Abstract
BACKGROUND AND PURPOSE:
An alternative technique, which is less influenced by tumor- and patient-related factors, is required to overcome the limits of GLM analysis of fMRI data in patients. The aim of this study was to statistically assess differences in the identification of language regions and hemispheric lateralization of language function between controls and patients as estimated by both the GLM and a novel combined ICA-GLM procedure.
MATERIALS AND METHODS:
We retrospectively evaluated 42 patients with pathologically confirmed brain gliomas of the left frontal and/or temporoparietal lobes and a control group of 14 age-matched healthy volunteers who underwent BOLD fMRI to lateralize language functions in the cerebral hemispheres. Data were processed by using a classic GLM and ICA-GLM.
RESULTS:
ICA-GLM demonstrated a higher sensitivity in detecting language activation, specifically in the left TPJ of patients. There were no significant differences between the GLM and ICA-GLM in controls; however, statistically significant differences were observed by using ICA-GLM for the LI in patients. For the computation of the LI, ICA-GLM was less influenced by the chosen statistical threshold compared with the GLM.
CONCLUSIONS:
We suggest the use of the ICA-GLM as a valid alternative to the classic GLM method for presurgical mapping in patients with brain tumors and to replicate the present results in a broader sample of patients.
Every year in the United States, approximately 200,000 patients are diagnosed with primary or metastatic brain tumors. Because preventive care is not possible, clinical interventions include correct diagnosis and, in most cases, surgery.1 The target of an effective surgical treatment is tumor removal while preserving the functional integrity of eloquent cortical regions and preventing undesirable postoperative functional deficits.2,3 Presurgical mapping by using BOLD fMRI is now a widely available procedure allowing noninvasive neurosurgical planning.4,5 Mapping language function distribution and identifying the dominant hemisphere are important for preserving the eloquent cortex.6
Despite the utility of fMRI for language mapping in clinical settings, it remains underused. Limits may be related to the technique itself, which indirectly measures cerebrovascular coupling through hemodynamic modifications during task-related activation. fMRI activations are usually obtained by fitting data to a predetermined hemodynamic response curve based on normal subjects according to the classic GLM.7 This assumes a normal hemodynamic response and accurate task performance. Both conditions may not always occur in patients with brain tumor due to a decoupled neurovascular response and hindered task-related performance.8
We propose the use of ICA in conjunction with the GLM to overcome the limits of classic fMRI data analysis and to minimize the risk of type II error (ie, failing to obtain statistically significant activations when effects are genuinely present).9 The methodologic strength of ICA consists of separating spatially independent patterns of synchronized neural activity. This separation occurs without any a priori knowledge and, therefore, does not rely on a predefined hemodynamic response model.10 Thus ICA should be less influenced by tumor-induced modifications of function and anatomy or by patient-related response factors. Because differences in the ICA and GLM can only be assessed at a qualitative level, we adopted a novel combined ICA-GLM approach, which allows a direct quantitative comparison. We independently evaluated differences in the identification of language regions and modifications in hemispheric lateralization of language in patients with brain tumors and healthy controls by using the GLM and ICA-GLM.
Materials and Methods
All participants gave written informed consent, and this study was approved by our local ethics committee. We retrospectively evaluated 42 consecutive patients without aphasia (20 women; age range, 18–72 years; mean age, 46.5 years) with nonoperated left frontal and/or temporoparietal lobe brain gliomas. Patients underwent BOLD fMRI. A control group of 14 age-matched healthy volunteers (6 women; age range, 19–69 years; mean age, 41.2 years) completed an identical fMRI protocol. All subjects had normal hearing and vision and were right-handed as determined by the Edinburgh Handedness Inventory test (laterality quotient of >80).11 Aphasia was evaluated by using the Test of Reception of Grammar.12 Training consisted of performing fMRI protocols during an “off-scanner” overt simulation session.
