SUMMARY:
DWI is a useful technique for the evaluation of cholesteatomas. It can be used to detect them when the physical examination is difficult and CT findings are equivocal, and it is especially useful in the evaluation of recurrent cholesteatoma. Initial DWI techniques only detected larger cholesteatomas, >5 mm, due to limitations of section thickness and prominent skull base artifacts. Newer techniques allow detection of smaller lesions and may be sufficient to replace second-look surgery in patients with prior cholesteatoma resection.
Cholesteatomas are enlarging collections of keratin within a sac of squamous epithelium and may be congenital or acquired.1 Acquired cholesteatomas generally occur in the middle ear and mastoid, whereas congenital cholesteatomas or epidermoids can occur in other locations, including the cerebellopontine angle, suprasellar cistern, calvarium, and multiple sites in the temporal bone. Congenital cholesteatomas compose only 2% of middle ear cholesteatomas.2
There are multiple theories regarding cholesteatoma development, but most authors believe there is a disruption of the normal process in which skin lining the tympanic membrane migrates externally within the external auditory canal. Retraction pockets, which are invaginations of the tympanic membrane into the middle ear cavity, develop and interfere with this process. These pockets are largely due to chronic otitis media and eustachian tube dysfunction, which can cause negative middle ear pressure. Retraction pockets occur most commonly in the pars flaccida of the membrane and less commonly in the pars tensa. Epithelial ingrowth may occur as a result of this process, and squamous debris can become trapped within these retraction pockets in the middle ear space.1,3 Many authors also believe that there is a hereditary predisposition to the development of acquired cholesteatomas.2
Complications of cholesteatomas are related to bony erosion. Erosion is generally thought to be related to mechanical pressure, though some believe that adjacent granulation tissue, an osteoclast stimulator, or collagenase production is necessary.1,2 Bony erosion can result in destruction of the ossicles, creating conductive hearing loss, labyrinthine fistulas with sensorineural hearing loss and vertigo, facial nerve canal erosion and facial paralysis, and rare intracranial complications, such as meningitis and abscess.1,2
The treatment for middle ear cholesteatomas is surgical excision. Small cholesteatomas limited to the Prussak space without significant bone erosion can often be effectively resected by using a transcanal atticotomy approach with subsequent tympanoplasty. Patients may undergo a canal wall up or canal wall down tympanomastoidectomy for more extensive disease, often requiring an ossiculoplasty to reconstruct the ossicular conductive mechanism of the middle ear. Canal wall down tympanomastoidectomy provides the surgeon with a larger surgical exposure and is associated with a lower recurrence rate, though this technique may be associated with worse postoperative conductive hearing than the canal wall up procedure.4 Both procedures can use a nontranslucent cartilage graft to reconstruct the tympanic membrane, which limits visualization of the middle ear in the postoperative setting.
Patients have traditionally undergone 2-stage operations for cholesteatoma removal, with a second-look procedure performed to check for residual or recurrent disease, often performed 6–18 months after the initial surgery.5,6 Most cholesteatomas recur within the first 2 postoperative years, with 60% occurring during the first year after surgery.7 Shelton and Sheehy5 found residual cholesteatoma in 43% of cases at re-exploration, with residual mastoid cholesteatoma being more prevalent with a canal wall up surgical technique. Gyo et al6 found 65 residual cholesteatomas in 48 of 167 ears (29%).
CT has widely been accepted for assessing the extent and location of disease and evaluating complications of cholesteatomas.8 Preoperative imaging is especially important for demonstrating disease in locations not easily visualized by the surgeon (such as the sinus tympani) and extension of disease into the epitympanum (attic) and mastoid antrum, and for revealing congenital anatomic variations (such as an aberrant course of the facial nerve).8 Occasionally CT will depict an unsuspected cholesteatoma, hidden from otoscopic view. CT is also used to look for recurrent disease following mastoidectomy, though granulation tissue and cholesteatoma have similar imaging characteristics on CT. CT is, therefore, most useful when the middle ear and mastoidectomy defect are aerated, but it lacks specificity when soft tissue is present.
Postcontrast T1-weighted MR imaging has been advocated as an effective technique for distinguishing granulation tissue from residual cholesteatoma.9–11 Cholesteatomas are avascular and do not enhance following contrast administration, whereas granulation tissue is poorly vascularized and does enhance on delayed images. With this technique, Ayache et al10 and Williams et al11 were able to detect larger cholesteatomas but often missed residual lesions <3 mm. Authors have typically advocated postcontrast imaging delays of 30–45 minutes, which is inconvenient for patients and decreases practice efficiency.
