Abstract
BACKGROUND AND PURPOSE:
IH can alter the configuration of anatomic structures of the central nervous system. We determined the sensitivity and specificity of MR imaging to detect these changes in patients with secondary IH.
MATERIALS AND METHODS:
Patients (n = 36) with IH were prospectively investigated with MR imaging and were matched to 36 controls. MR images were evaluated for elongation and edema of the optic nerves, protrusion of the optic disc, flattening of the posterior sclera, height of the pituitary gland, and width of the optic nerve sheath. On MRV, we recorded venous sinus abnormalities and measured the luminal width of the superior ophthalmic veins. A grading score was introduced to define cranial venous outflow obstruction.
RESULTS:
Cranial venous outflow obstruction and ONS hydrops were the most valid signs indicating IH with a sensitivity of 94% and 92% and a specificity of 100% and 89%, respectively. Sensitivities and specificities were 56% and 97% for reduced pituitary height, 64% and 78% for flattening of the posterior sclera, 31% and 97% for widening of the superior ophthalmic veins, 33% and 100% for optic disc protrusion, 14% and 100% for optic nerve edema, and 6% and 100% for elongation of the optic nerve. At least 2 MR imaging findings could be demonstrated in each patient but in none of the controls. The number of positive MR imaging findings correlated with CSF pressure (r = 0.62, P = .01).
CONCLUSIONS:
The combination of cranial and orbital MR imaging and MRV can be highly sensitive and specific in the diagnosis of patients with IH.
IH can be caused by numerous conditions (SIH) but can also be observed without any evidence of an underlying condition (pseudotumor cerebri, IIH1–4). The direct measurement of intracranial or lumbar CSF pressure is regarded as the criterion standard in the diagnostic work-up of IH, but due to its invasive nature, the dependence on patient compliance, and the susceptibility for false-positive or false-negative results, it has potential limitations.4 Furthermore, clinical signs and neurologic symptoms are variably expressed and might not be specific for IH.2,5–7 Therefore, a complementary noninvasive diagnosis of IH is preferable in clinical routine. Recently, venous sinus stenoses, which might serve as diagnostic markers, have been reported in patients with IIH but are not well-documented as occurring in patients with SIH.8–12 Other MR imaging signs of IH have been partly investigated but not in a larger series of patients with SIH and lacking an integrative imaging protocol.13–22 Reported findings are the following: elongation of the optic nerve, protrusion of the optic nerve head, widening of the ONS, deformation of the pituitary gland, and dilation of the SOV.
The aim of our study was to determine the sensitivity and specificity of quantitative and qualitative MR imaging findings for IH in patients with SIH, by using a standardized MR imaging protocol, which combines orbital, cranial, and venous imaging.
Materials and Methods
Patients
Thirty-six patients with SIH due to various causes were prospectively included in the study, among them 7 children. Clinical characteristics are given in Table 1. IH was proved by lumbar CSF pressure measurements in 22 patients, and in 3 patients, elevated pressure was reported intraoperatively with CSF gushing out at high pressure on opening the dura mater. In the remaining 11 patients without CSF pressure measurement, IH was diagnosed by positive funduscopy findings showing bilateral papilledema, clinical symptoms, and follow-up imaging showing a reduction of MR imaging signs of increased intracranial pressure going along with clinical improvement. Patients with visual disturbances (32/36) were grouped into 2 categories: 1) patients with little or transient visual disturbances and visual acuity of >70% (n = 20), and 2) patients with severe visual loss (n = 12). Four patients had undergone surgery before inclusion in the study, including 3 children (7–14 years of age) with ventriculoperitoneal shunt operations and 1 adult (51 years of age) with occlusion of the dominant IJV due to neck surgery. Patients with IIH were not included in this study.
Table 1:
Clinical characteristics in 36 patients with IH and 36 control subjects
| Patient Groupa | Opening CSF Pressure on LP in cm H2O (mean) (range) | Demographics |
Diagnosis, Clinical Signs, and Symptoms |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group Size, Sex | Patient Age (mean) (range) | Diagnosis | Papilledema | Mild VI | Severe VI | Headache | Nausea/Vomiting | Other Symptoms | ||
| All patients with IH | 39.8 (24–70) | n = 36 F (n = 23)M (n = 13) | 40.9 yr 2–76 yr | Meningeal disease (n = 10), thrombosis or occlusion of venous sinus or the dominant IJV (n = 10), intracranial tumor (n = 8), hydrocephalus (other than caused by tumor, n = 5), other (n = 5), see below, multiple (n = 3) | 23/35 66% | 20/36 56% | 12/36 33% | 30/36 83% | 9/36 25% | See below |
| Patient Subgroup I | 29.5 (22–38) | n = 12 F (n = 7)M (n = 5) | 41.6 yr 32–66 yr | Occlusion of a venous sinus or IJV by meningioma or postoperatively (n = 3), viral meningitis (n= 3), bacterial meningitis (n = 1), venous sinus thrombosis (n = 1), excessive arterial hypertension (n = 1), head trauma with hygromas (n = 1), hydrocephalus (n = 1), minocycline medication (n = 1) | 7/12 58% | 7/12 58% | 3/12 25% | 12/12 100% | 4/123 3% | Fever, meningism (n = 4), transient aphasia (n = 3), vertigo (n = 2), transient apraxia (n = 1), cough (n = 1), hypacusis (n = 1), tinnitus (n = 1), reduced consciousness (n= 1), paraesthesia (n = 1) |
| Patient Subgroup II | 52 40–70 | n = 10 F (n = 6)M (n = 4) | 34.5 yr 2–62 yr | Thrombosis of venous sinus or IJV (n = 4), hydrocephalus (n = 3), meningiosis (n = 2), neurosarcoid meningitis (n = 1) | 7/97 8% | 4/10 40% | 5/10 50% | 8/10 80% | 3/10 30% | Vertigo, dysaesthesia (n = 1), tinnitus (n = 1), double vision (n = 1), head enlargement (n = 1), unspecific feeling of illness (n = 1) |
| Patient Subgroup III | Not testedb | n = 14 F (n = 10)M (n = 4) | 44.8 yr 9–76 yr | Meningioma alone or combined with stenosis or occlusion of a venous sinus (n = 4), venous sinus thrombosis alone or combined with brain abscess or dural metastasis (n = 3), brain tumor with occlusive hydrocephalus (n = 2), meningeosis (n = 2), arterial hypertension, PRES (n = 1), hydrocephalus (n = 1) | 9/14 64% | 9/14 64% | 4/14 29% | 10/14 71% | 2/14 14% | Double vision (n = 4), paresis (n = 4), reduced consciousness (n = 3), nystagmus (n = 1). vertigo (n = 1), fever (n = 1) |
| Controls | Not tested. | n = 36 F (n = 23) M (n = 13) | 39 yr 2–80 yr | Mostly psychiatric | Not tested | 0/36 0% | 0/36 0% | 0/36 0% | 0/36 0% | |
Patients with IH (n = 36) were subgrouped into those with CSF pressure <40 cm H2O (n = 12, group I), those with >40 cm H2O (n = 10, group II), and those without direct pressure measurements (n = 14, group III).