An expert neuroradiologist (M.C.) used 3D T1-weighted high-resolution anatomic and pre- and postgadolinium images to manually segment and calculate tumor volumes. Brain gliomas were classified as histologically high (WHO III-IV) or low (WHO II) grade, “anterior” or “posterior” with respect to the AC, and “close” or “distant” with respect to language regions (based on a predefined distance of 15 mm from the left IFG or TPJ).13
Subjects silently performed 2 different orthographically cued block-designed lexical retrieval tasks: WGt and VGt. In VGt, five 20-second rest periods were alternated with four 30-second task periods during which subjects thought of pronouncing words beginning with letters presented at the center of the screen. In VGt, four 30-second task periods were alternated with five 20-second rest periods during which subjects thought for 2 seconds of pronouncing ≥1 verb associated with a noun, presented at the center of the screen for 1 second. During rest periods, subjects relaxed while fixating on a central cross. Visual stimuli were presented by using E-Prime, Volume 1.1 (Psychology Software Tools, Sharpburg, Pennsylvania) projected via an LCD projector and mirror.
Data were acquired with a 1.5T Magnetom Vision scanner (Siemens, Erlangen, Germany). BOLD contrast functional images were T2*-weighted echo-planar free induction decay sequences (TR/TE = 2000/60 ms, matrix = 64 × 64, FOV = 256 mm, in-plane voxel = 4 × 4 mm, flip angle = 90°, section thickness = 4 mm). A total of 105 and 115 volumes were acquired, respectively. Structural images were acquired with a sagittal 3D MPRAGE sequence (matrix = 256 × 256, FOV = 256 mm, section thickness = 1 mm, in-plane voxel = 1 × 1 mm, flip angle = 12°, TR/TE = 9.7/4 ms).
Brain Voyager QX 1.9 (Brain Innovation, Maastricht, the Netherlands) was used for data analysis. Time series were corrected for section timing and head motion and linearly detrended to remove slow signal-intensity drifts. Then, they were coregistered to the anatomic image and normalized to Talairach space at a 3-mm spatial resolution by a bounded-box rigid-body transformation14 for group comparisons. Spatial normalization of the structural volumes consisted of manually aligning the 3D MPRAGE dataset of each subject with the stereotaxic axes: AC-PC and 2 rotation parameters for midsagittal alignment. Then the extreme points of the brain (anterior, posterior, right/left lateral, and inferior/superior) were specified. The 8 coordinates were used to scale the 3D datasets to the standard brain of the Talairach and Tournaux atlas14 by using a piecewise affine and continuous transformation.
In the patient group, none of these extreme points contained the tumor. Therefore, the tumor was incorporated in the normalized space without influencing normalization. An expert neuroradiologist (C.B.) verified spatial normalization by calculating the distance between the AC and lateral points of the hemispheres; differences were evaluated by using a paired t test. No spatial smoothing or high-pass filtering was applied. Statistical activation maps were generated according to 2 different analysis methods: GLM and ICA-GLM (Fig 1). The GLM was based on a predictor obtained by the convolution of the boxcar waveform representing task and rest conditions with the Boynton hemodynamic response function implemented in BrainVoyager QX.15 The significance of voxel activation was measured by testing the correspondence between the BOLD time series with the predictor expressed in terms of t-scores.
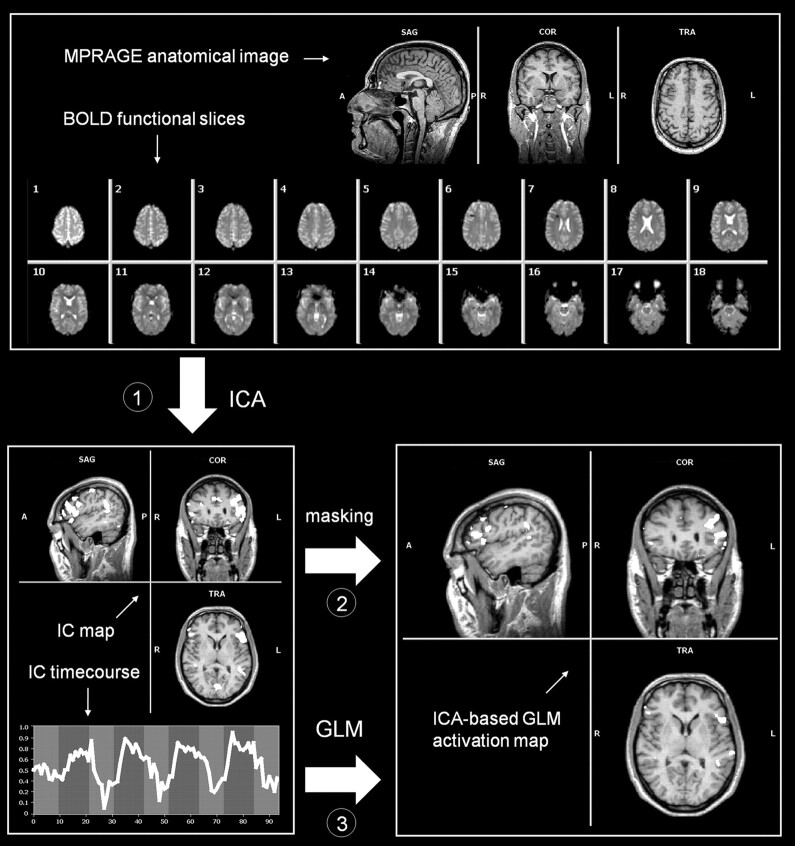
Fig 1.