During the past several years, data have been published advocating DWI for evaluation of residual or recurrent cholesteatoma following mastoidectomy. The DWI technique adds a preparation period before the image acquisition that enhances MR signal intensity attenuation in response to diffusion and other spin motion occurring during this period.12 Although not well understood, cholesteatomas are hyperintense on DWI images compared with CSF and brain parenchyma, like epidermoid cysts, which are histologically identical. This may be due to a combination of T2 and diffusion effects13 or predominately a T2 shinethrough effect.14,15 Despite compelling data, many practices in the United States have yet to adopt DWI for evaluation of residual/recurrent cholesteatoma. The purpose of this article is to discuss the utility of DWI for evaluation of cholesteatomas and review the technical parameters.
Technical Considerations
When applying DWI to the evaluation of cholesteatoma, investigators have used a variety of techniques ranging from traditional spin-echo EPI-based to TSE-based techniques such as HASTE and BLADE (Siemens, Erlangen, Germany). These techniques use a similar method for encoding diffusion, but differ in the method of image acquisition. This methodology strongly impacts the sensitivity of each to factors such as bulk or physiologic motion and field inhomogeneities, factors which can be significantly problematic when imaging near the skull base (Table).
Pertinent DWI features and artifacts when imaging near the skull base
| Imaging Parameter/Artifacts | DWI- EPI | DWI- HASTE | DWI- BLADE |
|---|---|---|---|
| Scanning timea | 0:40–3:40 | 4:00 | 4:09–5:20 |
| Resolution/contrast | Low | Moderateb | High |
| T2 blurringb | No effect | ↑ | ↓ |
| Motion sensitivity | ↓ | ↓ | ↓ |
| Off-resonance effects | ↑ | ↓ | ↓ |
| Susceptibility effects | ↑ | ↓ | ↓ |
| Ghosting | ↑ | ↓ | ↓ |
| Geometric distortion | ↑ | ↓ | ↓ |
Represents a (minutes/seconds) range found in the literature for this application as well as actual scanning times for protocols used in our practice. No effort was made to normalize protocol parameters across investigators.
HASTE image quality near the skull base is frequently degraded by T2 blurring. This impact can vary depending on T2 in the region and imaging parameters used.
EPI-DWI
SS TSE EPI is the traditional choice for DWI, due to its speed and relative insensitivity to motion. Image quality by using this technique, however, can be degraded due to low resolution, low SNR, chemical shift artifacts, susceptibility artifacts, ghosting, and geometric distortion (Fig 1A). Distortion and susceptibility in the temporal bone make this a challenging technique for cholesteatoma evaluation16 because the resulting artifacts can mask areas of restricted diffusion.17
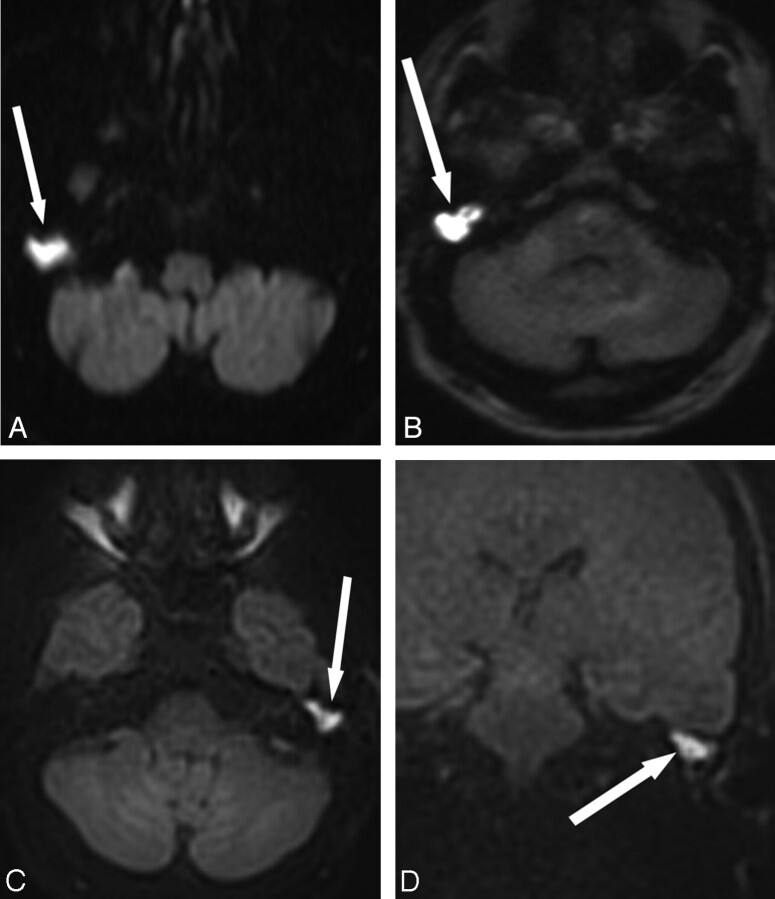
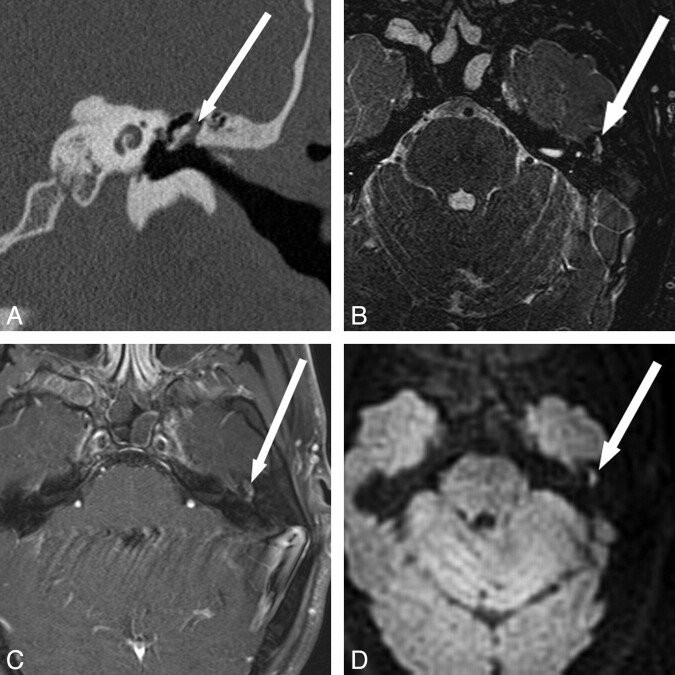
Fig 1.
