The causes of posterior reversible encephalopathy syndrome continue to be debated and other underlying issues are being added to its etiology. In this short and retrospective study, the authors assessed the role of low serum albumin in the development of PRES. The causes of PRES varied and the authors used diffusion-weighted and fluid-attenuated inversion recovery sequences to characterize the edema as vasogenic (N=22) or cytotoxic (N=6). All studies were done within 3 days of the onset of clinical symptoms. Review of laboratory values showed that during this time low serum albumin in 21 of 28 patients was more common and marked in those with vasogenic edema. Although all of this is simple and clear, the role of albumin is sort of like the “chicken and egg” question: Is there more leakage of fluids due to the low albumin, or is it low because it is actively leaking with fluid due to other processes affecting the blood vessel walls? Read the article to find out more!
SUMMARY:
PRES is a clinicoradiologic entity, combining seizures, blindness, and coma with MR imaging findings of predominantly vasogenic and occasional cytotoxic edema. In this clinical report, we determined the type of edema by using DWI and FLAIR sequences on MR imaging as well as ADC maps in 28 patients with PRES. The neuradiologic findings were correlated with levels of serum albumin, which is a main contributor to colloid osmotic pressure and vascular integrity. The presence of vasogenic edema was significantly associated with decreased serum albumin levels, which may be a particular risk factor for the development of PRES.
PRES is a clinicoradiologic entity with acute onset of seizures, blindness, alterations of consciousness, and headache. MR imaging is of great importance in the diagnosis of this clinically inhomogeneous syndrome, which typically shows bilateral signal-intensity alterations in cortical and subcortical regions of the posterior circulation, indicating vasogenic edema.1 A low proportion of patients show cytotoxic edema or lesions outside the posterior circulation.2 An association between the etiology of PRES and MR imaging findings has been negated in a recent investigation.3 Additionally, it remains unclear why patients develop either vasogenic or cytotoxic edema in PRES. Cytotoxic and vasogenic edema in PRES can be differentiated by using DWI and FLAIR sequences.4
It has been postulated that PRES is caused by endothelial damage and dysfunctional cerebral autoregulation due to excessive hypertension, leading to hyperperfusion and subsequent vasogenic edema in susceptible vessels.5,6 Other risk factors are pre-eclampsia, sepsis, cytotoxic and immunosuppressant drugs, and nephrotic syndrome.7 These conditions all involve high oxidative stress and a systemic proinflammatory process,8 hinting at additional mechanisms involved in PRES pathogenesis, such as vasoactive substances, vasospasm, and microinfarctions.9
As seen in nephrotic syndrome, most diseases associated with PRES exhibit significantly reduced serum albumin levels.10 Serum albumin accounts for 75% of plasma protein and, therefore, is a main contributor to COP, reducing perfusion pressure, which results in retention of fluid in the vessel.11 Vasogenic edema can be aggravated by a marked decrease in COP.12 Albumin protects vascular endothelial cells from oxidative stress and damage,13 acting as an important antioxidant that preserves vascular integrity.
This clinical report describes 28 patients with PRES and focuses on the type of edema in correlation with serum albumin. We show that vasogenic edema in PRES occurs significantly more often in patients with reduced serum albumin.
Case Series
Patients and Methods
Twenty-eight patients (7 men, 21 women; mean age, 43 ± 18.6 years) with typical clinical symptoms of PRES and characteristic MR imaging findings were included in this study. Primary clinical data were retrieved via chart review, and the exact date of primary symptom presentation was determined.
MR imaging was performed at a single institution on 1.5T and 3T MR imaging scanners between 1999 and 2009. Due to the extended period of study, protocols differed slightly with time, but FLAIR, DWI, and coronal T2-weighted sequences were available for all patients. While ADC maps were available in 17 patients, 11 had no ADC maps calculated. In those patients, the type of edema was determined by using DWI and FLAIR sequences.4
All MR imaging scans were reviewed by a single investigator (D.P.) blinded to clinical data. Vasogenic edema was diagnosed in cases of signal-intensity hyperintensity on FLAIR combined with signal-intensity hypointensity on DWI sequences and high signal intensity on ADC maps. Cytotoxic edema was diagnosed by the combination of DWI hyperintensity with hypo- or isointensity on FLAIR, T2-weighted sequences, and ADC maps.4,14 The location of lesions was classified as typical (uni- or bilateral occipital lesions, with or without involvement of frontal, temporal, or parietal lobes), atypical (supratentorial lesions without involvement of 1 or both occipital lobes), or extended (deep gray matter involvement and/or infratentorial involvement).