In subgroup III, 3 patients were reported to have elevated CSF pressure intraoperatively.
Control Group
Thirty-six individuals matched for age and sex with the SIH patient group served as controls. These individuals had cranial MR imaging performed for various reasons, mostly psychiatric disease, and did not have any clinical evidence of IH, such as headache or visual disturbances. Written informed consent was obtained from both patients with SIH and controls, and institutional review board approval was obtained. No individual fulfilled the usual exclusion criteria for MR imaging.
MR Imaging
MR imaging was performed in all patients by using a standardized protocol with a 3T unit and a head-sense-coil, including the following sequences: 1) T2 TSE axial: TR/TE, 4021/100 ms; section thickness, 3 mm; 2) STIR coronal: TR/TE/TI, 3838/90/180 ms; section thickness, 3 mm, covering the orbit and pituitary gland; and 3) 3D phase-contrast MRV: TR/TE, 17/7.9 ms; velocity encoding, 15 cm/s. Additional contrast-enhanced T1 sequences (n = 29), contrast-enhanced time-of-flight angiography (n = 3), time-resolved contrast-enhanced MR angiography (n = 1), digital subtraction angiography (n = 4), and/or CT venography (n = 1) were used to rule out venous sinus thrombosis in uncertain cases. Follow-up MR imaging was performed in 27 patients.
Analysis of MR Imaging Findings
Apart from the presence of space-occupying lesions with or without mass effect or obstruction of adjacent venous sinuses, special attention was paid to the following MR imaging findings: 1) nonquantitative cross-sectional findings: ventriculomegaly, periventricular edema caused by transependymal CSF flow (Fig 1A), flattening of the posterior sclera (Fig 2 A), optic papilla protrusion (Fig 1A), optic nerve edema (Fig 2B), and elongation of the optic nerve (Fig 1A); and 2) quantitative cross-sectional criteria: pituitary height (Fig 3 C, -F) and ONS width as measured at 4 different locations (3, 6, 10, and 20 mm behind the globe; Figs 2 and 4) by using coronal STIR sequences. With the source images of MRV, the maximum diameters of the SOV were measured (Fig 1C). Intracranial venous anatomy and pathology were judged by using MIPs, source images of MRV, and cross-sectional MR images. Gross venous anatomic variants, signal-intensity changes reflecting thrombosis within the venous sinuses (Fig 3A, -D), and venous sinus pathologies regarded as stenoses (but not typical for thrombosis) were further analyzed by using the primary MRV sections.
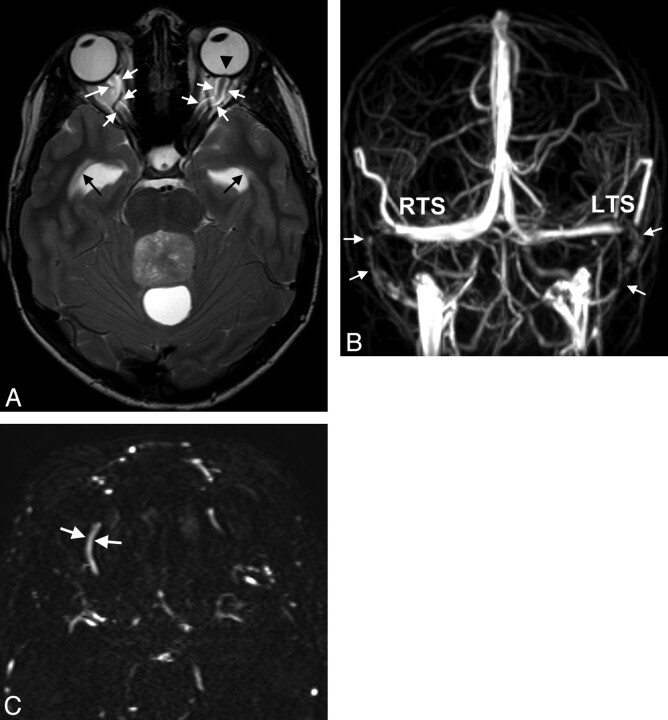
Fig 1.
A 15-year-old boy with double vision and reduced acuity has bilateral papilledema. A, On axial T2WI, ventriculomegaly with periventricular CSF flow (black arrows) due to an obstructing tumor within the fourth ventricle is found. Optic nerve sheath hydrops, elongation of the optic nerve (white arrows in A), and optic papilla protrusion are seen (black arrowhead). B, Anteroposterior view of MIP of MRV depicts bilateral narrowings of the lateral RTS and LTS and the sigmoid sinus (arrows). C, On primary axial sections of MRV, the SOVs are enlarged (arrows, right SOV, 2.8-mm width). Medulloblastoma was found at surgery.