Comparison of different DWI techniques. A, EPI DWI acquired in a patient undergoing evaluation for possible demyelinating disease. Abnormal DWI signal intensity in the right temporal bone (arrow) prompted further evaluation for cholesteatoma. The abnormal DWI signal intensity is clearly visible due to the large size of the lesion, but there are artifacts from the skull base. B, SS TSE (HASTE) DWI sequences obtained in a patient with obscured visual examination due to postoperative changes. Increased diffusion signal intensity is seen in the right middle ear and mastoid defect (arrow), with cholesteatoma confirmed at surgery. C, Multishot TSE DWI (BLADE) image in a patient with otoscopic examination obscured by cartilaginous reconstruction shows increased DWI signal intensity (arrow) in the left epitympanum, with cholesteatoma confirmed at surgery. D, The multishot TSE DWI has the additional advantage of generating images in a coronal plane, which can be especially useful when erosion of the tegmen tympani and/or intracranial extension is suspected. Arrow indicates increased DWI in the left epitympanum. Fig. 1B was reproduced with permission from Ear, Nose & Throat Journal (Schwartz KM, Lane JI, Neff BA, et al. Diffusion-weighted imaging for cholesteatoma evaluation. 2010;89:E14-19).32
DWI-HASTE
DWI-HASTE uses a SS TSE method for image acquisition (Fig 1B). As a single-shot technique, this sequence shares the low sensitivity to motion with EPI, albeit a slightly increased scanning time. Because the image acquisition of this technique is spin-echo-based, however, it does not exhibit the image distortion and susceptibility artifacts present in EPI-based techniques. The single TSE echo train is substantially longer than that in EPI, potentially causing image-quality degradation due to T2 decay during the acquisition.18 HASTE is designed to shorten the echo train, by using a half-Fourier acquisition, thereby reducing the impact of T2-blurring but with some negative impact on SNR.
DWI-BLADE
Multishot techniques can reduce the length of the echo train and mitigate T2 blurring effects; but with multiple echo trains contributing to a single diffusion measurement, the acquisition again becomes sensitive to motion. However, by using the BLADE sequence, sensitivity to bulk motion is greatly reduced (Fig 1C, -D). The BLADE sequence acquires k-space with several radially oriented TSE echo trains (blades) that overlap in the center of k-space. Because each blade crosses through the center of the k-space, each is essentially an independent single-shot low-resolution image with reduced motion sensitivity and little or no ghosting.17 The reconstruction of the high-resolution image from these low-resolution components retains these properties. The only drawback to DWI-BLADE is increased scanning time on the order of 4 times that of DWI-EPI. However, because the evaluation of cholesteatoma requires only limited coverage as opposed to whole-brain coverage, the resulting 4- to 5-minute scanning fits well into the imaging workflow.
Discussion
Patient Selection
Postoperative Ear.