Outline of the ICA-based GLM analysis. The procedure can be divided into 3 steps: 1) The fMRI time courses are decomposed by means of spatial ICA, and the IC showing the largest correspondence with the predictor is selected; 2) a spatial mask is created by thresholding the IC map, which is applied to the fMRI data; and 3) GLM analysis is performed on the masked fMRI time course as brain response data, by using the IC model.
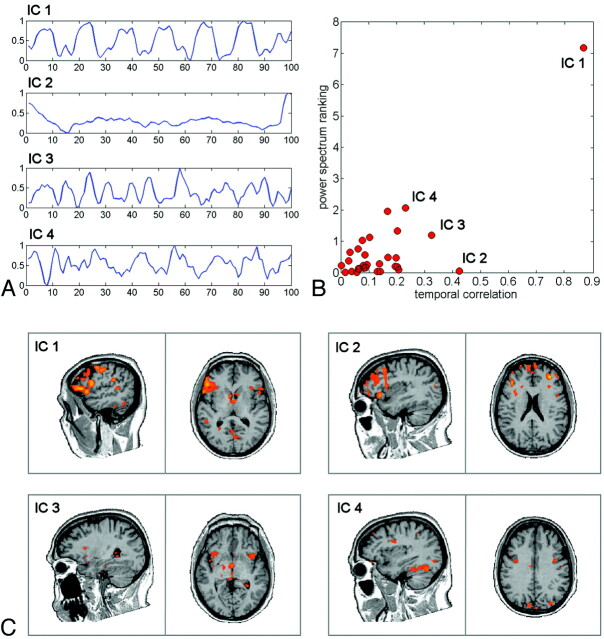
For ICA-GLM, the BOLD time series was decomposed into a set of independent spatiotemporal patterns, specifically ICs,10 by means of the Fast ICA algorithm.16 Each fMRI IC map was scaled to z scores10 and thresholded at z > 1.5 to display IC active voxels.10,17 The ICA decomposition provided ICs with substantially different temporal and spatial profiles (Fig 2 A, -C). After excluding artifactual ICs based on the IC fingerprint method,18 we selected the IC response curve showing the largest correlation coefficient with the predictor. In addition, we evaluated the power spectrum ranking as proposed by Moritz et al,19 to validate our approach and ensure that the same IC could be consistently selected with both methods (Fig 2B). Then we created a mask by using fMRI IC activations and performed the GLM on the masked fMRI data by using the IC time course as a predictor. This ICA-GLM analysis provided a statistical map in t-scores that were directly compared with those obtained with the GLM.
Fig 2.
Results of the ICA decomposition on a patient with a brain tumor. A, Time courses of the first 4 ranked ICs. B, Comparison between the results of power spectrum ranking and temporal correlation on all the separated ICs. C, Spatial maps of the first 4 ranked ICs, thresholded at z > 2.
Statistical analyses were performed by using the Statistical Package for the Social Sciences, Version 17.0 (SPSS, Chicago, Illinois). To test the hypothesis that patients showed different brain responses to experimental tasks with respect to healthy subjects, we analyzed correlation values between the GLM predictor and corresponding activation time course estimated with ICA as a measure of correspondence between an ideal and estimated brain response. Because correlation coefficients may not be normally distributed, they were converted to z scores by using the Fisher r-to-z transformation.20 The statistical significance of correlation coefficients for both groups was evaluated by using the Pearson correlation coefficient with a Bonferroni correction for multiple comparisons (P < .05). The differences in correlation coefficients between the control and patient groups were evaluated by using a 2-tailed t test (P < .05).
The GLM and ICA-GLM statistical maps were compared by testing their sensitivity in detecting significant activation in the right and left Broca (IFG) and Wernicke (TPJ) regions of controls and patients and by analyzing the imaging results of hemispheric lateralization of activated areas.