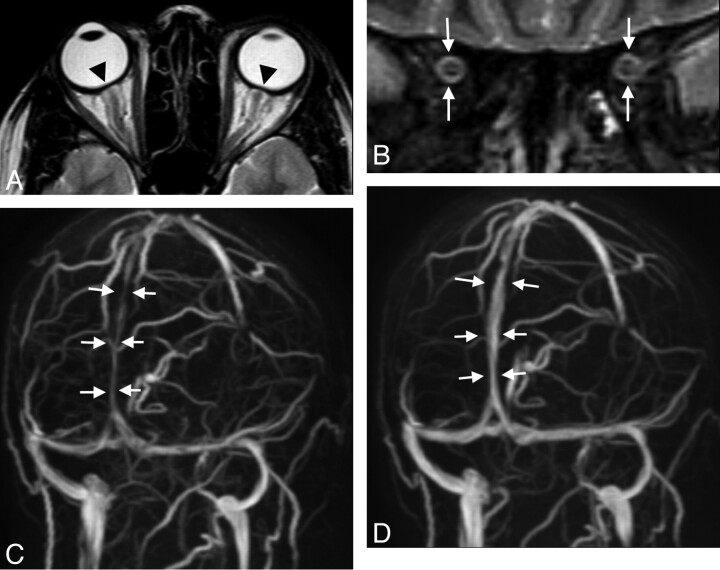
Fig 2.
A 30-year-old woman with arterial hypertension and visual disturbances has bilateral papilledema. A, MR imaging of the orbit displays a flattened posterior sclera (arrowheads). B, ONS hydrops is present, and there is edema of the optic nerve as seen on the coronal STIR sequence measured 20 mm behind the globe (white arrows, ONS width of 5.4 mm). C, Slightly oblique MIP of MRV shows lengthy narrowings of the intracranial venous sinuses, especially of the superior sagittal sinus (arrows). Vision returns to normal with successful treatment of hypertension. On follow-up MR imaging 3 months later, ONS width is reduced to 4.8 mm, and the posterior sclera appears normal (not shown). D, The intracranial venous sinuses regain normal caliber, as shown by MRV.
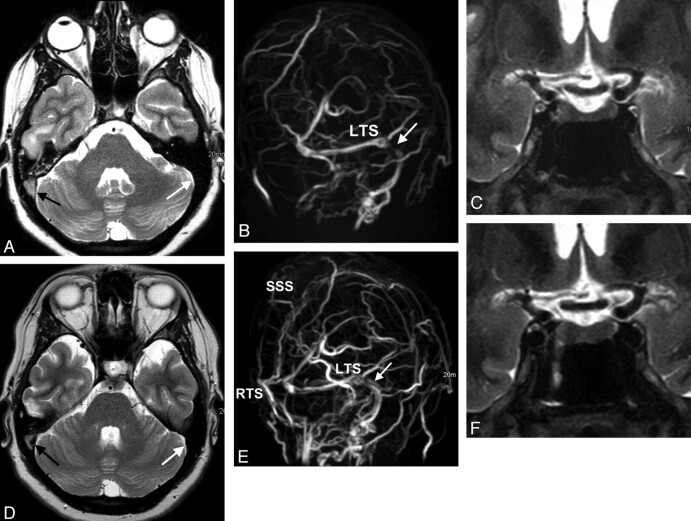
Fig 3.
A 28-year-old woman with venous sinus thrombosis secondary to in vitro fertilization. A, Signal intensity typical for thrombosed blood is seen in the SSS and the RTS on axial T2w (black arrow) but not in the LTS (white arrow). B, There is corresponding signal-intensity loss on MIP of MRV in the RTS and SSS (left oblique view). There is a stenosis in the LTS (white arrow) without evidence of a thrombus. C, ONS hydrops is present (not shown), and the height of the pituitary gland is reduced to 2.5 mm (coronal STIR). D and E, CSF pressure is 40 cm H2O. Six months later, partial recanalization of the RTS and SSS occurs following therapy with low-molecular heparin seen on T2WI (black arrow in D) and on MIPs of MRV (E). The stenosis in the LTS is believed to be the result of IH vanishing (white arrow in E). F, The pituitary height-weight returns to normal (4.5 mm).
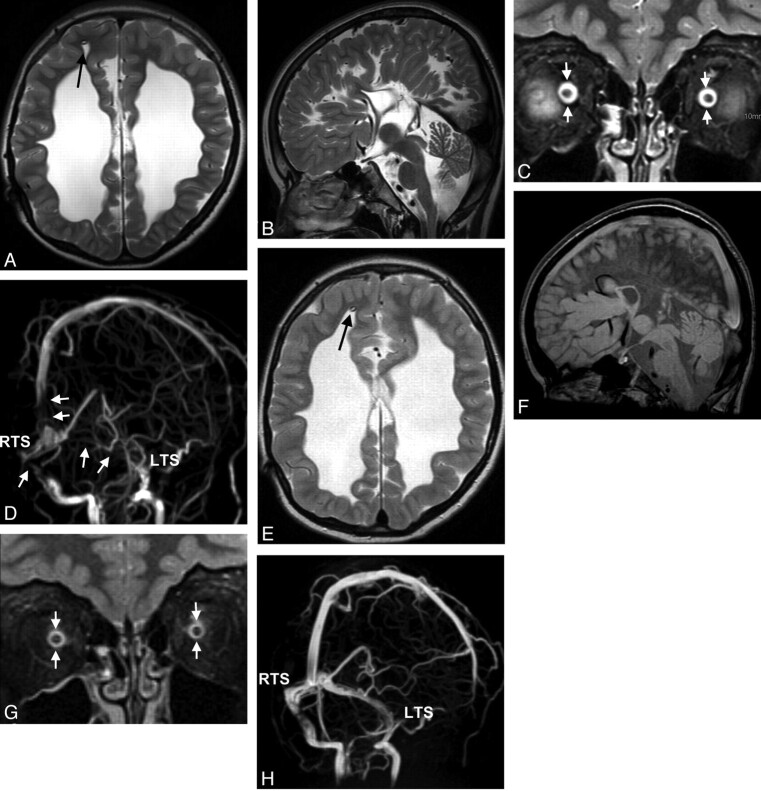
Fig 4.