Patients have traditionally undergone a second-look surgery 6–18 months following initial cholesteatoma surgery to evaluate for residual disease. This second surgery has been necessary due to limited visibility of the mastoid following canal wall up mastoidectomies or the middle ear due to tympanic membrane reconstruction using cartilage. CT is useful if no soft tissue is seen in the middle ear (or petrous apex or mastoid depending on original location of disease). If rounded soft tissue is present, then findings are suggestive of recurrent disease. However, if amorphous soft tissue or complete middle ear opacification is present, CT is nonspecific and cannot distinguish granulation tissue or scar tissue from recurrent disease.
Kimitsuki et al19 argued that spin-echo MR should not replace a second-look operation for the evaluation of recurrent cholesteatoma because of incorrect surgical correlation in 30% of their cases. Vanden Abeele et al20 also had disappointing results with MR imaging, with a surgical correlation of 50%–61%. However, in both studies, no DWI was used, and the postcontrast images were not delayed.
More recent studies have reported improved success in the detection of recurrent disease, with only small lesions missed when DWI sequences were used. Lesions of >5 mm have been reliably detected with the EPI-DWI technique,14,21–27 and even smaller lesions, with non-EPI techniques.15,28–31 In fact, De Foer et al28 argued that the SS TSE DWI sequence has high enough sensitivity, specificity, and positive and negative predictive values to replace routine second-stage surgery for the detection of residual cholesteatoma.
MR imaging with DWI sequences has been used at our institution for evaluation of patients with prior cholesteatoma resection with reliable results.32 This has been especially useful when the patient's otologic examination is obscured by an opaque tympanic membrane or cartilaginous reconstruction (Fig 2 ), when CT shows no definite bony erosion (Fig 2), when CT findings are equivocal (Fig 3), and to evaluate complications (Fig 3) and extent of disease (Fig 4 ). We did not find the apparent diffusion coefficient maps (in those cases in which they could be generated) helpful, a conclusion supporting the findings of Vercruysse et al14 and De Foer et al.15
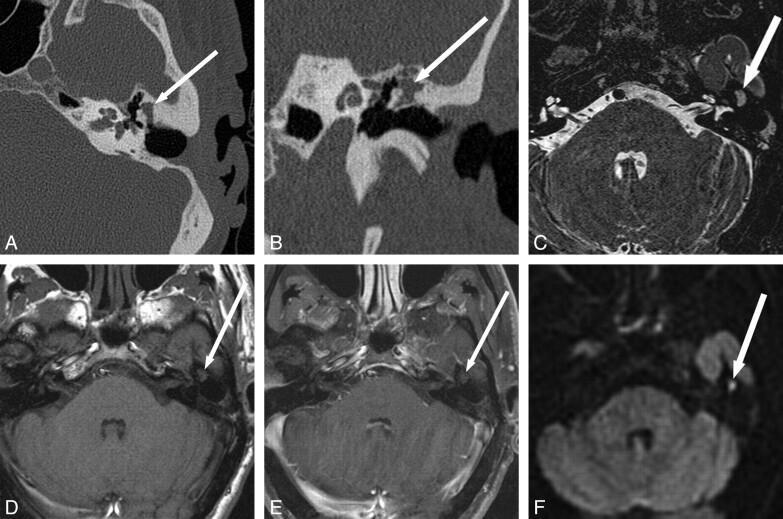
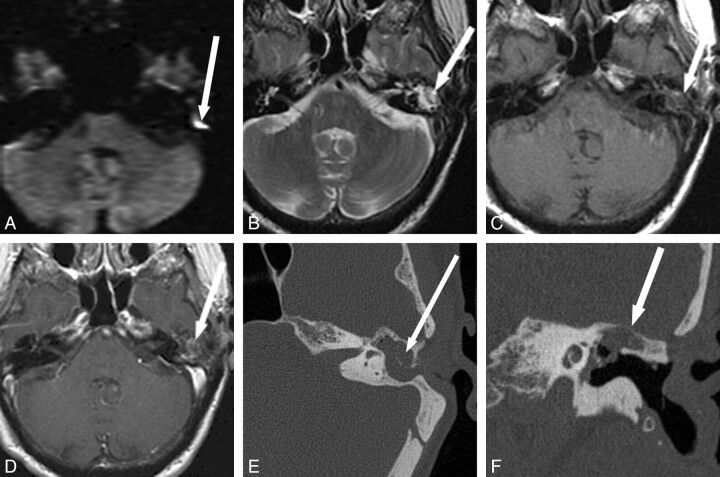
Fig 2.
