Abstract
BACKGROUND AND PURPOSE:
Subtle linguistic dysfunction and reorganization of the language network were described in patients with epilepsy, suggesting the occurrence of plasticity changes. We used resting state FC-MRI to investigate the effects induced by chronic epilepsy on the connectivity of the language-related brain regions and correlated it with language performance.
MATERIALS AND METHODS:
FC-MRI was evaluated in 22 right-handed patients with drug-resistant epilepsy (11 with LE and 11 with RE) and in 12 healthy volunteers. Neuropsychological assessment of verbal IQ was performed. Patients and controls underwent BOLD fMRI with a verb-generation task, and language function was lateralized by an LI. Intrinsic activity fluctuations for FC analysis were extracted from data collected during the task. Six seeding cortical regions for speech in both hemispheres were selected to obtain a measure of the connectivity pattern among the language networks.
RESULTS:
Patients with LE presented atypical language lateralization and an overall reduced connectivity of the language network with respect to controls. In patients with both LE and RE, the mean FC was significantly reduced within the left (dominant) hemisphere and between the 2 hemispheres. In patients with LE, there was a positive correlation between verbal IQ scores and the left intrahemispheric FC.
CONCLUSIONS:
In patients with intractable epilepsy, FC-MRI revealed an overall reduction and reorganization of the connectivity pattern within the language network. FC was reduced in the left hemisphere regardless of the epileptogenic focus side and was positively correlated with linguistic performance only in patients with LE.
MR imaging plays a crucial role in the preoperative assessment of patients with intractable epilepsy. Structural MR imaging is routinely used for the detection of epileptogenic brain lesions, whereas fMRI localizes brain functions on the cerebral cortex and establishes their spatial relationship with lesions. One of the most frequent applications of fMRI is presurgical mapping of language functions and identification of the dominant hemisphere for speech.1,2
Subtle linguistic dysfunction affects up to one-third of adults with chronic epilepsy,3–5 even in the absence of a direct injury to the brain language network. Functional neuroimaging has demonstrated different patterns of activation and reorganization of the language network in patients with epilepsy compared with healthy subjects. Using fMRI, Brázdil et al6 described a different activation pattern of the Broca area in a group of patients with left mesial-temporal epilepsy. In addition, task-evoked BOLD activity within the language network was found to be less unbalanced toward the dominant hemisphere in patients with epilepsy, compared with the healthy controls, especially in patients with early seizure onset.2 Taken as a whole, these results suggest the possibility that epilepsy may induce functional changes across remote cortical regions, including those specialized for speech.
In addition to task-evoked fMRI studies, resting state FC-MRI can reveal the cortical connectivity among language-network regions by evaluating correlations of spontaneous BOLD signal-intensity fluctuations.7–10 FC-MRI has attracted growing interest and new applications and can be reasonably considered a marker of neural connectivity.11 Most important, FC-MRI seems particularly suited for clinical populations because subjects lie in the scanner without any imposed task, thus eliminating the need for complying with task instructions.12 Similarly, FC analysis of intrinsic brain activity, typically performed on resting state data, can be performed on task-related data by subtracting the contribution of the BOLD activation response.12,13
Recently, Waites et al14 found a decreased resting state FC-MRI connectivity among language regions and an increased connectivity between the left inferior frontal gyrus and part of the DMN in a group of patients with left temporal lobe epilepsy compared with controls. These observations further confirm the influence of epilepsy on language-network activity and possibly account for subtle language impairments that may affect patients with epilepsy. However, we have little information about potential hemispheric differences in these patients; and most important, the changes in FC associated with verbal performance have not been studied previously, to our knowledge.
In the present retrospective analysis, we investigated the FC pattern among language-network nodes in a group of patients (n = 22) with either left- or right-lateralized drug-resistant epilepsy and in a group of matched healthy subjects (n = 12). First, we evaluated the pattern of cortical activation during a conventional block-designed verb-generation task assessing the degree of language function lateralization across hemispheres. We then used FC-MRI to investigate the pattern of functional connectivity among 6 regions of the language network in the 2 hemispheres. Finally, we investigated the correlation between the pattern of FC-MRI and the neuropsychological evaluation of language performance.
Materials and Methods
Subjects
All the participants gave written informed consent before enrollment in the study, which was approved by our local ethics committee. Twenty-two right-handed patients (10 males, 12 females) with drug-resistant epilepsy underwent an fMRI presurgical assessment to localize and lateralize language functions. Patient ages ranged from 16 to 51 years (mean, 29.8 ± 10.0 years). Presurgical diagnostic protocol was the following: clinical history, neurologic examination, video-electroencephalography monitoring, MR imaging, ictal and interictal single-photon emission CT, and neuropsychological assessment. The localization of the epileptogenic zone was based on the concordance of the electroclinical correlation with interictal positron-emission tomography data and MR imaging and neuropsychological changes, when present. According to the side of the epileptogenic focus, patients were divided in 2 subgroups: LE (n = 11) and RE (n = 11). The epileptogenic focus was detected in the temporal lobe in 16/22 (73%) patients and in the frontal lobe in 6/22 (27%). Epilepsy was cryptogenetic in 4 (18%) patients, whereas it was secondary to a cortical malformation in 3/22 (14%), vascular malformation in 2/22 (9%), DNET in 3/22 (14%), and MTS in 10/22 (45%) patients (Table 1). Large brain lesions causing epilepsy were not included in the study due to their tendency to destroy the normal anatomy of the brain and to directly affect (via contact or infiltration) the cortical regions for speech. Only patients with normal findings on MR imaging examinations of the brain or with small and no growing lesions distant from cortical regions for speech were included. Patient mean age at seizure onset was 16 years (range, 0.5–30 years).