Cranial MR imaging of a 7-year-old boy with congenital posthemorrhagic hydrocephalus before (A–D) and 7 days later after (E–H) correction of the distal part of an insufficient ventriculoperitoneal shunt (arrows in A and E). Standard imaging (axial T2WI in A and E; sagittal T2WI and FLAIR in B and F) does not reflect IH, and does not change after therapy. ONS hydrops (arrows in C, width of 6.8 mm measured in a plane 3 mm dorsal to the optic globe; upper normal limit is 6.3 mm) normalizes after therapy (arrows in G, width of 5.9 mm). Oblique views of MIP of MRV (D and H) display signal-intensity losses in the LTS and RTS and the SSS (arrows in D), also normalizing on follow-up (H). Bilateral papilledema resolves.
A simple classification system was used for grading venous sinus pathologies. A reduction of the diameter of a dural sinus of <50% was regarded as being within the normal range of variation (grade 0 lesion), whereas a more severe narrowing or a complete signal-intensity loss was categorized as a significant stenosis or occlusion (grade 1 lesion, Figs 1B, 2C, 3B, and 4D). CVOO was assumed in cases in which a grade 1 lesion affected the following: both TSs or at least the dominant TS if the opposite TS was severely hypoplastic or absent (type I), the mid or dorsal part of the SSS (type II), or a combination of the previous 2 types (type III). MR images were blinded for the presence of IH and were evaluated by 3 readers (A.C.R., an experienced board-certified radiologist/neuroradiologist; C.R., a board-certified radiologist and Specialist Registrar in neuroradiology; and M.-C.F., a senior medical student who was trained to evaluate cranial MR images with respect to features of IH). The results of the first reader were used for the primary analysis. The results of all 3 readers were used to calculate interobserver reliabilities.
Statistical Analysis
On the basis of cross-tabulations, the sensitivity, specificity, and OR of different MR imaging findings were calculated (95% CI). A Pearson correlation coefficient test was used to find correlations between CSF pressure and quantitative MR imaging signs of IH. A Spearman correlation coefficient test was chosen to find correlations between CSF pressure values and the number of positive MR imaging signs of IH. A Mann-Whitney U test was used to prove differences between mean values of measurements in patient subgroups and between patients and controls. For analysis of interobserver reliabilities, κ values and percentages of interobserver agreements (qualitative signs) and ICCs (quantitative signs) were calculated. For each test, the level of statistical significance was defined as P < .05. Statistical analysis was performed with the Statistical Package for the Social Sciences software, Version 13 (SPSS, Chicago, Illinois) except for computation of κ and ICC values, which was done with R by using the irr package (R Development Core Team, 2010; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).
Results
MR Imaging Findings in SIH
The main findings were CVOO, ONS hydrops, reduced pituitary height, and flattened posterior sclera (Tables 2 and 3). All the investigated findings differed significantly between patients and controls (P < .05).
Table 2:
Differences in MRI measurements between 36 patients with IH and 36 controlsa
| Diameter of the ONS (mm)b |
Pituitary Height (mm) | SOV Diameter (mm) | |||||
|---|---|---|---|---|---|---|---|
| Position 1 | Position 2 | Position 3 | Position 4 | ||||
| Patients with IH (all, n = 36) | Mean | 7.0 | 6.1 | 5.4 | 5.1 | 3.1 | 2.3 |
| SD | 0.9 | 0.9 | 1.0 | 0.7 | 1.6 | 0.7 | |
| 95% CI | 6.7–7.3 | 5.8–6.4 | 5.1–5.7 | 4.9–5.3 | 2.6–3.6 | 2.0–2.5 | |
| ICC | 0.832 | 0.84 | 0.866 | 0.84 | 0.863 | 0.389 | |
| Patient subgroup I (n = 12) | Mean | 7.2 | 6.2 | 5.3 | 5.1 | 3.2 | 2.2 |
| CSF pressure 22–38 cm H2O | SD | 0.6 | 0.7 | 1.1 | 0.5 | 1.0 | 0.8 |
| Patient subgroup II (n = 10) | Mean | 6.7 | 5.9 | 5.2 | 5.1 | 2.8 | 2.4 |
| CSF pressure 40–70 cm H2O | SD | 0.6 | 0.7 | 0.8 | 0.7 | 1.3 | 0.5 |
| Patient subgroup III (n = 14) | Mean | 7.0 | 6.2 | 5.7 | 5.0 | 3.2 | 2.2 |
| No CSF pressure measurements | SD | 1.2 | 1.1 | 1.0 | 0.8 | 2.0 | 0.7 |
| Controls (n = 36) | Mean | 5.8 | 4.9 | 4.4 | 4.1 | 5.2 | 1.7 |
| SD | 0.6 | 0.6 | 0.5 | 0.3 | 1.5 | 0.5 | |
| 95% CI | 5.6–6.0 | 4.7–5.1 | 4.3–4.6 | 4.0–4.3 | 4.7–5.6 | 1.5–1.8 | |
| ICC | 0.76 | 0.728 | 0.78 | 0.732 | 0.893 | 0.634 | |
Results of all measurements differed significantly between all patients and controls (P < .01) and also between the different patient subgroups (I∼III) and controls (P < .05). No significant differences existed among results of patient subgroups I, II, and III.