For statistical analysis, the Statistical Package for the Social Sciences software, Version 15.01 for Windows (SPSS, Chicago, Illinois) was used. Results are expressed as mean ± SD. Normal distribution of data was tested with the KS test. Intergroup comparison was performed by using the MWU test for variables not normally distributed, the Student t test for normally distributed variables, and the χ2 test for dichotomous variables. Stepwise multivariate logistic regression analysis was performed to identify factors related to PRES occurrence and to exclude possible confounders. Univariate ANOVA was performed to correct for multiple testing.
Results
A total of 28 patients with PRES were included in this analysis (7 men, 21 women; mean age, 43 ± 18.6 years). Risk factors for PRES, such as pregnancy and eclampsia, were present in 8/21 female patients (36%). Eleven patients were on cytotoxic medication at the onset (including cyclosporine, cyclophosphamide, vinchristine, and the combination of gemcitabine with oxaliplatin) either because of organ transplantation in 5 patients, malignancies in 5 patients, or autoimmune disorder (systemic lupus erythematosus) in 1 patient. Serum levels were within therapeutic range in all patients at onset. One patient was in the intensive care unit due to sepsis. Blood pressure was reported to be elevated at onset in 11/15 patients, with hypertension being the only risk factor for PRES in 3 of them.
Symptoms were delirium in 9/28 patients, qualitative changes of consciousness in 12/28 patients, and epileptic seizures in 19/28 patients. Furthermore, 10 patients reported deterioration of visual acuity, while headache was reported in 6 patients. No association between etiology, clinical presentation, or distribution of lesions on MR imaging could be identified.
According to the described MR imaging criteria, 22 patients had vasogenic and 6 patients had cytotoxic edema (Fig 1). MR imaging was performed on the day of onset in 21/28 patients, on day 1 after symptom onset in 3/28, on day 2 in 1, on day 3 in 1, and on day 5 in 2 patients. The type of edema was not associated with the interval between the onset of symptoms and MR imaging (MWU, P = .89).
Fig 1.
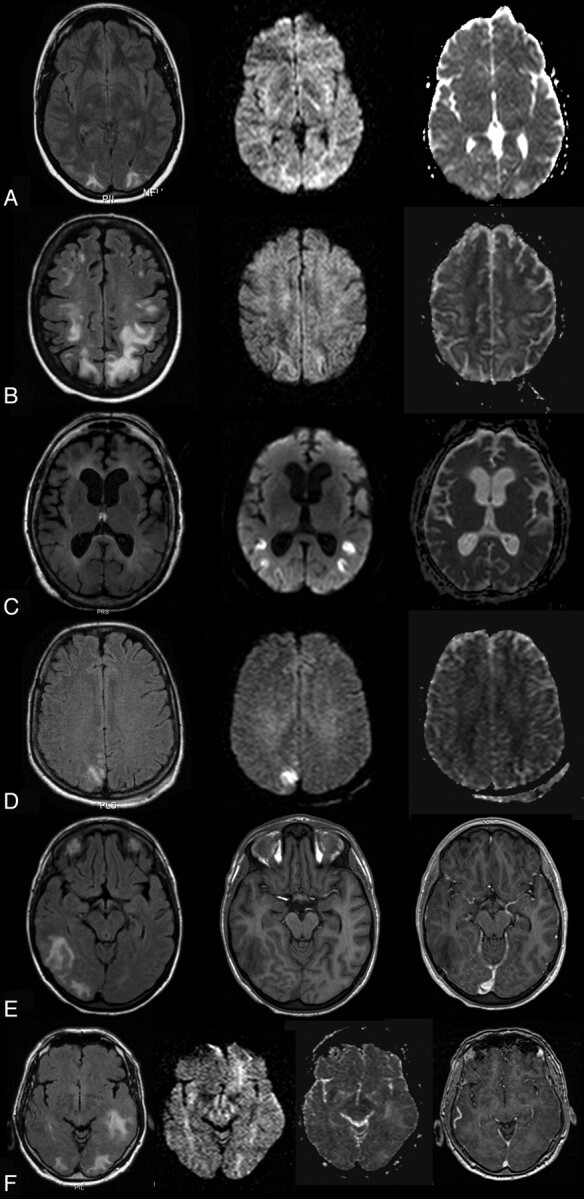
A, A 22-year-old woman with an albumin level of 24.7 mg/dL, on immunosuppressive medication (steroids and cyclosporine) and having systemic lupus erythematosus. Signal intensity is hyperintense bilaterally occipitally on FLAIR (left), isointense on DWI (middle), and hyperintense on ADC, indicating vasogenic edema. B, A 40-year-old woman with an albumin level of 21 mg/dL and blood pressure at the onset of 180/95 mm Hg and nephritis. Signal intensity is hyperintense bilaterally