Table 1:
Patient clinical data
| Patient No. | Age | SO (yr) | SF | Lobe | Etiology |
|---|---|---|---|---|---|
| 1 | 36 | 10 | W | Frontal | Micropolygyria |
| 2 | 49 | 10 | M | Temporal | MTS |
| 3 | 27 | 19 | M | Frontal | DNET |
| 4 | 51 | 28 | M | Temporal | Cortical dysplasia |
| 5 | 26 | 20 | W | Temporal | Cavernoma |
| 6 | 19 | 0.7 | M | Temporal | MTS |
| 7 | 29 | 2 | W | Temporal | MTS |
| 8 | 23 | 7 | W | Frontal | DNET |
| 9 | 32 | 10 | W | Temporal | MTS |
| 10 | 16 | 12 | D | Temporal | MTS |
| 11 | 16 | 8 | W | Temporal | MTS |
| 12 | 35 | 15 | M | Temporal | Cryptogenetic |
| 13 | 28 | 16 | M | Temporal | Cryptogenetic |
| 14 | 22 | 20 | D | Temporal | DNET |
| 15 | 22 | 0.5 | D | Frontal | Cryptogenetic |
| 16 | 33 | 30 | D | Frontal | Cryptogenetic |
| 17 | 34 | 26 | D | Temporal | MTS |
| 18 | 28 | 3 | W | Temporal | MTS |
| 19 | 46 | 38 | M | Frontal | AVM |
| 20 | 36 | 8 | M | Temporal | MTS |
| 21 | 29 | 12 | M | Temporal | Micropolygyria |
| 22 | 18 | 11 | W | Temporal | MTS |
The control group included 12 healthy right-handed subjects (mean age, 35 years; range, 25–44 years) presenting with normal neurologic examination findings, hearing, and sight. None of the controls experienced epilepsy during their lives. Right-handedness was determined by the Italian standardized version of the Edinburgh Handedness Inventory with an LI of >80.15,16
The Wechsler Adult Intelligence Scale IV-Revised was administered in 17 of the 22 patients (9 LE and 8 RE) by an expert neuropsychologist to measure verbal IQ.
fMRI Task and Imaging Acquisition
Patients and controls underwent BOLD fMRI to localize cortical regions involved in language functions and to provide a measure of language hemispheric lateralization. Each subject was asked to silently perform an orthographically cued, block-designed, lexical verb-generation task, in which 4 task periods (30 seconds each) were intermixed with 5 rest periods (20 seconds each). During each task period, subjects were required to think for 2 seconds of pronouncing ≥1 verb associated with a noun, which was presented at the center of the screen for 1 second. During rest periods, subjects were instructed to relax while maintaining central fixation. While this task does not provide a measure of performance, it is nonetheless widely used in the clinical setting for its proved efficacy17 and for not producing motion artifacts related to overt speech. Before scanning, all subjects executed a brief overt version of the task to ensure correct performance.
Visual stimuli were presented by using E-Prime software, Version 1.1 (Psychology Software Tools, Pittsburgh, Pennsylvania), projected via an LCD projector and viewed with a mirror placed above the subject's head.
Data were acquired on a 1.5T system (Magnetom Vision, Siemens, Erlangen, Germany; or Achieva, Philips Medical Systems, Best, the Netherlands). BOLD contrast functional images were acquired by using a T2*-weighted echo-planar free induction decay sequence (TR, 2000 ms; TE, 60 ms; matrix size, 64 × 64; FOV, 256 mm; in-plane voxel size, 4 × 4 mm; flip angle, 90°; section thickness, 4 mm; and no gap). The scan volume was oriented parallel to the anterior/posterior commissure plane and was acquired in ascending order covering the low and intermediate hemispheric convexity, including the classic cortical regions for language (the Broca and Wernicke areas). A total of 115 functional volumes per subject were collected. Structural images were acquired with an MPRAGE T1-weighted sequence (matrix, 256 × 256; FOV, 256 mm; section thickness, 1 mm; in-plane voxel size, 1 × 1 mm; flip angle, 12°; TR, 9.7 ms; TE, 4 ms).
Activation fMRI Analysis
BrainVoyager QX, Version1.9 (Brain Innovation BV, Maastricht, the Netherlands) was used for image preprocessing and statistical analysis. Functional image time-series was first corrected for the differences in section-acquisition times, detrended, realigned with the structural images, and warped into standard Talairach anatomic space.18 To assess the potential confound represented by group differences in the amount of motion artifacts, we quantified individual head shifts along the 3 rotation and translation anatomic axes. Statistical comparison across groups (patients versus controls) was performed by using a 2-tailed Student t test.
The fMRI statistical activation maps were generated according to a classic hypothesis-driven GLM method, combined with ICA, for the data-driven detection of the brain-activation response. Because the ICA approach does not rely on a predefined model of brain response, it is less influenced by modifications of BOLD activity induced by either disease or nonconformity of task execution.19 The BOLD time series was decomposed into a set of spatially independent ICs20 by means of the FastICA algorithm.21 Each fMRI IC map was scaled to z scores20 and was thresholded at z > 1.5 to display IC active voxels.22 After the exclusion of artifactual ICs based on the IC fingerprint method,23 we selected the IC showing the largest correlation coefficient between the predictor and respective time course, to obtain a data-driven estimate of the subject's task-related activation. Next, we created a mask by using the fMRI IC activations, and performed the standard GLM analysis on the masked fMRI data by using the IC time course as the predictor. This procedure generated statistical maps in z scores.
