SUMMARY:
We have encountered 2 cases of parathyroid adenomas that are atypical because of their large size, cystic character, and faint enhancement compared with the typical solid parathyroid adenomas. Specifically, the enhancement pattern of a typical parathyroid adenoma in a multiphasic scan demonstrates rapid arterial enhancement and rapid washout on delayed imaging, whereas, comparatively, the 2 cystic parathyroid adenomas we encountered demonstrated less arterial phase enhancement and little washout on venous and delayed-phase imaging.
Typically, the 4 parathyroid glands lie in close proximity to the thyroid gland but can lie anywhere along the path of descent of the pharyngeal pouches, from the mandible to the mediastinum. Each gland is composed mainly of chief cells, which respond to a decrease below a “set point” of circulating ionized calcium by releasing PTH in a homeostatic feedback loop mediated by calcium-sensing receptors on the cell surface.
PTH increases the level of circulating calcium by receptor-mediated tubular resorption of calcium in the kidney, increasing osteoclast activity to stimulate release of calcium from the bone, and increasing activity of renal 1-hydroxylase, resulting in production of 1,25-dihydroxyvitamin D and increasing bowel calcium absorption.1 Hyperparathyroidism in all its forms is characterized by an increase in the set point for serum calcium,2 which in turn leads to a serum calcium level above the reference range.
With the advent of advanced parathyroid imaging, standard 4-gland exploration in cases of primary hyperparathyroidism has been superseded by minimally invasive parathyroidectomy targeted at the parathyroid adenoma. At our institution, the primary imaging technique used for the detection of parathyroid adenoma is the dual isotope 123I/Tc99m sestamibi subtraction scan. Sonography has been the primary troubleshooting technique at our institution for the identification of parathyroid glands but has limited the visualization of ectopic parathyroid glands. With the increasing spatial resolution and speed of modern CT scanners, the utility of the multiphasic contrast-enhanced CT scan has been recognized and is more frequently requested by our surgeons in difficult cases.
The technique of multiphasic CT for parathyroid imaging and the enhancement patterns of parathyroid adenomas have been previously described3–5 and have proved useful in many cases for identification of parathyroid glands and preoperative planning. We have encountered 2 cases of parathyroid adenomas that are atypical because of their large size, cystic character, and faint enhancement.
Case Reports
Case 1
A 54-year-old female patient was initially found on medical evaluation to have an elevated calcium concentration of 11.8 mg/dL and inappropriate elevation of the PTH level at 169 pg/mL (normal, 15–50 pg/mL).
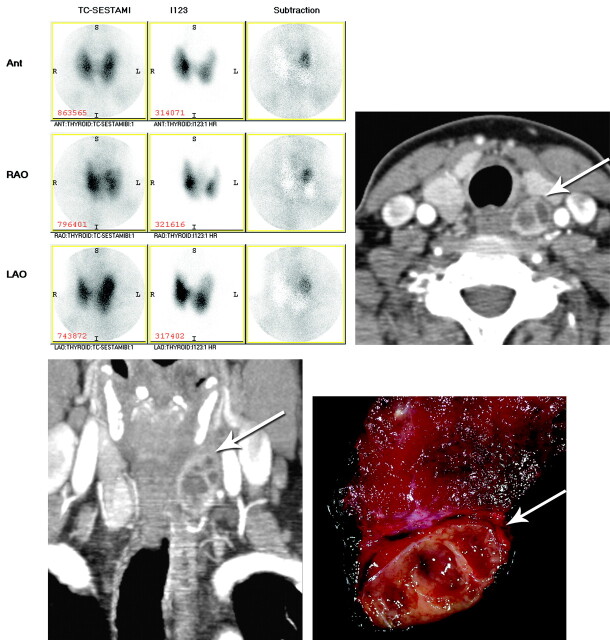
Preoperative imaging included a 123I/Tc99m sestamibi subtraction scan, which demonstrated discordant sestamibi uptake at the superior pole of the left thyroid lobe compatible with parathyroid adenoma or a thyroid nodule with discordant sestamibi/123I uptake. Neck sonography showed a large, partially solid, partially cystic nodule either arising from the posterior aspect of the upper pole of the left lobe of the thyroid or deep to the upper pole of the thyroid, measuring 1.6 × 3.1 cm. Dynamic CT confirmed a 2.5-cm cystic mass along but separate from the mid-to-superior left lobe of the thyroid, with mild arterial phase enhancement, which did not appreciably change on later phases of the examination acquisition (Fig 1).
Fig 1.
123I/Tc-99m sestamibi subtraction scan (top left); axial arterial phase CT scan (top right); coronal arterial phase CT scan (bottom left); and postoperative cut section of cystic parathyroid adenoma adherent to left lobe of thyroid (bottom right). Arrows denote the cystic parathyroid adenoma.
A minimally invasive targeted parathyroidectomy was performed along the anterior border of the left sternocleidomastoid muscle. Inflammatory reaction was found in the left tracheoesophageal groove, with the inflammatory mass adherent to the adjacent left lobe of the thyroid gland. Pathology demonstrated a large parathyroid adenoma with multilocular secondary cystic degeneration characterized by fibrosis and hemosiderosis between the parathyroid tumor and the nearby thyroid, without invasion of the thyroid parenchyma proper by the tumor.
Case 2
A 65-year-old woman with a history of previous neck surgery for hyperparathyroidism was diagnosed with recurrent hyperparathyroidism with negative findings on outside imaging (including the sestamibi scan). Her neck sonography before her first surgery reportedly showed a multinodular thyroid gland but no evidence of parathyroid tissue. She underwent subsequent full-neck exploration at the outside institution, where a single large parathyroid gland was removed with a transient return of her serum calcium level to normal. A month after surgery, her serum calcium concentration was found to be increased again at 11.1 mg/dL, and her PTH level had increased to 185 pg/mL.