occipitally on FLAIR (left), isointense on DWI (middle), and hyperintense on ADC (right), indicating vasogenic edema. C, A 68-year-old woman with albumin levels of 38.3 mg/dL and chronic alcohol abuse. Her blood pressure at onset was 180/90 mm Hg. Signal intensity is isointense on FLAIR (left), hyperintense on DWI (middle), and hypointense on ADC (right), indicating cytotoxic edema. D, A 29-year-old woman with albumin levels of 37.8 mg/dL and eclampsia. Signal intensity is slightly hyperintense on FLAIR (left), hyperintense on DWI (middle), and isointense on ADC (right), indicating cytotoxic edema but also vasogenic components. E, A 19-year-old woman with an albumin level of 28.9 mg/dL, a hypertensive episode of 170/90 mm Hg, and renal failure. Signal intensity is hyperintense on FLAIR (left); there are no signal-intensity abnormalities on T1 (middle) and no gadolinium enhancement (left). F, A 78-year-old man with albumin levels of 22.6 mg/dL with immunosuppression (cyclosporine), renal transplantation, and blood pressure of 160/70 mm Hg. Signal intensity is hyperintense on FLAIR (left), isointense on DWI (middle left), hyperintense on ADC (middle right), and isointense on T1, indicating vasogenic edema.
Serum albumin was normally distributed (P = 0.941, KS test). Decreased serum albumin concentration (<35 mg/mL) was noted in 21/28 patients (71%; mean, 31 ± 7 mg/dL) in general. Serum albumin decrease was more common in patients with vasogenic edema (19/22, 86%) compared with edema with cytotoxic components (1/6, 17%), (χ2, P = .001). In cases of pure vasogenic edema, serum albumin concentration was significantly lower (29.3 ± 4.2 mg/dL) than in cases with cytotoxic components (38.4 ± 6.7 mg/dL; Student t test, P = .004; 95% CI, 3.2–15.1) (Fig 2). None of the other serum parameters tested were significantly associated with the type of edema (Table). Regarding etiologic factors, the presence of malignancies only showed a weak association with cytotoxic edema (χ2, P = .05) (Table). Clinically, only headaches were significantly associated with the presence of cytotoxic edema (χ2, P = .023). After logistic regression (parameters: albumin, presence of tumor, age, and sex), only the influence of reduced serum albumin concentration on the type of edema remained statistically significant (P = .019; adjusted odds ratio, 0.87; 95% CI, 0.63–0.96). Albumin also remained the only parameter significantly associated with the type of edema after correction for multiple testing by using ANOVA (P = .004). No association was found between albumin levels and other laboratory parameters or etiology.
Fig 2.
Boxplots show serum albumin levels (milligrams per deciliter) in cytotoxic (left) and vasogenic (right) edema.