For each subject, the LI was calculated by counting the number of active voxels in the left and right hemispheres by using the following formula: LI = (Vl − Vr) / (Vl + Vr).24,25 By definition, LI values range between −1 and +1, where LI > +0.20 indicates left lateralization; <−0.20, right lateralization; and +0.20 and −0.20 inclusive, bilateral or not lateralized. Because LI varies with the statistical threshold, we calculated the MLI values obtained at multiple thresholds between P < .164 and the least stringent P value, according to which either LI = ±1, or no activation was detected.24 The MLI value of each subject was used for statistical comparisons. To exclude voxels not specifically related to language function, we repeated the analysis, limiting the voxel count within the 4 regions of interest (centered on anterior and posterior language areas of each hemisphere) obtained by grouping the task-evoked BOLD activation of controls and patients, thresholded at P < .001. LI was computed by summing the active voxels of anterior and posterior language regions of each hemisphere.
FC Analysis
Regions of interest were independently identified on the basis of previous studies26–29 to allow an unbiased intergroup comparison of FC-MRI. Three regions of interest (6-mm radius) were defined on the left hemisphere corresponding to the following: the pars triangularis of the IFG, Talairach coordinates: x = −49, y = +17, z = +5; T2 mL: x = −55, y = −35, z = +3; and T1p: x = −55, y = −36, z = +24). The corresponding homologous coordinates were used to define regions of interest on the contralateral hemisphere, and the resulting 6 regions of interest were used in subsequent FC-MRI analysis.
Using a conjunction analysis,9 we verified that the coordinates of the regions of interest were included in the fMRI activation maps that were observed in our subjects during the execution of the language task. With this method, group-level statistical maps obtained from the GLM analysis of the verb-generation task time course were derived by a random-effect analysis (P < .01, corrected with the FDR).30
Before FC analysis, fMRI time series were further preprocessed, according to the procedures described by He et al12 and Fair et al.13 The preprocessing steps included the following: 1) band-pass filtering between 0.009 and 0.08 Hz, 2) regression of white matter and ventricle signals and their first derivatives, and 3) regression of 3D motion parameters and their first derivatives. In addition, to remove the contribution of the BOLD activation response from the signal-intensity time series, we performed a regression of the deterministic task-evoked components, by including a regressor corresponding to the verb-generation periods of the paradigm. Therefore, the FC-MRI analysis was performed over the entire residual dataset. While we cannot be sure that this procedure completely removed all the task-related components of the BOLD signal intensity, the resting state data obtained from block-designed fMRI studies, such as in our case, were reported to be well-suited for resting state FC-MRI analyses.13 A GLM analysis on the residual dataset was performed in controls to ensure that no residual BOLD task-evoked activity was present in language-network regions. The resulting BOLD signal-intensity time course was averaged across all voxels of each region of interest. We calculated the Pearson correlation coefficients (r) between the signal-intensity time course of each region of interest pair (pair-wise correlation) and between each region of interest and every voxel of the acquired volume (voxel-wise correlation maps).
Statistical Analysis
To obtain an approximately normal distribution of the FC values and thus apply parametric statistics, we converted the FC correlation r coefficients into z scores by means of the Fisher r-to-z transformation.31 mFC values were estimated among all the seed regions (overall FC), within (intrahemispheric), and between (interhemispheric) hemispheres, by calculating respective correlation coefficients, according to the method proposed by Carter et al.32 Group-level comparisons among mFC scores were performed by using analysis of variance and independent-sample t tests.
The correlation between mFC results and MLIs in patients and controls and the correlation between mFC results and verbal IQ scores in patients were estimated by using Pearson r coefficients because the mFC values, MLIs, and verbal IQ scores had an approximate normal distribution.
Results
Control Analyses
Statistical comparison between the amount of head movement (mean shifts along rotation and translation axes) of patients and control groups did not yield significant differences (P > .05, 2-sample t test). At a threshold of P < .001, no residual task-evoked BOLD activity was present in language-network regions of the control group.
Task-Dependent Activations and MLIs
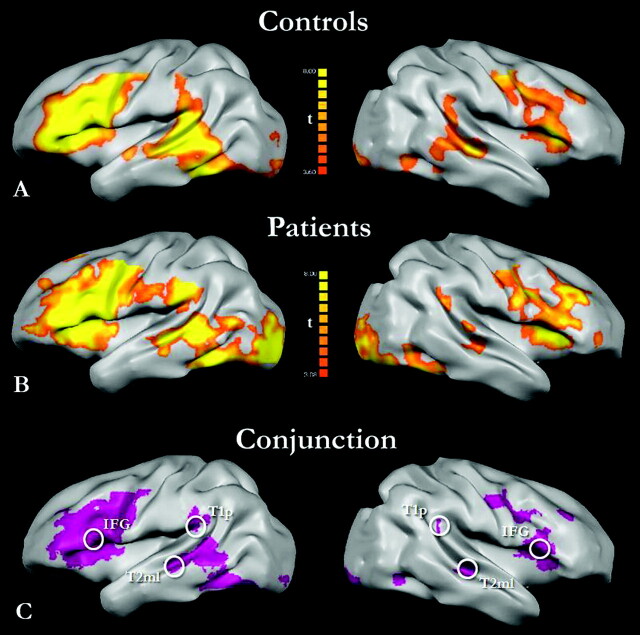
In both control and patient groups, ICA-GLM analysis of BOLD data for the verb-generation task showed statistically significant activations (random effect analysis, P < .01 corrected with FDR) of the main language-related regions, including the left IFG, T2 mL, and T1p and their contralateral homologues (Fig 1A, -B). The combined conjunction map of patients and controls confirmed the common activation of language-related regions, including the predefined seed regions for subsequent FC analysis (Fig 1C).