Two blinded neuroradiolgists (M.C., R.E.), in consensus, verified the presence of significant activations (P < .001; minimum cluster size, 4 voxels) within the IFG and TPJ in each hemisphere of each subject, separately for the GLM and ICA-GLM. The presence/absence of activation in each area was expressed in terms of a yes/no judgment (Table). Differences were assessed with the McNemar nonparametric test (P < .05).
Comparison of the GLM and ICA-GLMa
| Left IFG |
Left TPJ |
Right IFG |
Right TPJ |
|||||
|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | |
| GLM | 40 (95) | 14 (100) | 27 (64) | 13 (93) | 36 (86) | 14 (100) | 13 (31) | 27 (64) |
| ICA-GLM | 42 (100) | 14 (100) | 38 (90) | 13 (93) | 36 (86) | 13 (93) | 13 (31) | 10 (71) |
Number (percentage) of subjects with BOLD activity detected in the 4 classic language regions.
To assess the hemispheric lateralization for language, statistical maps were obtained by computing the number of active voxels in the left (Vl) and right (Vr) hemisphere and calculating the LI = (Vl − Vr) /(Vl + Vr). By definition, the LI ranges between −1 and +1: left lateralized if LI > is +0.20, right lateralized if it is <−0.20, and bilateral (ie, not lateralized) if it is between +0.20 and −0.20.21 Because the LI varies at different statistical thresholds, we calculated the MLI obtained at multiple thresholds between P < .164 and the least stringent P value defined as either LI = ±1 or no activation.22 The MLIs of each subject for the 2 language tasks were used for statistical comparisons. ANOVA was used to test the effect induced by task (WGt, VGt) and group (controls, patients) on MLI obtained by using the GLM and ICA-GLM. To assess the influence of motion artifacts in LI computations with the GLM, we created an additional model in which movement parameters were added as covariates, and we evaluated differences with a paired t test. To test the influence of the selected z score (z = >1.5) in the LI computation with ICA-GLM, we compared LIs obtained by using lower (z = >1) or higher (z = >3) thresholds (paired t test; P < .001).
Next, to examine potential differences of MLI induced by the use of the GLM and ICA-GLM in patient and control groups, we performed a 2-way ANOVA, with task (WGt, VGt) and method (GLM, ICA-GLM) as factors. In the patient group, we further investigated the effects on the LI induced by different thresholds with a 3-way ANOVA with task (WGt, VGt), method (GLM, ICA-GLM), and threshold (13 levels) as factors.
Statistical differences due to tumor grade, position, and relationship to cortical language regions were evaluated by using the Student paired t test. The relationship between the MLI and tumor size was evaluated by using linear regression analysis.
Results
None of the patients or controls were classified as aphasic. Everyone comprehended and performed the language protocols during training sessions.
Gliomas were classified as low in 18/42 and high in 24/42 patients.23 The volumes of gliomas ranged between 739 and 141,000 mm3 (mean, 29,855 ± 30,729 mm3). Twenty-four of 42 tumors were classified as anterior and 18/42, as posterior. Thirteen of 42 tumors were classified close to and 29/42, as distant from cortical language regions.
In no case did the tumor determine either a considerable shift of the hemispheric midline or an anatomic deformation of the AC or PC. No statistical differences were observed in the distances between the AC and lateral points of controls and patients, indicating that tumors did not compromise spatial normalization.
Statistically significant correlations between the GLM predictor and the corresponding activation time course estimated by ICA were obtained for patients and controls with WGt and VGt (P < .001, Bonferroni-corrected). In addition, we observed a statistically significant reduction of average correlations for patients with respect to controls for both WGt (P = .02) and VGt (P = .02).
The GLM detected significant activations of the left IFG in 40/42 and the left TPJ in 27/42 patients; lower frequencies were observed in right homologous regions of patients (IFG, 36/42; TPJ, 13/42). ICA-GLM detected significant activations of the left IFG in 42/42, the left TPJ in 38/42, the right IFG in 36/42, and the right TPJ in 13/42 patients (Table and Fig 3 ). The McNemar nonparametric test revealed a significant difference between methods in left TPJ activation (P < .005), demonstrating a higher sensitivity of ICA-GLM in detecting language activations specifically in this area. No significant differences across methods were observed in language areas in controls.