ONS diameter was measured 3, 6, 10, and 20 mm behind the globe (positions 1–4).
Table 3:
MR imaging signs in 36 patients with IH and 36 control subjectsa
| Patient Group | Opening CSF Pressure on LP in cm H2O (mean) (range) | CVOO | ONS Hydropsb | Reduced Pituitary Height (<2.6 mm) | Flattened Posterior Sclera | Optic Disc Protrusion | Dilated SOV (<2.6 mm in diameter) | Ventriculomegaly | Periventricular CSF Flow | Optic Nerve Edema | Optic Nerve Elongation | No. of Positive MRI Signs (mean) (range) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | 39.8 | Frequency, | 34/36 | 33/36 | 20/36 | 23/36 | 12/36 | 11/36 | 7/36 | 5/36 | 5/36 | 2/36 | 4.3 (2–9) |
| 24–70 | Sensitivity, | 94% | 92% | 56% | 64% | 33% | 31% | 19% | 14% | 14% | 6% | ||
| CI 95%, | 81%–99% | 76%–98% | 38%–72% | 46%–79% | 19%–91% | 16%–48% | 8%–36% | 5%–29% | 5%–29% | 1%–19% | |||
| Specificity, | 100% | 89% | 97% | 78% | 100% | 97% | 100% | 100% | 100% | 100% | |||
| 95% CI, | 90%–100% | 73%–96% | 85%–100% | 61%–90% | 90%–100% | 85%–100% | 90%–100% | 90%–100% | 90%–100% | 90%–100% | |||
| OR | 1007 | 88 | 44 | 6 | 37 | 15 | 18 | 13 | 13 | 5 | |||
| 95% CI, | 47–21,741 | 18–424 | 5–355 | 2–17 | 2–658 | 2–127 | 1–338 | 1–240 | 1–240 | .3–114 | |||
| Agreement,c | 100% | 100% | 91.7% | 63.9% | 77.8% | 67% | 91.7% | 91.7% | 69.4% | 80.6% | |||
| κc | 1 | 1 | 0.888 | 0.484 | 0.675 | 0.338 | 0.852 | 0.79 | 0.47 | 0.533 | |||
| Patient | 29.5 | Frequency | 10/12 | 11/12 | 4/12 | 5/12 | 2/12 | 4/12 | 1/12 | 1/12 | 0/12 | 0/12 | 3.3 (2–6) |
| subgroup I | 22–38 | 83% | 92% | 33% | 42% | 17% | 33% | 8% | 8% | 0% | 0% | ||
| Patient | 52 | Frequency | 10/10 | 9/10 | 7/10 | 8/10 | 6/10 | 3/10 | 3/10 | 1/10 | 3/10 | 1/10 | 5.1 (3–7) |
| subgroup II | 40–70 | 100% | 90% | 70% | 80% | 60% | 30% | 30% | 10% | 30% | 10% | ||
| Patient | N.T. | Frequency | 14/14 | 13/14 | 9/14 | 10/14 | 4/14 | 4/14 | 3/14 | 3/14 | 2/14 | 1/14 | 4.5 (2–9) |
| subgroup III | 100% | 93% | 64% | 71% | 29% | 29% | 21% | 21% | 14% | 7% | |||
| Controls | N.T. | Frequency | 0/36 | 4/36 | 35/36 | 8/36 | 0/36 | 1/36 | 0/36 | 0/36 | 0/36 | 0/36 | 0.4 (0–1) |
| 0% | 11% | 3% | 22% | 0% | 3% | 0% | 0% | 0% | 0% | ||||
| Agreementc | 100% | 78% | 100% | 69.4% | 94.4% | 94.4% | 94.4% | 97.2% | 97.2% | 94.4% |
Frequency of all MRI signs differed between patients and controls (P< .01). Frequency of the MRI signs “optic disc protrusion” and “optic nerve edema” were more often found in patient group II compared with patient group I (P< .05). Frequencies of all MRI signs did not differ between patient subgroup III and the other patient groups.
ONS hydrops was assumed if the diameter was above normal limits in ≥1 different position. Normal upper limits of ONS diameters were 6.4, 5.8, 5.1, and 4.8 mm in measurement positions 1, 2, 3, and 4 (3, 6, 10, and 20 mm behind the globe).
Results of 3 readers used to determine the interobserver-reliability.
CVOO
All except 2 patients (n = 34, sensitivity 94%) had CVOO. Venous sinus stenoses were apparent in sinuses distant from thrombosed sinuses in 6/8 patients (75%, Fig 3) and distant from sinuses compressed by a tumor in 7/8 patients (88%), or occurred in patients without any primary pathology of a venous sinus (18/20 patients, 90%; Figs 1, 2, and 4). Most patients (31/36, 86%) had stenoses that could not be directly attributed to a primary underlying pathology (ie, compression by a tumor or acute thrombosis). Type I CVOO (Fig 1B) was found in 41%; type II (Fig 2C), in 12%; and type III (Fig 4D), in 47%. In the control group, CVOO was not detected, but flow irregularities were seen in 44%.
ONS Hydrops
Mean diameters of the ONS were greater in patients compared with controls in all 4 positions (P < .01, Table 2). Using upper normal limits of the width of the ONS of 6.4, 5.8, 5.1, and 4.8 mm in positions 1–4 as proposed,23we observed ONS hydrops with sensitivities of 78%, 75%, 75%, and 75% and specificities of 89%, 100%, 97%, and 100%. In 33/36 patients, ONS hydrops occurred in at least 1 of the 4 positions of the optic nerve (sensitivity 92%), which could be detected in only 4/36 controls (specificity, 89%; Table 3). ONS hydrops was always bilateral (Figs 2 and 4).