Detection of recurrent cholesteatoma when physical examination is obscured and CT is indeterminate. A 53-year-old man with 3 prior left tympanomastoidectomies presented for routine follow-up with mildly progressive decreased hearing. Otologic examination was obscured by cartilage reconstruction and an opaque tympanic membrane. A and B, CT shows soft-tissue opacification of the Prussak space without definite bony erosion (arrow), considered indeterminate for recurrent disease versus postoperative scar or granulation tissue. C−E, MR imaging shows a corresponding area in the left middle ear (arrow) that is isointense on T2 (C) and T1 (D), without definite enhancement (E). BLADE DWI shows hyperintensity (arrow) in this same area, consistent with recurrent cholesteatoma. F, Cholesteatoma (arrow) was found in this location at surgery, confirmed by pathology. Fig 2A, C, and F reproduced with permission from Ear, Nose & Throat Journal (Schwartz KM, Lane JI, Neff BA, et al. Diffusion-weighted imaging for cholesteatoma evaluation. 2010;89:E14-19).32
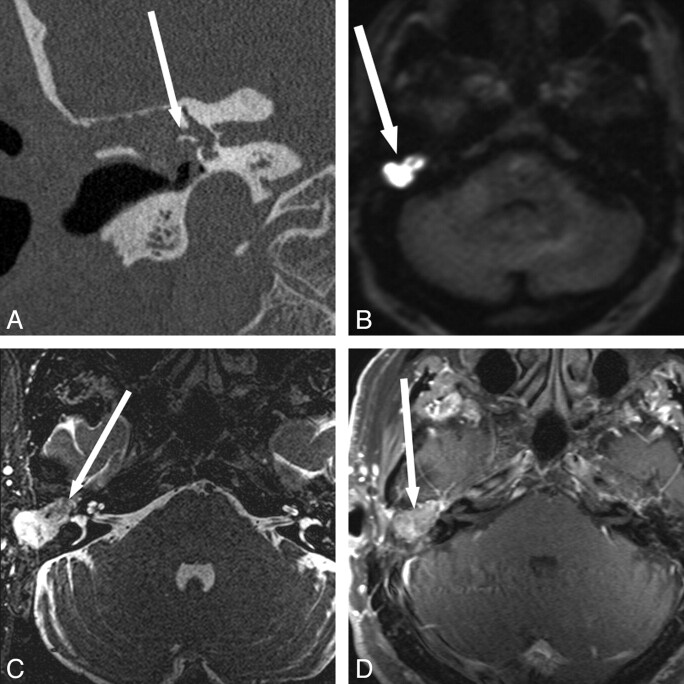
Fig 3.
Detection of recurrent disease and intracranial extension when otologic evaluation is obscured and CT is nonspecific. A 14-year-old girl with a long history of recurrent cholesteatoma and multiple surgeries. The middle ear is obscured due to a stenotic external auditory canal (A and B). CT shows nonspecific diffuse opacification of the mastoidectomy and middle ear (arrow). C−F, MR imaging shows T2 hyperintense (arrow, C) and T1 hypointense (arrow, D) regions with hyperintensity on BLADE DWI (arrow, E and F) along the superior aspect of the right temporal bone, suspicious for recurrent cholesteatoma with intracranial extension. The patient declined contrast material. At surgery, intradural extension of disease was confirmed.
Fig 4.
Evaluation of disease extent in a patient with lateral semicircular canal fistula. A 61-year-old man with multiple prior ear surgeries, including a right mastoidectomy for unknown reasons, presented with vertigo and imbalance. Visual inspection of the middle ear cavities was obscured by postoperative changes, but no cholesteatoma was seen. A and B, CT shows soft tissue (arrow) in the mastoid defect, external auditory canal, and epitympanum with bony erosion of the lateral semicircular canal. C and D, MR images show the extent of cholesteatoma and demonstrate a large area of hyperintensity on HASTE DWI in the mastoid defect and middle ear (B in Fig 1) with T2 hypointensity (arrow, C), and mild T1 hyperintensity but no definite enhancement (arrow, D). A portion of the right lateral semicircular canal is obscured by the soft-tissue mass (C), again consistent with the fistula shown on CT. Cholesteatoma and lateral semicircular canal fistula were confirmed at surgery. Fig 4A, B, and C reproduced with permission from Ear, Nose & Throat Journal (Schwartz KM, Lane JI, Neff BA, et al. Diffusion-weighted imaging for cholesteatoma evaluation. 2010;89:E14-19).32
Newly Diagnosed Cholesteatoma.
The initial diagnosis of cholesteatoma is generally made by otoscopic examination. A pearly white mass is seen behind the tympanic membrane, which is frequently retracted. CT may be performed to evaluate complications or extent of disease. MR imaging, specifically DWI, is not necessary in most of these patients. MR imaging is useful if there is erosion of the tegmen tympani to determine if intracranial extension is present (Fig 3) or if there is an associated meningocele/encephalocele, facial nerve canal dehiscence, and semicircular canal fistula (Fig 4). MR imaging can be helpful in evaluating a chronically draining ear with inflammation and polypoid disease that obscures physical examination with a nonspecific CT (Fig 5). We favor the BLADE DWI technique in these cases due to improved resolution compared with the HASTE technique and improved resolution and decreased artifacts at the skull base compared with EPI DWI images. Occasionally, the diagnosis of cholesteatoma may not be suspected, and restricted diffusion may incidentally be seen in the middle ear on MR imaging performed for an unrelated indication (Fig 6).