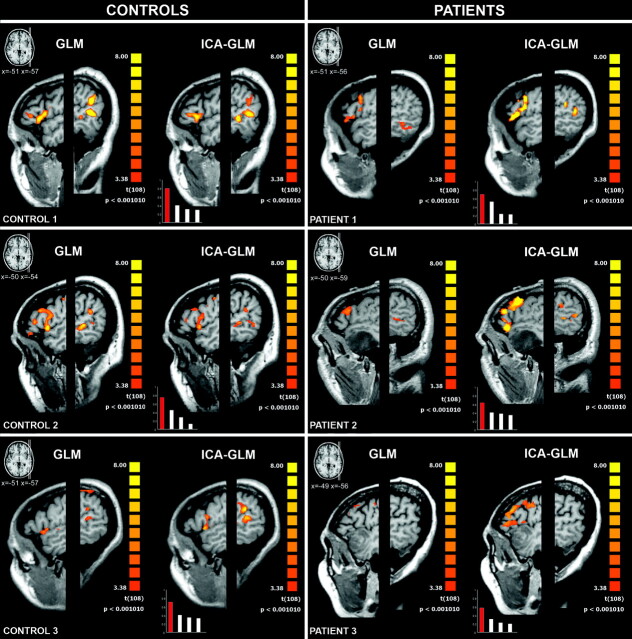
Fig 3.
GLM (left column) and ICA-GLM (right column) activations (P < .001, uncorrected) during VGt in representative controls and patients. The left hemispheres are displayed with different Talairach x-values, to visualize the IFG (Broca area) and TPJ (Wernicke area). The bar charts in the lower left-hand corners show the correlation coefficients of the first 4 ICs and the task predictor; the chosen IC is displayed in red. In controls, both the GLM and ICA-GLM show activation in the IFG and TPJ. In patient 1 (left parietal glioma), the ICA-GLM activates both the IFG and TPJ, whereas the GLM activates only the IFG. In patient 2 (left temporal glioma), the ICA-GLM activates both the IFG and TPJ, whereas no language regions are detected with the GLM. In patient 3 (left temporal glioma), the ICA-GLM activates only the IFG, whereas no language regions are detected with the GLM.
In controls, the GLM in both tasks provided left-lateralized maps in 12/14 subjects, with a mean MLI of 0.50 ± 0.25 for WGt and 0.49 ± 0.22 for VGt (Fig 4). With WGt, 21/42 patients were left-lateralized, 20/42 were nonlateralized, and 1/42 was right-lateralized. With VGt, 24/42 patients were left-lateralized, 16/42 were nonlateralized, and 2/42 were right-lateralized (Fig 4D). On average, patients showed an MLI of 0.23 ± 0.25 and 0.28 ± 0.26 for WGt and VGt, respectively. A 2-way ANOVA with group (control, patients) as the between-factor and task (WGt, VGt) as the within-factor revealed only a main effect for group [F(1,54) = 12.45, P < .001] without a significant interaction between-factors. In general, the GLM showed significantly lower MLI in patients compared with controls in both tasks. This is consistent with the idea of a reorganization of the global amount of language activation toward the nondominant right hemisphere induced by tumors. Results did not change when adding movement parameters as covariates in the GLM, in both controls and patients; this finding suggests that differences of MLI in the 2 groups cannot be attributed to movement alone.
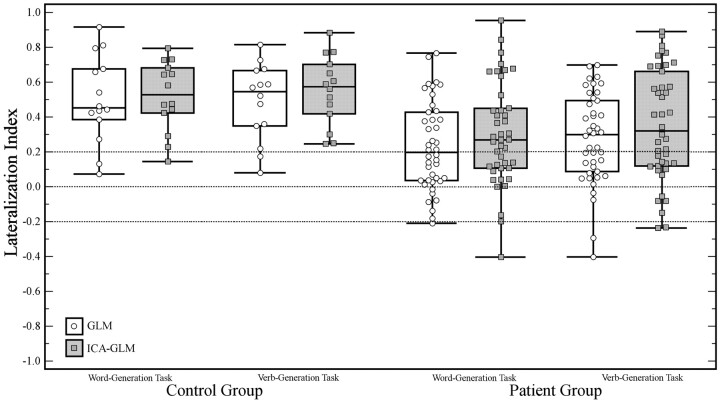
Fig 4.