Fig 1.
A and B, Verb-generation task statistical activations from group-level ICA-GLM analysis in the control (n = 12) and patient (n = 22) groups, respectively (P < .01, FDR-corrected for multiple comparisons; only clusters >10 mm2 are represented). C, Predefined seed regions of interest for the FC analysis are superimposed on the conjunction plots, displaying common activated voxels in controls and in patients in both LE and RE subgroups. All seed centers were found to be included within the activated shared voxels.
MLI values indicated that language functions were left-lateralized in all except 1 of the control subjects (mean, 0.43 ± 0.2; range, 0.13–0.78). In the patient group, the mean MLI was 0.23, and 1 patient had a right-lateralized index (range, −0.32–0.94; SD, 0.3). The difference in mean LI (P < .05, 2-tailed t test) between patients with LE (mean, 0.11 ± 0.28; range, −0.32–0.57) and those with RE (mean, 0.36 ± 0.27; range, −0.2–0.94) was statistically significant. Most important, only the LE group showed a less lateralized language representation2 with respect to controls (P < .05, 2-tailed t test). Results obtained by using the region-of-interest approach (see “Material and Methods”) provided very similar findings. In particular, the LI of controls (LI = 0.70 ± 0.24) was greater than the LI observed in all patients (LI = 0.45 ± 0.38, P = .05, 2-tailed t test). Specifically, a difference was observed for the LE group (LI = 0.36 ± 0.51, P = .05) but not for the RE group (LI = 0.54 ± 0.16, P = .10).
FC of the Language Network
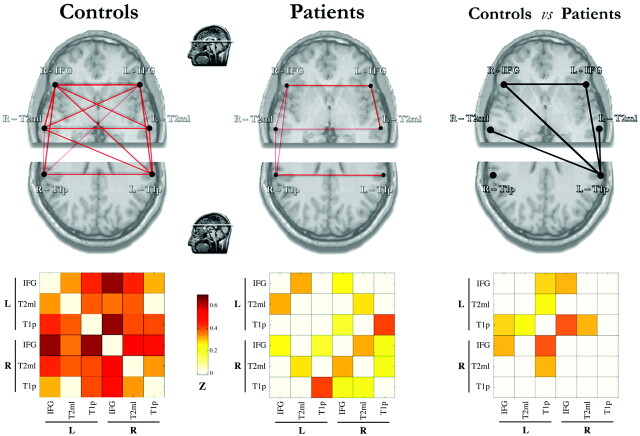
We analyzed the pattern of FC in language networks by using 3 cortical regions involved in language functions and their corresponding homologous coordinates of the controlateral hemisphere (see “Materials and Methods” section). Diagrams and cross-correlation matrices representing FC for control and patient groups are shown in Fig 2 (left panel). Healthy subjects showed significant connectivity among all of the language-network nodes, except for the L-T2 mL to R-T1p link (14 of 15 possible links among the 6 nodes); the strongest correlations were seen between the 2 IFGs and between the R-IFG and L-T1p nodes (r > 0.6). In the patient group (n = 22), only 6 of 15 FC links were significant, including interhomologous connections and IFG-T2 mL links in both hemispheres.
Fig 2.
Plots and matrices representing FC links among the 6 language-network regions in both hemispheres evaluated in this study (left panel) in controls (n = 12) and all patients (n = 22). Connectivity plots (upper row) show statistically significant correlations by means of red lines of variable thickness according to the z scores, from Pearson coefficient values. These are further represented in the corresponding color-coded matrices (lower row), ranging from white (z = 0) to dark red (z = 0.7). Only correlations significant at P < .01 are shown. The right panel shows a statistical comparison between the groups (independent t test, P < .01). Significantly reduced FC links in patients are indicated by black lines and quantified in the corresponding color-coded matrices (color scale for z score representation is the same as that in the left panel). Line thickness varies according to the reduction of correlation values.
To identify the most weakened functional connections, we performed a group comparison of the FC strength across region-of-interest pairs (2-tailed t tests). Significantly (P < .01) reduced FC values are indicated by black lines in Fig 2 (right panel), which illustrate that the most affected node is L-T1p. Overall, these results demonstrate that the strong FC among the language network in healthy subjects was significantly weakened in patients with epilepsy, especially in the L-T1p node.