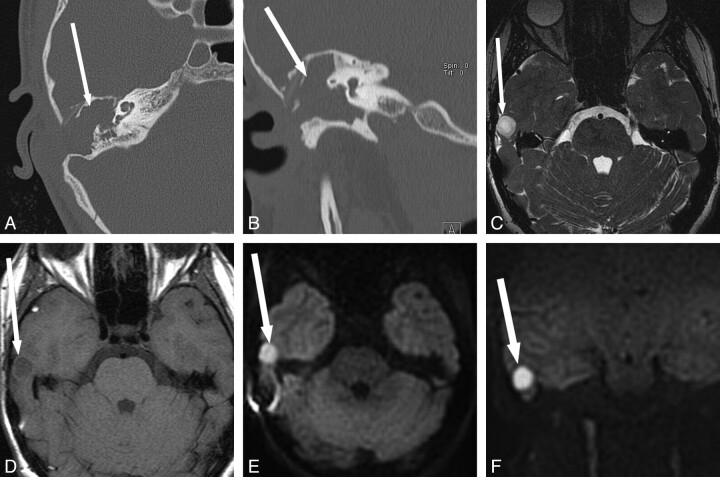
Fig 5.
Detection of de novo cholesteatoma in a patient with indeterminate findings on physical examination and CT. A 20-year-old man presented with left-sided hearing loss and prior otorrhea following traumatic tympanic membrane perforation. Visual inspection showed slight retraction of the pars flaccida of the tympanic membrane on the left without a definite cholesteatoma seen. A, Temporal bone CT scan shows soft-tissue opacification (arrow) in the left Prussak space without definite bony erosion. B and C, MR images show corresponding mild T2 hyperintensity (arrow, B), peripheral enhancement (arrow, C), and subtle increased DWI signal intensity (arrow, D) in the left epitympanum. Cholesteatoma was confirmed in this location at surgery.
Fig 6.
Incidental detection of recurrent cholesteatoma in a patient being evaluated for an unrelated indication. A, A 68-year-old woman with a history of 2 prior tympanomastoidectomies for cholesteatoma underwent MR imaging for unrelated reasons (meningioma evaluation) and was found to have increased DWI signal intensity (arrow) in the left temporal bone on EPI-DWI. B−D, This area is isointense on T2 (arrow, B) and T1 (arrow, C) and shows mild peripheral enhancement (arrow, D). E and F, Temporal bone CT shows soft-tissue opacification of the mastoid bowl, epitympanum, and mesotympanum (arrow, E) with thinning of the tegmen tympani (arrow, F). Surgery confirmed cholesteatoma in the area of DWI hyperintensity, with surrounding granulation tissue and encephalocele in the areas of soft-tissue opacification on CT without corresponding DWI abnormality. Fig 6A, B, and E reproduced with permission from Ear, Nose & Throat Journal (Schwartz KM, Lane JI, Neff BA, et al. Diffusion-weighted imaging for cholesteatoma evaluation. 2010;89:E14-19).32
Review of the Literature
Initial attempts at DWI for cholesteatoma evaluation used EPI-DWI techniques.14,16,21–25,27 The EPI-DWI technique was limited by large section thickness, susceptibility artifacts from the skull base, and low resolution. These EPI images were generally effective for detection of lesions ≥4 or 5 mm, but EPI frequently missed smaller lesions.14,21–26 This led Vercruysse et al14 and Venail et al22to advocate concurrent use of DWI, considered more specific, and postcontrast T1-weighted images, which were more sensitive.
Non-EPI techniques have more recently been proposed for the reliable detection of smaller cholesteatomas.15,28–31 These non-EPI DWI techniques have the advantage of smaller section thickness and better resolution and are less degraded by susceptibility artifacts.
In 1 study, De Foer et al15 evaluated, with SS TSE DWI, 21 patients strongly suspected of having a middle ear cholesteatoma and found 19 of 21 cholesteatomas. The false-negative cases included a cholesteatoma sac and a cholesteatoma in a child whose images had motion artifacts. The authors did note that lack of anatomic landmarks of the temporal bone on this sequence was a drawback.15 De Foer et al,28 in a different study, evaluated 32 consecutive patients with SS TSE DWI sequences 10–18 months after primary cholesteatoma surgery with canal wall up mastoidectomy and detected 9 of 10 residual cholesteatomas, measuring 2-6 mm, missing only one 2-mm lesion in a motion-degraded study. Dhepnorrarat et al29 detected and localized cholesteatomas by using SS TSE DWI in all 7 of 22 patients undergoing second-look surgery with recurrent disease, with cholesteatomas ranging from 3 to 9 mm.