Laboratory and clinical parameters
| KS Testa | Vasogenic Edemac | Cytotoxic Edemac | Pd | |
|---|---|---|---|---|
| Age (yr) | .54b | 40.9 (16.76) | 50.67 (24) | .38 |
| Female | 16 (72) | 6 (100) | .149 | |
| Sodium | .904b | 137.5 (3.4) | 134.8 (5.5) | .54 |
| Potassium | .945b | 4.2 (0.32) | 3.6 (0.32) | .153 |
| Chloridee | .608b | 104.5 (3.92) | 98.2 (7.33) | .07 |
| Calcium | .890b | 2.16 (0.31) | 2.2 (0.23) | .9 |
| Creatinine | .046 | 0.92 (0.66–2.4) | 0.91 (0.62–1.67) | .76 |
| Albuminf | .941b | 29.26 (6.71) | 38.43 (4.2) | .004 |
| Aspartate transaminase | .024 | 45 (24–79) | 32 (21–45) | .22 |
| Alanine aminotransferase | .008 | 23 (10–145) | 23 (18–41) | .98 |
| γ-Glutamyltransferase | .012 | 73 (18–178) | 130 (79–200) | .37 |
| Red blood cells | .718b | 3.61 (0.78) | 4.11 (0.60) | .26 |
| Hemoglobin | .937b | 11 (2.56) | 12 (2.15) | .14 |
| Hematocrit | .985b | 33.16 (7.72) | 36.65 (5.74) | .25 |
| White blood cells | .809b | 11.16 (5.69) | 7.62 (5.11) | .18 |
| Threonine | .957b | 175.03 (110.45) | 202.58 (155.77) | .7 |
| Hypertension | 8 (73) | 3 (75) | .73 | |
| Pregnancy | 7 (44) | 1 (16) | .35 | |
| Immunosuppression | 9 (41) | 2 (33) | .56 | |
| Tumore | 2 (9) | 3 (50) | .05 | |
| Transplantation | 4 (18) | 1 (16) | .72 | |
| Coma, delirium | 17 (90) | 4 (80) | .52 | |
| Seizure | 15 (83) | 4 (80) | .65 | |
| Disturbance of vision | 7 (64) | 3 (60) | .65 | |
| Headachee | 2 (17) | 4 (80) | .03 |
P value of KS test.
Normally distributed variables.
Mean (± SD) for normally distributed variables, median (25%–75% quartile) for variables not normally distributed, or n (%) for nominal variables.
P values of the Student t test for normally distributed variables, the MWU test for variables not normally distributed, and the χ2 test for paired variables. Significance is indicated as P < .05 and P < .01.
P < 0.05.
P < .01.
Three patients had pure vasogenic edema despite normal serum albumin levels at onset. In 2 of these 3 patients, the MR imaging had been performed 3 and 5 days after onset, respectively, when the type of edema may possibly already have changed. The third patient had undergone pulmonary transplantation 1 year before onset of PRES. Chronic graft rejection had been diagnosed before the onset of PRES, and the patient died several months after primary PRES presentation as a result of the graft rejection. The vasogenic edema in this patient might have been related to excessive inflammatory activity with consequent vascular damage (see “Discussion”).
Typical PRES with uni- or bilateral occipital involvement was present in all patients, and no patient had atypical PRES without occipital involvement. Nine patients had extended PRES with additional involvement of deep gray matter, infratentorial involvement, or both. Patients with extended PRES had lower serum albumin concentrations (28.8 ± 7.0) than patients with typical PRES (32.3 ± 7.3) without reaching statistical significance (Student t test, P = .23; 95% CI, −2.37–9.62).
A follow-up MR imaging was performed in 22/28 patients, between day 1 and day 307. Complete edema remission was achieved in 10 patients (45%); partial remission, in the remainder.
Visual disturbances recovered in 10/13 patients. The level of consciousness recovered in 14/16 patients, while 2/21 patients were still in delirium at follow-up. Additionally, 10/14 patients were free of seizures at follow-up. Complete edema remission on MR imaging was associated with complete remission of seizures (χ2, P = .018). There was no statistical association between complete edema remission on MR imaging and etiology, type of edema, serum albumin level, or clinical presentation at onset.
Discussion
In a clinical report of 28 consecutive patients with PRES, we focused on the type of edema and serum albumin levels. We found that patients with vasogenic edema had significantly decreased serum albumin levels compared with edema with cytotoxic components, indicating that low serum albumin levels may be involved in the development of vasogenic edema in PRES.
Vasogenic edema in PRES has been attributed so far to the hypothesis that increased perfusion pressure overwhelms cerebral autoregulation with consecutive hyperperfusion and fluid extravasation. However, PRES has been increasingly observed in patients on immunosuppressive therapy or chemotherapy and in patients with infection, sepsis, and shock without elevation of systemic blood pressure,15 suggesting additional pathophysiologic mechanisms.2 As mentioned below, many conditions prone to PRES are characterized by decreased serum albumin levels and a proinflammatory systemic process, as has been recently reviewed by Bartynski8: Proinflammatory cytokines such as IL-1, IL-6, interferon-γ, and tumor necrosis factor-α are elevated during sepsis and preeclampsia. The proinflammatory cytokine milieu and, in some conditions, additional T-cell activation lead to endothelial cell activation and eventually to T-cell trafficking,16 endothelial cell swelling, and production of vasoactive substances resulting in disturbed microcirculation with either increased vascular permeability or vasoconstriction.8 Albumin plays an integral part in this systemic process. In addition to inflammation, clinical conditions particularly prone to PRES are often associated with high oxidative stress and decreased serum albumin levels.17,18
Serum albumin has a mean life span of 14–18 days.19 In healthy individuals, 30%–40% of serum albumin is maintained within the vascular compartment; it leaks from plasma at a rate of 5% per hour and is returned at an equivalent rate.20 Albumin can be reduced as a consequence of an elevated metabolic albumin turnover, accelerated aging of the molecules through oxidative stress, or increased leakage due to immune-mediated vasculopathy.20,21 In line with these characteristics, 71% of our patients had decreased serum albumin levels.