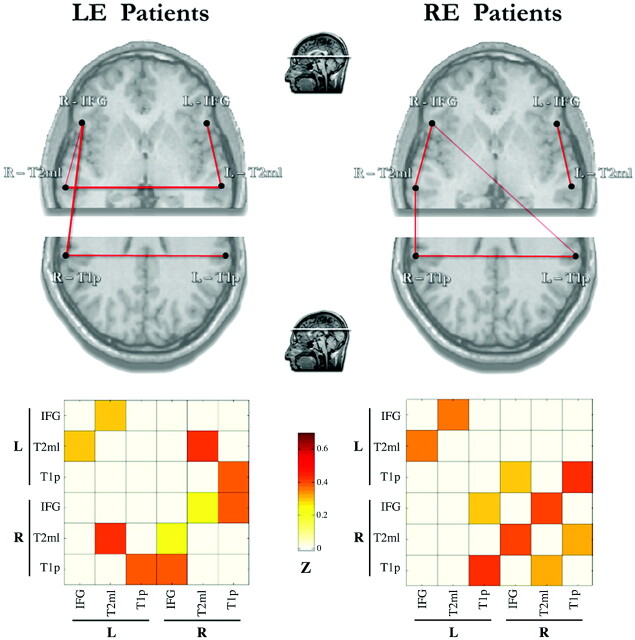
We tested possible effects of the epileptogenic focus side on the language-network connectivity by splitting the group of patients according to the epileptogenic focus side (LE or RE) (Fig 3). Both subgroups presented several missing FC links, both within and between hemispheres (P < .01). Regardless of epileptogenic focus side, 2 significant language-network links were present in the right hemisphere, whereas only a single significant link was present in the left hemisphere. The inter-T1p and inter-IFG-T2 mL links were preserved bilaterally in patients with both LE and RE.
Fig 3.
Plots and matrices representing FC links among the 6 language-network regions in both hemispheres evaluated in this study (left panel) in patients with LE (n = 11) and RE (n = 11). Connectivity plots (upper row) show statistically significant correlations by means of red lines of variable thickness according to the z scores, from Pearson coefficient values. These are further represented in the corresponding color-coded matrices (lower row), ranging from white (z = 0) to dark red (z = 0.7). Only correlations significant at P < .01 are shown.
Next, we compared the pattern of FC between controls and each of the patient subgroups, estimating the mFC within and between hemispheres. The results are presented in Table 2. Regardless of the epileptogenic focus side, the overall, inter-, and left-intra mFCs were significantly reduced in both subgroups, whereas the right intrahemispheric mFC did not differ compared with controls. Thus, epilepsy seems to generally affect the normal FC pattern, by particularly disrupting language-network connectivity within the left (dominant) hemisphere, regardless of epileptogenic focus side.
Table 2:
Mean FC summary
<.05, 2-tailed t test.
Voxel-Wise FC Maps
We also analyzed the spatial pattern of widespread cortical connectivity by using each language-network region as a seed for the creation of group-level FC statistical voxel-wise maps. Compared with the regional analysis, these maps provided a test of the spatial specificity of the correlation among the language network and could potentially reveal connectivity with other cortical regions. Figure 4 illustrates the connectivity map generated by using the L-T1p as a seed, a region that was found to be particularly compromised in regional analysis. First, the map of the control group demonstrated that the seed showed correlation almost exclusively with other regions belonging to the language network, indicating a high degree of spatial specificity. Furthermore, in accordance with the regional analysis, voxel-wise maps of the group of patients confirmed a significant reduction of connectivity between L-T1p and other language-network regions, compared with healthy subjects. Most interesting, L-T1p was functionally connected with the PCC only in the patient group (white circles in Fig 4). This finding suggested that, besides the disruption of several FC links within the language network, patients may establish peculiar connections with other brain regions that are not only unrelated to language functions but are even part of a cortical network that typically shows deactivation during the execution of externally oriented tasks (the so-called default mode network33).
Fig 4.
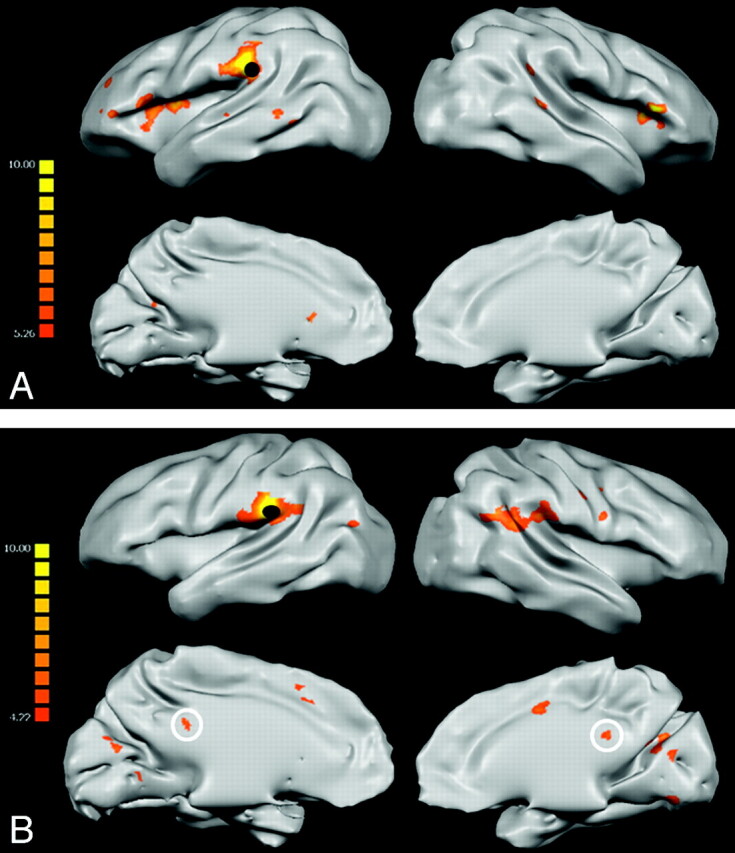
FC statistical maps displaying correlation z scores between L-T1p (black dots) and the rest of the brain, superimposed on inflated 3D templates for anatomic reference (P < .01, FDR-corrected for multiple comparisons). Patient maps (B) lack several connectivity links with the rest of the language network compared with controls (A). However, contrary to controls, the patient group presents statistically significant correlations with the left PCC (white circles).