Most of the literature has focused on DWI images with 1.5T imaging units. Lehmann et al33 compared PROPELLER DWI with ASSET single-shot EPI-DWI by using a 3T imaging unit. The 3T PROPELLER technique was associated with better sensitivity, specificity, and positive and negative predictive values for the detection of recurrent cholesteatoma. This improvement over the ASSET technique was thought to be due to artifact reduction, especially important at 3T, though PROPELLER DWI can be performed only with axial sections, which does not optimize visualization of the tegmen region.
Conclusions
DWI has proven utility in the evaluation of cholesteatomas. It can be used for distinguishing scar tissue, granulation tissue, and inflammatory changes from cholesteatoma in patients with prior cholesteatoma resection, particularly when CT findings are equivocal. Newer DWI techniques with thinner section acquisition and decreased susceptibility artifacts allow detection of small lesions. DWI can be useful as the primary imaging technique when visualization is impaired by canal wall up mastoidectomy or cartilaginous reconstruction. The DWI technique may be used in place of second-look surgery, sparing patients the morbidity of repeat exploration.
Acknowledgments
We acknowledge Alto Stemmer of Siemens Healthcare, who developed the diffusion-weighted BLADE application. We also thank Kevin Johnson, Registered Technologist in Radiography and Magnetic Resonance Imaging, of Siemens Healthcare for his significant contribution toward developing the MR imaging cholesteatoma evaluation protocols discussed in this work.
Abbreviations
- ASSET
array spatial sensitivity encoding technique
- DWI
diffusion-weighted imaging
- EPI
echo-planar imaging
- HASTE
half-Fourier acquired single-shot turbo spin-echo
- PROPELLER
periodically rotated overlapping parallel lines with enhanced reconstruction
- SNR
signal intensity–to-noise ratio
- SS TSE
single-shot TSE
- TSE
turbo spin-echo
References
- 1. Swartz JD. Temporal bone inflammatory disease. In: Som PM, Curtin HD. eds. Head and Neck Imaging. 3rd ed. St. Louis: Mosby; 1996:1391–424 [Google Scholar]
- 2. Swartz JD. Cholesteatomas of the middle ear: diagnosis, etiology, and complications. Radiol Clin North Am 1984:22;15–35 [PubMed] [Google Scholar]
- 3. Michaels L. Biology of cholesteatoma. Otolaryngol Clin North Am 1989:22;869–81 [PubMed] [Google Scholar]
- 4. Ajalloueyan M. Experience with surgical management of cholesteatomas. Arch Otolaryngol Head Neck Surg 2006:132;931–33 [DOI] [PubMed] [Google Scholar]
- 5. Shelton C, Sheehy JL. Tympanoplasty: review of 400 staged cases. Laryngoscope 1990:100; 679–81 [DOI] [PubMed] [Google Scholar]
- 6. Gyo K, Sasaki Y, Hinohira Y, et al. Residue of middle ear cholesteatoma after intact canal wall tympanoplasty: surgical findings at one year. Ann Otol Rhinol Laryngol 1996:105;615–19 [DOI] [PubMed] [Google Scholar]
- 7. Migirov L, Tal S, Eyal A, et al. MRI, not CT, to rule out recurrent cholesteatoma and avoid unnecessary second-look mastoidectomy. Isr Med Assoc J 2009:11;144–46 [PubMed] [Google Scholar]
- 8. Chee NW, Tan TY. The value of pre-operative high resolution CT scans in cholesteatoma surgery. Singapore Med J 2001:42;155–59 [PubMed] [Google Scholar]
- 9. Martin N, Sterkers O, Nahum H. Chronic inflammatory disease of the middle ear cavities: Gd-DTPA-enhanced MR imaging. Radiology 1990:176;399–405 [DOI] [PubMed] [Google Scholar]
- 10. Ayache D, Williams MT, Lejeune D, et al. Usefulness of delayed postcontrast magnetic resonance imaging in the detection of residual cholesteatoma after canal wall-up tympanoplasty. Laryngoscope 2005:155;607–10 [DOI] [PubMed] [Google Scholar]
- 11. Williams MT, Ayache D, Alberti C, et al. Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: initial findings. Eur Radiol 2003:13;169–74 [DOI] [PubMed] [Google Scholar]
- 12. Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986:161;401–07 [DOI] [PubMed] [Google Scholar]
- 13. Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000:217;331–45 [DOI] [PubMed] [Google Scholar]
- 14. Vercruysse J-P, De Foer B, Pouillon M, et al. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol 2006:16;1461–67 [DOI] [PubMed] [Google Scholar]