Serum albumin normally accounts for 75% of protein in blood plasma and, therefore, also for 75% of COP. COP affects osmotic pressure because the negative charges attract sodium, thus holding water and exerting an antagonistic effect on perfusion pressure.12,18 In conditions with endothelial damage due to inflammatory processes, reduction of COP may facilitate fluid extravasation and development of vasogenic edema.
Albumin is one of the most important plasma antioxidants, scavenging and detoxifying ROS.22 Excessive production of ROS, as seen in conditions predisposing to PRES, can lead to damage of several molecules, including albumin, and endothelial dysfunction, and may contribute to disease progression.21 Low albumin levels or damage to albumin molecules themselves in the context of abundant oxidative stress (eg, in proinflammatory conditions) may blunt the antioxidative properties of albumin and promote endothelial damage.10 Moreover, albumin may directly influence vascular integrity by binding to the extracellular matrix and subendothelium and by reducing the permeability of these layers to large molecules.13,18
In accordance, vasogenic edema in PRES was significantly more frequent in patients with decreased serum albumin levels, whereas cytotoxic components occurred more frequently in patients with normal serum albumin levels.
A possible correlation between low albumin levels and PRES is supported by Ishikura et al,23 who reported 7 pediatric patients who developed PRES during nephrotic syndrome and low levels of serum albumin. In these patients, PRES rapidly abated after substitution of albumin, despite further treatment with cytotoxic substances. Similarly, ifosfamide-induced cerebral vasogenic edema, a condition resembling PRES, seems to occur exclusively in patients with low serum albumin levels.24 In contrast, cytotoxic edema in PRES may contribute to cerebral vasospasm, with endothelial activation or injury leading to reduced cerebral blood flow with consequent hypoxia, as described in several PRES studies using MR imaging or conventional angiography.9,25
Due to the retrospective character of our case series, we acknowledge certain limitations of our results. MR imaging protocols differed between patients, and ADC maps were not available for 9 of our patients. In those patients, the type of edema was determined by using only DWI and FLAIR sequences. Clinical data were obtained from medical reports. Therefore, it is conceded that certain minimal symptoms at presentation may have been missed due to a lack of documentation. Caution is needed in interpreting blood pressure in the context of albumin in our patient sample, because blood pressure was documented at the onset of symptoms in only 15 patients. Unfortunately, our retrospective sample size (N = 28) does not provide sufficient information to draw statistically sound conclusions on whether PRES present outside the posterior circulation is associated with decreased serum albumin levels and, second, whether the extent of vasogenic edema might be associated with reduction of serum albumin levels.
Patients developing a decrease in serum albumin levels seem to be at particular risk for the development of vasogenic edema and eventually PRES. Because PRES is not necessarily benign and reversible in all patients, more focused and accurate treatment strategies are needed. Infusion of human serum albumin at an early and reversible phase to increase oncotic pressure and restore antioxidative potential could theoretically prevent widespread oxidative and/or ischemic damage to vulnerable vascular structures in PRES and should be subjected to prospective studies.
Abbreviations
- ADC
apparent diffusion coefficient
- ANOVA
analysis of variance
- CI
confidence interval
- COP
colloid osmotic pressure
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- IL
Interleukin
- KS
Kolmogorov-Smirnov
- MWU
Mann Whitney U
- NO
nitric oxide
- PRES
posterior reversible encephalopathy syndrome
- ROS
reactive oxygen species
Footnotes
Paper previously presented at: Symposium Neuroradiologicum and The World Congress of Neuroradiology, October 4–9, 2010; Bologna, Italy.