Comparison of LI values obtained with the classic GLM and ICA-GLM of controls and patients for the following tasks: With WGt, 2 controls presented a bilateral LI (−0.2 < LI < 0.2) with the GLM. The same 2 subjects showed a left dominance with ICA-GLM, while the third showed a bilateral organization with ICA-GLM. With VGt, the same 2 controls with bilateral LI with GLM. Both presented a left dominance with ICA-GLM. With WGt, 20 patients had an LI indicating a bilateral language organization with the GLM: Seven presented left dominance with ICA-GLM and 13 remained bilateral. Three patients showed a left dominance with the GLM but a bilateral dominance with ICA-GLM. Eighteen of the remaining patients had a left dominance with both the GLM and ICA-GLM, and 1, a concordant right dominance. With VGt, 16 patients presented a bilateral LI indicated by the GLM, of which 6 had left and 1 right dominance with ICA-GLM. Four patients showed a left dominance with the GLM but a bilateral dominance with ICA-GLM. One patient presented a right dominance with the GLM but a bilateral dominance with ICA-GLM. Of the remaining patients, 20 had a left dominance with both the GLM and ICA-GLM and 1 patient had a concordant right dominance.
Next, we analyzed MLIs obtained with ICA-GLM. IC maps were thresholded at z > 1.5 because no differences in LIs were observed by using a lower (z > 1; P = .11) or higher (z > 3; P = .57) threshold. In controls, WGt lateralized language to the left hemisphere in 13/14 subjects, while 1 of 14 subjects was nonlateralized (Fig 4). With VGt, all control subjects were left-lateralized. The mean MLI was 0.52 ± 0.20 for WGt and 0.54 ± 0.21 for VGt. In patients, the WGt showed that 25/42 individuals were left-lateralized, 16/42 were nonlateralized, and 1/42 was right-lateralized. With the VGt, 26/42 patients were left-lateralized, 14/42 were nonlateralized, and 2/42 were right-lateralized. The mean MLI was 0.30 ± 0.29 for WGt and 0.36 ± 0.32 for VGt. A 2 (group) × 2 (task) ANOVA revealed only a significant main effect of group [F(1,54) = 7.70, P < .01], indicating a significant reduction of the MLI in patients compared with controls, regardless of task.
A paired samples t tests comparing the effect of tumor location and grading did not yield statistically significant differences in the MLI. In addition, linear regression analysis did not indicate the presence of a statistically significant correlation between tumor size and the MLI in either group for both tasks.
Next, we tested potential differences in the MLI between the GLM and ICA-GLM, separately for patient and control groups. A 2-way repeated-measures ANOVA, with task (WGt, VGt) and method (GLM, ICA-GLM) as factors was performed in each group. In controls, we did not find any significant effects or interactions, suggesting that the 2 methods did not provide significantly different results in assessing MLI. However, when the same analysis was performed in the patient group, a significant reliable effect of method was found [F(1,41) = 9.59, P < .005], with no interaction with task. These results suggest that in patients with brain tumors, the ICA-GLM approach provided MLIs more lateralized to the dominant hemisphere, regardless of the language task used.
We further investigated differences across methods in the patient group by evaluating the variation of LIs at different statistical thresholds. MLI represents the median of the LI scores obtained at different thresholds, a procedure that minimizes the effect of the arbitrary choice of a particular threshold. When one refers to patient studies, this is particularly crucial due to clinical implications. However, it is also possible to track the evolution of the LI from very lenient uncorrected to very strict thresholds, especially because patients are usually studied individually.