Correlation with Clinical Data
We investigated the relationship between the FC within the language network and patient performance at language tasks. The mean verbal IQ score of patients was 79.6; there was no significant difference between patients with LE (76.7) and RE (82.7) (P = .54, 2-tailed t test). Because we found a significant reduction of the intrahemispheric mFC only in the left hemisphere, regardless of the epileptogenic focus side, we evaluated the correlation between the amount of left intrahemispheric connectivity and verbal IQ scores. The verbal IQ scores significantly correlated with the L-Intra mFC in patients with LE (r = 0.7, P < .05) but not in patients with RE (r = 0.05; P = .44). There was no correlation between verbal IQ and the right intrahemispheric mFC for either subgroup. We did not find a significant correlation between verbal IQ performance and LIs. Thus, while patients with both LE and RE exhibited compromised connectivity within language-network nodes of the left hemisphere compared with controls, the degree of connectivity was related to language performance exclusively in patients with LE.
Discussion
Mild linguistic impairments, often occurring in chronic drug-resistant epilepsy, are not associated with evident changes in structural neuroimaging. Previous task-evoked fMRI2,6,34 and magnetoencephalography35 studies found aberrant localization of language functions in selected groups of patients with epilepsy. As a complementary procedure to the analysis of task-evoked brain activity, FC-MRI can evaluate the integrity of the entire network supporting specific functions during resting state. In patients with left temporal lobe epilepsy, Waites et al14 described the disruption of several FC links between otherwise normally task-activated brain regions; however, they only evaluated patients with left MTS. Furthermore, the study did not include left temporal regions, which are crucially related to semantic language processing,29,36 nor homologous regions of the right hemisphere, among the FC seeds.
In the present study, FC was estimated in 12 healthy subjects and 22 patients with either LE or RE among a network including 3 homologous cortical regions of both hemispheres. In addition, we investigated potential correlations between the strength of FC among the language networks and language performance.
Intersubject Comparison
There are multiple potential limitations when comparing task-evoked BOLD activity between groups of patients and controls. The main issue is that a difference in task performance, rather than a neurologic condition, may explain a difference in the BOLD task-evoked response. A variation in the way patients, compared with controls, cooperate with task instructions, especially when they continuously receive antiepileptic medications that may affect general arousal, has already been considered in previous studies.14,37 Crucially, the FC low-frequency fluctuations of the BOLD signal intensity during the resting state do not depend on task execution7 and are, therefore, less influenced by patient attentive capability and cognitive performance.12 In the present study, we extracted the intrinsic signal-intensity fluctuations from a conventional verb-generation block paradigm, regressing out the deterministic components from the BOLD time course.12,13
We also investigated the presence of group differences in terms of head motion during imaging acquisition because patients may have exhibited more movement artifacts. The quantity of head translational and rotational movements did not differ between patients and controls. Finally, we tried to preserve the intersubject comparability by only including either patients without lesions or with lesions that did not broadly alter the normal cortical anatomy.
Task-Dependent Language Activations
In both healthy subjects and patients, task-evoked BOLD activity was observed in anterior and posterior brain regions classically associated with language functions,38 with a larger recruitment of right cortical areas in patients with epilepsy compared with controls. This was not unexpected because previous fMRI studies2,34,39 found a significant correlation between epilepsy and atypical language lateralization, especially in patients with early-onset epilepsy. Consistent with several of these reports,34,39 we found a significant difference in the MLIs between patients with LE and those with RE: Patients with LE had lower MLIs with respect to either controls or patients with RE, confirming a selective atypical lateralization.
FC of the Language Network
We tested the amount of FC of the language network across both hemispheres for 2 main reasons: Language reorganization in patients with epilepsy involves both hemispheres, and language activations also spread through the nondominant hemisphere in healthy subjects.40 We, therefore, chose 3 classical cortical regions related to semantic and phonologic language processing in the dominant hemisphere,26–29 together with their contralateral homologues. These areas represent the essential brain regions for speech known to be functionally and structurally connected.41–43 Most interesting, the control group exhibited a high degree of interhemispheric connectivity while showing a left-hemisphere language lateralization, supporting a difference between task-evoked BOLD activity and the underlying pattern of intrinsic FC.
Patients with epilepsy generally showed a reduced pattern of connectivity across the language network at the same statistical threshold, in accordance with previous studies,14,42 demonstrating a reduction of FC between language areas. In the present work, we could further observe a reduction of several links both within and between hemispheres (Fig 2). When we compared the FC strength between patients and controls (Fig 2, right panel), the left T1p was found to be the most weakened node within the language network (4 significantly reduced links). Remarkably, Alstott et al,44 by using a computational model from diffusivity-based tractography data, described dramatic effects on brain network connectivity when simulating a lesion that disrupted the region of the temporoparietal junction. Although these results come from radically different experimental environments, they bolster the crucial role of the posterior temporal region for functional network integrity and also suggest a particular vulnerability of this cortical area to nonlocal disturbances over the connectivity network.
