. [DOI] [PubMed] [Google Scholar]
- 65. Candon S, Thervet E, Lebon P, et al. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant 2009; 9: 2346. [DOI] [PubMed] [Google Scholar]
- 66. Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Danziger‐Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant 2019; 33: e13563. [DOI] [PubMed] [Google Scholar]