Preoperative imaging performed at our institution included a 123I/Tc99m sestamibi subtraction scan, which demonstrated an area of increased sestamibi uptake without radioiodine uptake, adjacent to the midportion of the right thyroid lobe on subtraction images. Neck sonography showed no evidence of a parathyroid adenoma. Dynamic CT visualized a large peripherally enhancing centrally cystic structure without significant washout, adjacent to the esophagus, and an equivocal area of enhancement in the right thyroid (Fig 2).
Fig 2.
123I/Tc-99m sestamibi subtraction scan (top left); neck sonogram in region of cystic mass (top right); axial arterial phase CT scan (bottom left); and coronal arterial phase CT scan (bottom right). Arrows denote the cystic parathyroid adenoma.
Given the equivocal results of imaging, a secondary collar incision was made, and the right lobe of the thyroid was elevated to reveal a superior parathyroid adenoma located low in the groove well away from the thyroid. A 4800-mg parathyroid gland, which also had secondary cystic degeneration, was confirmed by pathology.
Discussion
Approximately 350 cases (or 0.5%–1% of all parathyroid pathologies) of cystic parathyroid lesions have been described in the literature.6,7 Approximately 90% of these are classified as nonfunctioning cystic lesions, being found in individuals with normal calcium concentrations. The typical incidental nonfunctional cystic parathyroid lesion is anechoic, containing clear colorless fluid with a high intralesional PTH level. In approximately 10% of cases (larger percentages being reported in hyperparathyroidism referral centers), the cystic parathyroid lesions were functional, responsible for hyperparathyroidism.
As reported by McCoy et al,6 SPECT sestamibi imaging failed to localize functional parathyroid cysts in as many as 32% of patients in their study, probably because of the diffuse enlargement and subsequent diffusion of activity within the cystic parathyroid gland. There is debate in the literature concerning whether these lesions represent embryonic remnants of the branchial apparatus (lined with epithelial tissue) or arise from central degeneration and liquefaction of a functional parathyroid adenoma. A third possibility would be the coalescence of parathyroid microcysts. At our institution, the histologic distinction between a cystic parathyroid adenoma and the rare functional parathyroid cyst is made by the former having a preponderance of chief cells with multilocular degenerative thick-walled cysts and the latter usually consisting of a unilocular thin-walled cyst. In any case, the functional parathyroid cyst must be removed carefully to avoid rupture and spillage of PTH into the operative bed, possibly precipitating parathyromatosis, in which release of massive amounts of free PTH into the bloodstream precipitates a hypercalcemic crisis. Ideally, if a cystic parathyroid gland is encountered in preoperative imaging, it should be targeted for removal regardless of whether it is responsible for the patient's hyperparathyroidism, to prevent future confusion over the source of recurrent elevated PTH levels.7
We found these parathyroid adenomas atypical because of their large size, cystic nonenhancing central components, and unusual enhancement pattern compared with solid parathyroid adenomas.3 Specifically, the enhancement pattern of a typical parathyroid adenoma in a multiphasic scan demonstrates rapid arterial enhancement and rapid washout on delayed imaging, while comparatively the 2 cystic parathyroid adenomas we encountered demonstrated less arterial phase enhancement and little washout on venous and delayed-phase imaging. As a result, in the absence of a more typical candidate parathyroid adenoma, we now routinely describe enhancing cystic lesions as candidate parathyroid adenomas, particularly if 123I/Tc99m sestamibi subtraction imaging supports the presence of parathyroid tissue.
Abbreviations
- ANT
anterior
- I
iodine
- LAO
left anterior oblique
- PTH
parathyroid hormone
- RAO
right anterior oblique
- SPECT
single-photon emission tomography
- Tc
technetium
Footnotes
Paper previously presented at: 48th Annual Meeting of the American Society of Neuroradiology (Excerpta Extraordinaire), May 15–20, 2010; Boston, Massachusetts.
References
- 1. Fraser WD. Hyperparathyroidism. Lancet 2009; 374: 145– 58 [DOI] [PubMed] [Google Scholar]
- 2. Strewler GJ. A 64-year-old woman with primary hyperparathyroidism. JAMA 2005; 293: 1772– 79 [DOI] [PubMed] [Google Scholar]
- 3. Randall GJ, Zald PB, Cohen JI, et al. Contrast-enhanced MDCT characteristics of parathyroid adenomas. AJR Am J Roentgenol 2009; 193: W139– 43 [DOI] [PubMed] [Google Scholar]
- 4. Rodgers SE, Hunter GJ, Hamberg LM, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006; 140: 932– 41 [DOI] [PubMed] [Google Scholar]
- 5. Mortenson MM, Evans DB, Lee JE, et al. Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography. J Am Coll Surg 2008; 206: 888– 95 [DOI] [PubMed] [Google Scholar]
- 6. McCoy KL, Yim JH, Zuckerbraun BS, et al. Cystic parathyroid lesions: functional and nonfunctional parathyroid cysts. Arch Surg 2009; 144: 52– 56 [DOI] [PubMed] [Google Scholar]
- 7. Armstrong J, Leteurtre E, Proye C. Intraparathyroid cyst: a tumor of branchial origin and a possible pitfall for targeted parathyroid surgery. ANZ J Surg 2003; 73: 1048– 51 [DOI] [PubMed] [Google Scholar]