The significant reduction of connectivity in patients compared with controls should not be considered a marker of a generalized reduction of cortical connectivity due to epilepsy. As a matter of fact, the voxel-wise analysis demonstrated that patients also exhibit increased connectivity between the L-T1p node and the PCC, replicating the results of Waites et al,14 who found greater resting state connectivity between another region of the language network, the left IFG, and the PCC in patients with left temporal lobe epilepsy compared with controls. The PCC is part of the DMN, defined as the set of cortical regions that are consistently deactivated during externally oriented task execution.33,45 Most interesting, the DMN also exhibits a negative correlation with “antagonist” attentive brain networks, both at rest9 and during task execution,46 suggesting that the negative correlation between the DMN and task-positive networks is a marker of an efficient cortical organization. In our patients, the presence of a diminished resting state connectivity among the language networks, coupled with an increased connectivity between the language network and the DMN, may be part of plasticity changes induced by epilepsy.47 Whether this correlation is part of a compensatory mechanism to establish new functional pathways replacing the damaged ones or if it represents the unsuppressed interference from DMN regions with the language network is a matter for further investigation. Recently, the degree of negative correlation between DMN and attentive networks was found to be significantly related to an individual's task performance.46 We can, therefore, hypothesize that the presence of a T1p-PCC positive correlation in our patients underlies unfavorable dysfunctional mechanisms.
When we investigated the effect of the epileptogenic focus side on the FC pattern by separating the group of patients into LE and RE subgroups, we found that the intrahemispheric mFC was always significantly reduced in the left (dominant) hemisphere regardless the epileptogenic focus side (Table 1 and Fig 3). This finding was unpredicted because some significant epileptogenic focus effects on the ipsilateral FC could be hypothesized when considering the anatomic proximity to the focus. Mechanisms other than proximity justify the presence of a language-network-wise effect of epilepsy48: 1) Interictal and ictal discharges may propagate from the seizure focus to other brain regions through existing fiber tracts49; 2) the postulated attempt of the brain to inhibit epileptic seizures by upregulating inhibitory functions may have the side effect of suppressing neuronal activity, including the language system50; and 3) antiepileptic drugs may alter the physiologic neurotransmission, causing changes in the resting state FC.51
BOLD-Neuropsychological Correlations
We additionally investigated the presence of potential correlations between the pattern of FC and the neuropsychological assessment in patients with LE and RE. Although the reduction of FC across the language network seems to largely exceed the amount of speech impairment, because patient scores were within the normal range at the group level, it may, nonetheless, be more subtly related to language performance and explain part of the interindividual variability of the neuropsychological assessment.
What is crucial, despite both patient subgroups showing decreased intrahemispheric connectivity within the left hemisphere, only patients with LE exhibited a significant correlation between left hemispheric mFC and verbal IQ scores. This result has 2 important implications: First, the reduction of left intrahemispheric connectivity in patients with LE is related to language performance. Second, the reduction of left intrahemispheric connectivity per se does not account for language performance because no correlation was observed in patients with RE. We offer an explanation of this result by simultaneously considering both the lateralization index, which is a measure of the task-evoked hemisphere dominance, and the pattern of FC. In fact, only the patients with LE exhibited atypical language lateralization compared with controls (mean MLI, 0.11). We reasoned that verbal performance is explained only when the reduction of the left intrahemispheric FC is observed simultaneously with an atypical language lateralization pattern.
Conclusions
The 2 subgroups of patients with LE and RE showed a selective reduction of left intrahemispheric FC of the language network, confirming both a local and distant influence of epileptogenic foci on cortical networks. The left intrahemispheric FC of the language network correlated with verbal performance only in patients with LE.
These results indicate the advantages of including the assessment of the FC, beyond the simple analysis of task-evoked activations, for a more comprehensive understanding of functional modifications induced by epilepsy. Larger study groups, together with postoperative follow-ups, are required to definitively assess the role of FC-MRI in the presurgical work-up of patients with intractable epilepsy.
Abbreviations
- AVM
arteriovenous malformation
- BOLD
blood oxygen level–dependent
- D
daily
- DMN
default mode network
- DNET
dysembryoplastic neuroepithelial tumors
- FC
functional connectivity
- FC-MRI
functional connectivity MR imaging
- FDR
false discovery rate
- fMRI
functional MR imaging
- GLM
general linear model
- IC
independent component
- ICA
independent component analysis
- IFG
inferior frontal gyrus
- IQ
intelligence quotient
- L
left
- LE
left-epileptogenic focus
- LI
lateralization index
- M
monthly
- mFC
mean functional connectivity
- MLI
median of the LI
- MPRAGE
magnetization-prepared rapid acquisition of gradient echo
- MTS
mesial temporal sclerosis
- PCC
posterior cingulate cortex
- R
right
- RE
right-epileptogenic focus
- SF
seizure frequency
- SO
age at seizure onset
- T1p
posterior part of the superior temporal gyrus
- T2 ml
middle-lateral part of the middle temporal gyrus
- Vl
left hemisphere active voxel
- Vr
right hemisphere active voxel
- W
weekly
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 15–20, 2010; Boston, Massachusetts.