Reduced Pituitary Height
Mean pituitary height was lower in patients than in controls (Table 2). A pituitary height <2.6 mm was observed in 20/36 patients and in 1/36 controls (56% sensitivity, 97% specificity; Table 3 and Fig 3C). When we took into account age- and sex-adapted lower normal limits of the pituitary height of 3 mm for children, 2.7 mm for men of all ages, 3.3 mm for women 18–62 years of age, and 1.8 mm for women older than 62 years of age,23 22 patient and 2 control measurements were abnormal, resulting in a sensitivity and specificity of 61% and 94%, respectively.
Flattened Posterior Sclera, Optic Papilla Protrusion, Elongation, and Edema of the Optic Nerve
The posterior sclera was flattened in 23 patients and in 8 controls. Protrusion of the optic disc, optic nerve edema, and optic nerve elongation were observed in 33%, 14%, and 6% of patients but in none of the controls (specificity of 100% each). Funduscopy was performed in 11 of 12 patients with protrusion of the disc, and bilateral papilledema was found in all of them. Optic disc protrusion was found in only 12/23 patients with proved papilledema (sensitivity of 52%).
Dilation of the SOV
The mean diameter of the SOV was greater in patients with SIH than in controls (Table 2). In 11 patients and in 1 control, the maximum diameter of the SOV exceeded 2.6 mm (Table 3).
Ventriculomegaly
Ventriculomegaly was noted in 7 patients. In 2 patients with obstructive hydrocephalus and 1 patient with hydrocephalus of unknown origin, periventricular edema was present. In all of these 7 patients, CVOO was present; in 7/8 patients, cross-sectional MR imaging signs of IH were seen.
Correlating CSF Pressure with MR Imaging Findings and Vision Loss
CSF pressure was significantly higher in patients with optic disc protrusion (48.3 ± 13.4 cm H2O) and optic nerve edema (54.7 ± 8.1 cm H2O) than in patients without these findings (mean, 36.1 ± 10.9 cm H2O and 38.3 ± 12.3 cm H2O, respectively; P < .05 each). In patients with CVOO, CSF pressure was higher in those having CVOO type III (54.4 ± 12.7 cm H2O) than in those having either CVOO type I or II (36.7 ± 11.2 cm H2O, P < .05). ONS diameter, height of the pituitary gland, and diameter of the SOV did not significantly correlate with the degree of CSF pressure increase. There was a positive correlation of CSF pressure and the number of positive MR imaging findings of IH (Spearman correlation coefficient, r = 0.62; P = .01). At least 2 MR imaging findings could be demonstrated in each patient. Concerning visual impairment, no significant differences in CSF pressure values were found between patients in groups 1 (little or transient impairment) and 2 (severe impairment) (mean, 37.4 ± 10.7 cm H2O versus 43.3 ± 14.0 cm H2O), but patients in group 2 had significantly more frequent MR imaging features of optic disc protrusion (58%) and optic nerve edema (33%) than patients in group 1 (25% and 5%, P < .05 each). There were no noted differences between patients with and without direct pressure measurements (Tables 2 and 3).
Interobserver Reliability
Concerning quantitative signs, the interobserver reliability was best for measurements of the pituitary height, was good for measurements of the ONS diameters, and was worst for measurements of the SOVs (Table 2). Concerning qualitive signs, the interobserver reliability was excellent for CVOO, ventriculomegaly, and periventricular CSF flow; was mediocre for optic disc protrusion, optic nerve elongation, and “optic nerve edema; and was worst for flattened posterior sclera (Table 3).
Follow-Up Investigations
Follow-up MR images were available in 27 patients. After CSF pressure was lowered, 20 patients improved clinically with associated decrease in MR imaging signs of IH (Fig 3). One patient remained stable clinically and on MR imaging. Six patients improved clinically, but MR imaging findings did not change. The rate of agreement (clinical versus neuroradiologic), therefore, was 21/27 (78%). Follow-up MR images after shunt revisions were available in 2 of 3 children with hydrocephalus and demonstrated normalization of MR imaging features except for the presence of hydrocephalus (Fig 4).
Discussion
Our study shows that the imaging parameters CVOO, ONS hydrops, and reduction in pituitary height discriminated best between patients with IH and controls. Most other signs studied did show a lesser sensitivity but a high specificity.
Intracranial Venous Morphology in IH
Thrombosis or occlusion of venous sinuses (primary CVOO) can result in IH,23–26 probably by inducing venous hypertension and subsequent impairment of CSF absorption.25 In patients with IIH, bilateral narrowing of the TS not associated with thrombosis has been found regularly.27–31 Because stent angioplasty can help control IH in these patients, a causative role of these stenoses is favored by some.29,30 On the other hand, lowering the intracranial pressure can result in normalization of venous morphology, suggesting that these stenoses might be induced by IH itself (secondary CVOO).25,26 We have shown that CVOO is not a specific feature of patients with IIH but is a universal phenomenon in most patients with IH irrespective of the underlying cause (Figs 2–4). Also, stenoses are not restricted to both lateral TSs as in IH but can occur in a variety of locations and present with different shapes. A widespread involvement of sinuses was associated with very high CSF pressure. Most interesting, in patients with primary CVOO, additional stenoses also occur in sinuses not affected by primary obstruction. We hypothesize that IH compresses the dural sleeve of the venous sinuses in all patients. This compression might lead to lengthy narrowing or—in the case of rather short bilateral TS stenoses—enlargement and invagination of arachnoid granulations into the sinus lumen or might induce a collapse of “vulnerable” sinus segments. The latter phenomenon might induce a vicious circle because the sinus stenoses themselves worsen IH via the mechanism of prestenotic venous hypertension. Because principally all segments of the venous sinuses can be affected, failures of venous sinus stent placement can occur.11
Analysis of MRVs can be challenging because flow signal-intensity irregularities occurred in a significant number of our controls (44%). Similar results have been published by Higgins et al.32 Nevertheless, by using a simple new scoring system, stenoses were found to lead to CVOO in 94% of our patients with SIH but in none of the controls. Widening of the SOV in patients with IH has been demonstrated with T1WI16 and could also be shown in our patients by using MRV. The reason for this is not clear but could be due to increased collateral venous outflow.