- 15. De Foer B, Vercruysse J-P, Bernaerts A, et al. The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology 2007;49:841–48. Epub 2007 Sep 3 [DOI] [PubMed] [Google Scholar]
- 16. Maheshwari S, Mukherji S. Diffusion-weighted imaging for differentiating recurrent cholesteatoma from granulation tissue after mastoidectomy: case report. AJNR Am J Neuroradiology 2002:23;847–49 [PMC free article] [PubMed] [Google Scholar]
- 17. Attenberger UI, Runge VM, Stemmer A, et al. Diffusion weighted imaging: a comprehensive evaluation of a fast spin-echo DWI sequence with BLADE (PROPELLER) k-space sampling at 3T, using a 32-channel head coil in acute brain ischemia. Invest Radiol 2009:44;656–61 [DOI] [PubMed] [Google Scholar]
- 18. Constable RT, Gore JC. The loss of small objects in variable TE imaging: implications for RSE, RARE, and EPI. Magn Reson Med 1992:28;9–24 [DOI] [PubMed] [Google Scholar]
- 19. Kimitsuki T, Suda Y, Kawano H, et al. Correlation between MRI findings and second-look operation in cholesteatoma surgery. ORL J Otorhinolaryngol Relat Spec 2001:63l;291–93 [DOI] [PubMed] [Google Scholar]
- 20. Vanden Abeele D, Coen E, Parizel PM, et al. Can MRI replace a second look operation in cholesteatoma surgery? Acta Otolaryngol 1999:119;555–61 [DOI] [PubMed] [Google Scholar]
- 21. Aikele P, Kittner T, Offergeld C, et al. Diffusion-weighted MR imaging of cholesteatoma in pediatric and adult patients who have undergone middle ear surgery. AJR Am J Roentgenol 2003:181;261–65 [DOI] [PubMed] [Google Scholar]
- 22. Venail F, Bonafe A, Poirrier V, et al. Comparison of echo-planar diffusion-weighted imaging and delayed postcontrast T1-weighted MR imaging for detection of residual cholesteatoma. AJNR Am J Neuroradiol 2008:29;1363–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stasolla A, Magliulo G, Parrotto D, et al. Detection of postoperative relapsing/residual cholesteatomas with diffusion-weighted echo-planar magnetic resonance imaging. Otol Neurotol 2004:25;879–84 [DOI] [PubMed] [Google Scholar]
- 24. De Foer B, Vercruysee J-P, Pouillon M, et al. Value of high-resolution computed tomography and magnetic resonance imaging in the detection of residual cholesteatomas in primary bony obliterated mastoids. Am J Otolaryngol 2007:28;230–34 [DOI] [PubMed] [Google Scholar]
- 25. Jeunen G, Desloovere C, Hermans R, et al. The value of magnetic resonance imaging in the diagnosis of residual or recurrent acquired cholesteatoma after canal wall-up tympanoplasty. Otol Neurotol 2007:29;16–18 [DOI] [PubMed] [Google Scholar]
- 26. Jindal M, Doshi J, Srivastav M, et al. Diffusion-weighted magnetic resonance imaging in the management of cholesteatoma. Eur Arch Otorhinolaryngol 2010:267;181–85 [DOI] [PubMed] [Google Scholar]
- 27. Fitzek C, Mewes T, Fitzek S, et al. Diffusion-weighted MRI of cholesteatomas of the petrous bone. J Magn Reson Imaging 2002:15;636–41 [DOI] [PubMed] [Google Scholar]
- 28. De Foer B, Vercruysse JP, Bernaerts A, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol 2008;29:513–17 [DOI] [PubMed] [Google Scholar]
- 29. Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol 2008:30;54–58 [DOI] [PubMed] [Google Scholar]
- 30. De Foer B, Vercruysse J-P, Pilet B, et al. Single-shot, turbo spin-echo, diffusion-weighted imaging versus spin-echo-planar, diffusion-weighted imaging in the detection of acquired middle ear cholesteatoma. AJNR Am J Neuroradiol 2006:27;1480–82 [PMC free article] [PubMed] [Google Scholar]
- 31. Dubrulle F, Souillard R, Chechin D, et al. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology 2006;238:604–10 [DOI] [PubMed] [Google Scholar]
- 32. Schwartz KM, Lane JI, Neff BA, et al. Diffusion-weighted imaging for cholesteatoma evaluation. ENT J. 2010;89:E14–19 [PubMed] [Google Scholar]
- 33. Lehmann P, Saliou G, Brochart C, et al. 3T MR imaging of postoperative recurrent middle ear cholesteatomas: value of periodically rotated overlapping parallel lines with enhanced reconstruction diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2009:30;423–27 [DOI] [PMC free article] [PubMed] [Google Scholar]