References
- 1. Swanson SJ, Sabsevitz DS, Hammeke TA, et al. Functional magnetic resonance imaging of language in epilepsy. Neuropsychol Rev 2007;17:491–04 [DOI] [PubMed] [Google Scholar]
- 2. Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 1999;122:2033–46 [DOI] [PubMed] [Google Scholar]
- 3. Wheless JW, Simos PG, Butler IJ. Language dysfunction in epileptic conditions. Semin Pediatr Neurol 2002;9:218–28 [DOI] [PubMed] [Google Scholar]
- 4. Hermann BP, Bell B, Seidenberg M, et al. Learning disabilities and language function in epilepsy. Epilepsia 2001;42:21–23 [DOI] [PubMed] [Google Scholar]
- 5. Davey D, Thompson D. Interictal language functioning in chronic epilepsy. J Neurolinguistics 1991;6:381–99 [Google Scholar]
- 6. Brázdil M, Chlebus P, Mikl M, et al. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy: an fMRI study. Eur J Neurol 2005;12:268–75 [DOI] [PubMed] [Google Scholar]
- 7. Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41 [DOI] [PubMed] [Google Scholar]
- 8. Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol 2000;21:1636–44 [PMC free article] [PubMed] [Google Scholar]
- 9. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003;100:253–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greicius MD, Supekar K, Menon V, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009;19:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He BJ, Snyder AZ, Vincent JL, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007;53:905–18 [DOI] [PubMed] [Google Scholar]
- 13. Fair DA, Schlaggar BL, Cohen AL, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 2007;35:396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waites AB, Briellmann RS, Saling MM, et al. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol 2006;59:335–43 [DOI] [PubMed] [Google Scholar]
- 15. Salmaso D, Longoni AM. Problems in the assessment of hand preference. Cortex 1985;21:533–49 [DOI] [PubMed] [Google Scholar]
- 16. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 17. Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 1999;52:798–809 [DOI] [PubMed] [Google Scholar]
- 18. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988 [Google Scholar]
- 19. Quigley MA, Haughton VM, Carew J, et al. Comparison of independent component analysis and conventional hypothesis-driven analysis for clinical functional MR image processing. AJNR Am J Neuroradiol 2002;23:49–58 [PMC free article] [PubMed] [Google Scholar]
- 20. McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 1998;6:160–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 1999;10:626–34 [DOI] [PubMed] [Google Scholar]
- 22. Mantini D, Perrucci MG, Del Gratta C, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 2007;104:13170–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Martino F, Gentile F, Esposito F, et al. Classification of fMRI independent components using IC-fingerprints and support vector machine classifiers. Neuroimage 2007;34:177–94 [DOI] [PubMed] [Google Scholar]
- 24. Ruff IM, Petrovich Brennan NM, Peck KK, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol 2008;29:528–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 2008;26:594–01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heim S, Opitz B, Friederici AD. Broca's area in the human brain is involved in the selection of grammatical gender for language production: evidence from event-related functional magnetic resonance imaging. Neurosci Lett 2002;328:101–14 [DOI] [PubMed] [Google Scholar]
- 27. Seghier ML, Lazeyras F, Pegna AJ, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp 2004;23:140–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luke KK, Liu HL, Wai YY, et al. Functional anatomy of syntactic and semantic processing in language comprehension. Hum Brain Mapp 2002;16:133–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vigneau M, Beaucousin V, Hervé PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 2006;30:1414–32 [DOI] [PubMed] [Google Scholar]
- 30. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–78 [DOI] [PubMed] [Google Scholar]
- 31. Zar JH. Biostatistical Analysis. Upper Saddle River, New Jersey: Prentice-Hall; 1996:768–72 [Google Scholar]
- 32. Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 2010;67:365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berl MM, Balsamo LM, Xu B, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology 2005;65:1604–11 [DOI] [PubMed] [Google Scholar]
- 35. Pataraia E, Simos PG, Castillo EM, et al. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology 2004;63:1825–32 [DOI] [PubMed] [Google Scholar]
- 36. Geschwind N. The organization of language and the brain. Science 1970;170:940–44 [DOI] [PubMed] [Google Scholar]
- 37. Jokeit H, Okujava M, Woermann FG. Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 2001;1:6. Epub 2001 Dec 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Démonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev 2005;85:49–95 [DOI] [PubMed] [Google Scholar]
- 39. Thivard L, Hombrouck J, du Montcel ST, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage 2005;24:841–51 [DOI] [PubMed] [Google Scholar]
- 40. Binder JR, Frost JA, Hammeke TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 1997;17:353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol 2005;57:8–16 [DOI] [PubMed] [Google Scholar]
- 42. Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci U S A 1998;95:8939–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hampson M, Tokoglu F, Sun Z, et al. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage 2006;31:513–19 [DOI] [PubMed] [Google Scholar]
- 44. Alstott J, Breakspear M, Hagmann P, et al. Modeling the impact of lesions in the human brain. PLoS Comput Biol 2009;5:e1000408. Epub 2009 Jun 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: Ii. decreases in cerebral cortex J Cognitive Neurosci 1997;9:648–63 [DOI] [PubMed] [Google Scholar]
- 46. Kelly AM, Uddin LQ, Biswal BB, et al. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008;39:527–37 [DOI] [PubMed] [Google Scholar]
- 47. Buser P, Bancaud J. Unilateral connections between amygdala and hippocampus in man: a study of epileptic patients with depth electrodes. Electroencephalogr Clin Neurophysiol 1983;55:1–12 [DOI] [PubMed] [Google Scholar]
- 48. Avanzini G. System epilepsy: reply to Lüders, et al, 2009. Epilepsia 2009;50:2326. [DOI] [PubMed] [Google Scholar]
- 49. Lowenstein DH. Recent advances related to basic mechanisms of epileptogenesis. Epilepsy Res Suppl 1996;11:45–60 [PubMed] [Google Scholar]
- 50. Juhàsz C, Nagy F, Watson C, et al. Glucose and [11C]flumazenil positron emission tomography abnormalities of thalamic nuclei in temporal lobe epilepsy. Neurology 1999;53:2037–45 [DOI] [PubMed] [Google Scholar]
- 51. Gaillard WD, Zeffiro T, Fazilat S, et al. Effect of valproate on cerebral metabolism and blood flow: an 18F-2-deoxyglucose and 15O water positron emission tomography study. Epilepsia 1996;37:515–21 [DOI] [PubMed] [Google Scholar]