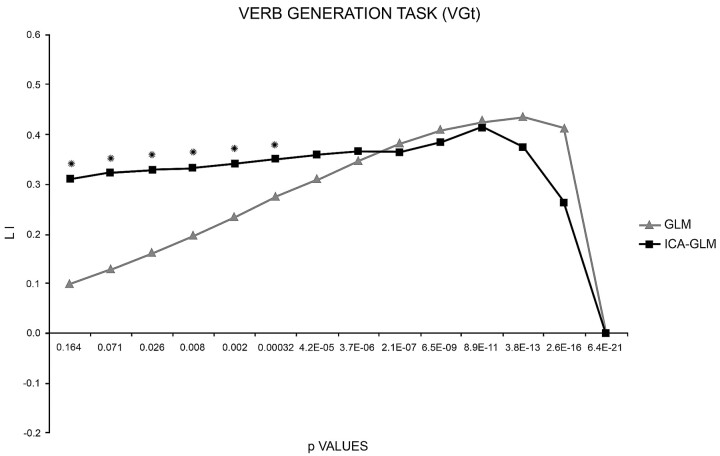
We performed a 3-way ANOVA on the patient group with task (WGt, VGt), method (GLM, ICA-GLM), and threshold (13 levels) as factors and found a significant main effect for method [F(1,41) = 4.20, P < .05] and threshold [F(12,492) = 13.22, P < .0001]. These 2 factors interacted [F(12,492) = 2.88, P < .001], indicating a stronger dependence of the GLM, compared with ICA-GLM, on the statistical threshold. However, the results also showed a significant 3-way interaction, implying that this pattern differed across tasks. Therefore, we analyzed the relationship between the LI and threshold separately for each task, by using a 2-way ANOVA (method, threshold). A significant interaction between method and threshold [F(12,492) = 5.55, P < .0001] was found only for VGt (Fig 5). When restricting the analysis to ICA-GLM, we found no main effect of threshold, consistent with the idea that this method was independent of threshold.
Fig 5.
LI curves of patients performing the VGt analyzed with the 2 methods (GLM and ICA-GLM). With the GLM, the LI curve starts near low values and increases as the threshold rises toward stricter values. With ICA-GLM, the LI curve starts from higher values and remains constant toward stricter values. Both curves eventually drop to zero when no activated voxels resist. Asterisks indicate the threshold values at which the 2 methods are statistically different.
Discussion
Although invasive and spatially limited, intraoperative electrocortical stimulation continues to be the reference method for surgical brain mapping. Similarly, the Wada test remains the criterion standard for determining hemispheric dominance.24,25 Several attempts have been made to replace these with less invasive modalities. Thus fMRI is now considered a valid and reliable alternative for brain mapping and language lateralization.26,27
Although fMRI is an appropriate technique for detecting functional reorganization induced by the presence of a brain tumor, its clinical use can be affected by biases.28,29 Classic fMRI analysis (GLM) measures cortical activity by fitting data to a predetermined normal hemodynamic response curve.7 This normal condition does not necessarily occur in the presence of overt neuropathology such as glioma. Patients may differ from controls in the way they execute tasks. Brain tumors, growing in a rigid structure, cause a buildup of intracranial pressure, altering the normal hemodynamic process of hemoglobin oxygenation in surrounding veins.30,31 Brain tumors may influence BOLD effect by releasing vasoactive substances and/or neurotransmitters.32 Disease-induced pathology and/or medications may alter psychological factors such as cognition and attention; this change compromises normal execution of tasks and subsequently renders rigid data analysis ineffective.
To evaluate the effects of the above-mentioned limitations, we compared 2 different methods of fMRI data analysis in this study, a classic hypothesis-driven method (GLM) and an alternative combined method, a novel ICA-GLM–based approach.9,33
ICA was previously used in fMRI studies of language to map transient randomly occurring neuropsychological events without an a priori knowledge of the paradigm.34,35
Our ICA-GLM approach called for selecting the IC that presented the largest temporal correlation coefficient with the language task predictor and which, consistent with a recent study,36 usually revealed the contemporary activation of the left IFG and TPJ (Table). The fact that a single IC visualized both the anterior and posterior regions for speech is also consistent with studies on resting-state functional-connectivity MR imaging, which reported that these regions were functionally connected.37
We observed significantly lower correlation values between ideal and observed activation time courses in patients, implying that brain responses in patients with glioma are less predictable. However, on the basis of observed results, we cannot determine the extent to which these differences reflect a modification in cerebral hemodynamic response or an intrinsic difficulty experienced by patients in performing tasks. We intentionally included only patients without aphasia capable of performing language tasks adequately, to limit the influence of task performance. The most likely explanation was that both factors influenced the GLM and ICA-GLM results. Despite the causal mechanism, an analysis referring to an ICA estimate of task response would be less influenced by unpredictable modifications of the BOLD signal intensity−time course.
Analysis of sensitivity revealed that ICA-GLM performed better in detecting language-related BOLD activity of only the left TPJ. With the GLM, activation of this region was only observed in 27/42 patients. This confirmed the greater difficulty of the GLM in detecting BOLD activity in the posterior compared with anterior areas and the superior performance of the hybrid approach. The fact that a significant difference was observed only in patients and not in controls suggested that ICA-GLM was less influenced by pathologic modifications of cortical and behavioral responses. Therefore, it is particularly suited for clinical populations (Fig 3).