References
- 1. Hinchey J, Chaves C, Appignan B, et al. A reversible posterior encephalopathy syndrome. N Engl J Med 1996;334:494–500 [DOI] [PubMed] [Google Scholar]
- 2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiological findings. Mayo Clin Proc 2010;85:427–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mueller-Mang C, Mang T, Pirker A, et al. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiol 2009;51:373–85 [DOI] [PubMed] [Google Scholar]
- 4. Doelken M, Lanz S, Rennert J, et al. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol 2007;13:125–28 [PubMed] [Google Scholar]
- 5. Ijima T, Kubota Y, Kuroiwa T, et al. Blood-brain barrier opening following transient reflex sympathetic hypertension. Acta Neurochir Suppl 1994;60:142–44 [DOI] [PubMed] [Google Scholar]
- 6. Sheth R, Rigs J, Bodensteiner J, et al. Parietal occipital edema in hypertensive encephalopathy: a pathologic mechanism. Eur Neurol 1996;36:25–28 [DOI] [PubMed] [Google Scholar]
- 7. Gocmen R, Ozgen B, Oguz K. Widening the spectrum of PRES: a series from a tertiary care center. Eur J Radiol 2007;62:454–59 [DOI] [PubMed] [Google Scholar]
- 8. Bartynski WS. Posterior reversible encephalopathy syndrome. Part 2. Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043–49. Epub 2008 Apr 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weidauer S, Gaa J, Sitzer M, et al. Posterior encephalopathy with vasospasm: MRI and angiography. Neuroradiology 2003;45:869–76 [DOI] [PubMed] [Google Scholar]
- 10. Lewis D, Tooke J, Meaman M, et al. Peripheral microvascular parameters in the nephrotic syndrome. Kidney Int 1998;54:1261–67 [DOI] [PubMed] [Google Scholar]
- 11. Quinlan G, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211–19 [DOI] [PubMed] [Google Scholar]
- 12. Vande Walle J, Dockerwolcke R. Pathogenesis of edema formation in the nephrotic syndrome. Pediatr Nephrol 2001;16:283–93 [DOI] [PubMed] [Google Scholar]
- 13. Vogel SM, Minshall RD, Pilipovic M, et al. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol 2001;281:L512–22 [DOI] [PubMed] [Google Scholar]
- 14. Chou M, Lai P, Yen L, et al. Posterior reversible encephalopathy syndrome: magnetic resonance imaging and diffusion-weighted imaging in 12 cases. Kaoshiung J Med Sci 2004;20:381–88 [DOI] [PubMed] [Google Scholar]
- 15. Bartynski WS, Boardman J, Zeiger Z, et al. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 2006;27:2179–90 [PMC free article] [PubMed] [Google Scholar]
- 16. Horbinski C, Bartynski WS, Carson-Walter E, et al. Reversible encephalopathy after cardiac transplantation: histologic evidence of endothelial activation, T-cell specific trafficking, and vascular endothelial growth factor expression. AJNR Am J Neuroradiol 2009;30:588–90. Epub 2008 Oct 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaysen G. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin Am Soc Nephrol 2009;4(suppl 1):S56–63 [DOI] [PubMed] [Google Scholar]
- 18. Margarson M, Soni N. Serum albumin: touchstone or totem. Anaesthesia 1998;53:789–803 [DOI] [PubMed] [Google Scholar]
- 19. Peters T. All About Albumin: Biochemistry, Genetics and Medical Applications. San Diego: Academic Press; 1996 [Google Scholar]
- 20. Nicholson J, Wolmarans M, Park G. The role of albumin in critical illness. Br J Anaesthes 2000;85:599–610 [DOI] [PubMed] [Google Scholar]
- 21. Bito R, Hino S, Baba A, et al. Degradation of oxidative stress induced denatured serum albumin in rat liver endothelial cells. Am J Physiol Cell Physiol 2005;289–91:C531–642. Epub 2005 May 4 [DOI] [PubMed] [Google Scholar]
- 22. Fernandez J, Navasa M, Garcia-Pagan JC, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vasoactive neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol 2004;41:384–90 [DOI] [PubMed] [Google Scholar]
- 23. Ishikura K, Ikeda M, Hamasaki Y, et al. Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis 2006;48:231–39 [DOI] [PubMed] [Google Scholar]
- 24. Sweiss K, Beri R, Shord SS. Encephalopathy after high dose ifosfamide: a retrospective cohort study and review of the literature. Drug Saf 2008;31:989–96 [DOI] [PubMed] [Google Scholar]
- 25. Bartynski WS, Boardman J. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]