Cross-Sectional MR Imaging Signs of IH
Cross-sectional MR imaging and CT signs of IH have been studied in patients with IIH13,14,17–22,33,34 with varying results. In a recent study, only 1 MR imaging sign (flattening of the posterior sclera) was found to be useful.21 This could be due to the different nature of IIH compared with SIH but could also be due to technical limitations of that study: Although most MR imaging signs were derived from the orbital content, no detailed orbital studies were performed and authors did not describe at which location the width of the ONS was measured. However, this is an important aspect because this width varies significantly from anterior to posterior.35,36 Changes of the pituitary shape were judged subjectively despite age- and sex-dependent variations of the pituitary size.35,37
With our approach of using quantitative markers and by using MR imaging sequences optimized for orbital imaging, we found several parameters to be useful for predicting IH, especially ONS hydrops and a decrease of the pituitary height. Flattening of the posterior sclera was a sign less sensitive and not specific for IH in our study, and interobserver reliability for this sign was low. In our opinion, judgment of this sign is delicate and prone to be influenced by partial volume effects. Protrusion of the optic disc indicates papilledema. This sign and optic nerve edema were associated with very high CSF pressure and with severe visual impairment. We favor a diagnostic approach that considers multiple indirect MR imaging signs of IH, because we found that the number of positive signs correlates with the degree of pressure increase and because pathologies of the orbit or sella might prevent using a single sign.
Making use of MR imaging signs of IH in patients with SIH has several possible implications: Because unlike in IIH, patients with SIH display a wider range of signs and symptoms due to their underlying disease, the clinical diagnosis of IH can be difficult and thus could be substantiated by MR imaging. In patients lacking obvious pathology on neuroimaging (Fig 2), MR imaging signs of IH might be the only indicators of IH and may lead to effective treatment (CSF diversion). Some patients with venous sinus thrombosis develop visual problems that can be attributed to IH. We found MR imaging to be helpful in diagnosing IH in these cases (Fig 3). In patients with hydrocephalus with shunt surgery procedures, ventriculomegaly can persist irrespective of the actual intracranial pressure (Fig 4). To our knowledge, this is the first report to show that the presence of venous sinus narrowing on MRV can easily demonstrate an increase in intracranial pressure in these patients. In the case of severe noncommunicating hydrocephalus, additional MR imaging features of IH probably do not influence patient treatment (Fig 1), but they may be of use on follow-up. In our study, these cases provide evidence that MR imaging signs of IH work in a variety of settings (communicating and noncommunicating hydrocephalus). Our preliminary data by using follow-up MR images indicate that MR imaging signs of IH are at least partially reversible on normalization of intracranial pressure.
Larger numbers of patients and controls are needed to substantiate our findings, especially with regard to children with shunt placements and patients acutely affected by IH.
Conclusions
With a standardized protocol, MR imaging is sensitive and specific in the diagnosis of patients with IH irrespective of the underlying cause. In our cohort, all patients but none of the controls displayed at least 2 MR imaging signs of increased intracranial pressure. This technique can be potentially helpful in increasing the diagnostic reliability and in detecting patients who need invasive procedures in IH. Venous sinus narrowings are not a specific feature of IIH. In patients with secondary IH, these narrowings occur in different locations and with different shapes than those reported for IIH. We speculate that they are caused by IH but might contribute to the increase in intracranial pressure. Venous sinus narrowing might be a sensitive sign for diagnosing ventriculoperitoneal shunt failure but larger numbers of patients are needed to confirm this possibility.
Abbreviations
- CI
confidence interval
- CVOO
cranial venous outflow obstruction
- FLAIR
fluid-attenuated inversion recovery
- ICC
intraclass correlation coefficient
- IH
intracranial hypertension
- IIH
idiopathic intracranial hypertension
- IJV
internal jugular vein
- LP
lumbar puncture
- LTS
left transverse sinus
- MIP
maximum intensity projection
- MRI
MR imaging
- MRV
MR venography
- ONS
optic nerve sheath
- OR
odds ratio
- PRES
posterior reversible encephalopathy syndrome
- RTS
right transverse sinus
- SIH
secondary intracranial hypertension
- SOV
superior ophthalmic vein
- SSS
superior sagittal sinus
- STIR
short tau inversion recovery
- T1WI
T1-weighted imaging
- T2WI
T2-weighted imaging
- TS
transverse/sigmoid sinus
- TSE
turbo spin-echo
- VI
visual impairment
Footnotes
This work was supported by Archimedes Pharma (approximately 30,000 Euro).
Disclosure: Lutz Doerner is a member of the Speaker Bureau for Archimedes Pharma. He earned approximately 5000 Euro.
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology and the Neuroradiology Education and Research Foundation Symposium, May 16–21, 2009; Vancouver, British Columbia, Canada.
References
- 1. Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol 2006; 5: 433– 42 [DOI] [PubMed] [Google Scholar]
- 2. Binder DK, Horton JC, Lawton MT, et al. Idiopathic intracranial hypertension. Neurosurgery 2004; 54: 538– 51, discussion 551–32 [DOI] [PubMed] [Google Scholar]
- 3. Skau M, Brennum J, Gjerris F, et al. What is new about idiopathic intracranial hypertension? An updated review of mechanism and treatment. Cephalalgia 2006; 26: 384– 99 [DOI] [PubMed] [Google Scholar]
- 4. Bershad EM, Humphreis WE, Suarez JI. Intracranial hypertension. Semin Neurol 2008; 28: 690– 702 [DOI] [PubMed] [Google Scholar]
- 5. Algahtani HA, Baeesa SS, Obeid TH, et al. Idiopathic intracranial hypertension: atypical presentation. Saudi Med J 2007; 28: 762– 65 [PubMed] [Google Scholar]
- 6. Duggal HS. Idiopathic intracranial hypertension presenting with psychiatric symptoms. J Neuropsychiatry Clin Neurosci 2005; 17: 426– 27 [DOI] [PubMed] [Google Scholar]
- 7. Marcelis J, Silberstein SD. Idiopathic intracranial hypertension without papilledema. Arch Neurol 1991; 48: 392– 99 [DOI] [PubMed] [Google Scholar]
- 8. Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 2003; 98: 1045– 55 [DOI] [PubMed] [Google Scholar]
- 9. Higgins JN, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004; 62: 1907– 08 [DOI] [PubMed] [Google Scholar]
- 10. King JO, Mitchell PJ, Thomson KR, et al. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002; 58: 26– 30 [DOI] [PubMed] [Google Scholar]
- 11. Rohr A, Dorner L, Stingele R, et al. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2007; 28: 656– 59 [PMC free article] [PubMed] [Google Scholar]
- 12. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003; 60: 1418–24 [DOI] [PubMed] [Google Scholar]
- 13. Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 1998; 105: 1686– 93 [DOI] [PubMed] [Google Scholar]
- 14. Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging 2000; 12: 808– 13 [DOI] [PubMed] [Google Scholar]
- 15. Bono F, Messina D, Giliberto C, et al. Bilateral transverse sinus stenosis predicts IIH without papilledema in patients with migraine. Neurology 2006; 67: 419– 23 [DOI] [PubMed] [Google Scholar]
- 16. Lirng JF, Fuh JL, Wu ZA, et al. Diameter of the superior ophthalmic vein in relation to intracranial pressure. AJNR Am J Neuroradiol 2003; 24: 700– 03 [PMC free article] [PubMed] [Google Scholar]
- 17. Gibby WA, Cohen MS, Goldberg HI, et al. Pseudotumor cerebri: CT findings and correlation with vision loss. AJR Am J Roentgenol 1993; 160: 143– 46 [DOI] [PubMed] [Google Scholar]
- 18. Silbergleit R, Junck L, Gebarski SS, et al. Idiopathic intracranial hypertension (pseudotumor cerebri): MR imaging. Radiology 1989; 170: 207– 09 [DOI] [PubMed] [Google Scholar]
- 19. Zagardo MT, Cail WS, Kelman SE, et al. Reversible empty sella in idiopathic intracranial hypertension: an indicator of successful therapy? AJNR Am J Neuroradiol 1996; 17: 1953– 56 [PMC free article] [PubMed] [Google Scholar]
- 20. Jinkins JR, Athale S, Xiong L, et al. MR of optic papilla protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol 1996; 17: 665– 68 [PMC free article] [PubMed] [Google Scholar]
- 21. Agid R, Farb RI, Willinsky RA, et al. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology 2006; 48: 521– 27 [DOI] [PubMed] [Google Scholar]
- 22. Weisberg LA. Computed tomography in benign intracranial hypertension. Neurology 1985; 35: 1075– 78 [DOI] [PubMed] [Google Scholar]
- 23. Fuentes S, Metellus P, Levrier O, et al. Depressed skull fracture overlying the superior sagittal sinus causing benign intracranial hypertension: description of two cases and review of the literature. Br J Neurosurg 2005; 19: 438– 42 [DOI] [PubMed] [Google Scholar]
- 24. Duke BJ, Ryu RK, Brega KE, et al. Traumatic bilateral jugular vein thrombosis: case report and review of the literature. Neurosurgery 1997; 41: 680– 83 [DOI] [PubMed] [Google Scholar]
- 25. Karahalios DG, Rekate HL, Khayata MH, et al. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996; 46: 198– 202 [DOI] [PubMed] [Google Scholar]
- 26. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 1999; 53: 1537– 42 [DOI] [PubMed] [Google Scholar]
- 27. Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004; 62: 1445– 46 [DOI] [PubMed] [Google Scholar]
- 28. McGonigal A, Bone I, Teasdale E. Resolution of transverse sinus stenosis in idiopathic intracranial hypertension after L-P shunt. Neurology 2004; 62: 514– 15 [DOI] [PubMed] [Google Scholar]
- 29. Donnet A, Metellus P, Levrier O, et al. Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology 2008; 70: 641– 47 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JN, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003; 74: 1662– 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol 2005; 252: 1021– 25 [DOI] [PubMed] [Google Scholar]
- 32. Higgins JN, Gillard JH, Owler BK, et al. MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Psychiatry 2004; 75: 621– 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brodsky MC. MR of papilledema. AJNR Am J Neuroradiol 1997; 18: 1592– 94 [PubMed] [Google Scholar]
- 34. Brodsky MC, Glasier CM. Magnetic resonance visualization of the swollen optic disc in papilledema. J Neuroophthalmol 1995; 15: 122– 24 [PubMed] [Google Scholar]
- 35. Rohr A, Riedel C, Reimann G, et al. Pseudotumor cerebri: quantitative in-vivo measurements of markers of intracranial hypertension [in German]. Rofo 2008; 180: 884– 90 [DOI] [PubMed] [Google Scholar]
- 36. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves: an ultrasound study of the optic nerve sheath. Surg Radiol Anat 1996; 18: 323– 28 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki M, Takashima T, Kadoya M, et al. Height of normal pituitary gland on MR imaging: age and sex differentiation. J Comput Assist Tomogr 1990; 14: 36– 39 [DOI] [PubMed] [Google Scholar]