The use of ICA in ICA-GLM may reduce an artifactual contribution because the selected ICA maps are related to brain activity only.33 The selective activation of language-related voxels also accounts for the significantly lower dependence of ICA-GLM on statistical thresholds used for determining the LI. This property has a profound clinical value by permitting the rapid and reproducible identification of the dominant hemisphere in patients (Fig 5). Accordingly, differences obtained between ICA-GLM and the GLM, in particular in terms of MLI, could be related to better quality of the ICA-GLM maps. The differences obtained were not particularly evident at the single dataset level. In this regard, the MLI analysis quantified the improvement achieved by ICA-GLM.
Nonetheless, the differences in MLI obtained with the GLM and ICA-GLM were not statistically significant in the control group but were in the patient group. This indicated a greater flexibility of ICA-GLM, and, consequently, a greater effectiveness in detecting task-related activations when real brain response did not match the ideal one. These results are in agreement with a previous study conducted by Quigley et al,38 who qualitatively compared ICA and the GLM and recommended the use of ICA when patients either incorrectly performed the task or moved during data acquisition.
To calculate the LIs, we entered all active voxels of each hemisphere, thus bypassing difficulties that would have been encountered positioning predetermined regions of interest in patients with modified/obliterated anatomic structures or landmarks induced by tumors.30 Therefore, we determined hemispheric dominance for language on the basis of interhemispheric differences of activated voxels at variable statistical thresholds.22
Language was definitively lateralized to the left/dominant hemisphere as opposed to a decreased number of patients with nonlateralized language (Fig 4). We interpreted this shift from nonlateralized to left-lateralized as secondary to recruitment of BOLD signal intensity in the affected hemisphere, which resulted from the use of ICA-GLM (attributable to a decrease in type II errors).9
As a counterpart to a decrease in type II errors, the use of the ICA-GLM method may affect specificity if the ICA-based template deviates significantly from the GLM predictor. In this condition, the activation maps may include regions not related to language functions. To control for the risk of incurring in a type II error, we verified that the first IC and the task predictor were always significantly correlated and that in those patients having the lowest correlation, the chosen IC was still able to detect activation of the classic regions for speech. However, we affirm the need for a visual inspection of ICA-based templates, especially in patients with low correlation coefficients, to verify the overall correspondence between the template and the classic language network topography.
Tumor size, location, and grade did not influence lateralization. This might be related to he following: 1) Low- and high-grade gliomas are equally slow-growing lesions compared with the time required for brain plasticity; 2) tumor size mainly influences the type of intrahemispheric functional reorganization (local/distant to classic language regions) but not interhemispheric redistribution of functions30; and 3) we can also hypothesize that tumor size did not influence lateralization because patients with aphasia (perhaps reflecting larger tumors) were excluded from this study. Furthermore, any effect related to the type of task was excluded. Differences in language lateralization were only due to the data analysis method. However, in the patient group, the comparison of the GLM and ICA-GLM yielded significant differences only with VGt and not with WGt. This result is in agreement with a previous study in which tasks requiring semantic processing, such as VGt, were considered the most reliable predictors of laterality.39
Conclusions
We propose ICA-GLM as a valid alternative to the classic GLM method for mapping language regions and assessing language lateralization in patients with tumors, on the basis of 2 main findings: First, ICA-GLM was more sensitive in detecting BOLD activity in eloquent areas for language. Second, ICA-GLM provided greater LIs toward the dominant hemisphere and more consistent results across different statistical thresholds. The latter point is of extreme importance because a single subject analysis is required for mapping protocols in patients. Future studies on various clinical populations will be needed to test whether ICA-GLM should always be applied in clinical studies.
Abbreviations
- AC
anterior commissure
- ANOVA
analysis of variance
- BOLD
blood oxygen level–dependent
- fMRI
functional MR imaging
- GLM
general linear model
- IC
independent component
- ICA
independent component analysis
- IFG
inferior frontal gyrus
- LI
lateralization index
- MLI
median of the lateralization index
- MPRAGE
magnetization-prepared rapid acquisition of gradient echo
- PC
posterior commissure
- TPJ
temporoparietal junction
- VGt
verb-generation task
- WGt
word-generation task
- WHO
World Health Organization
References
- 1. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 2008; 26: 1338– 45 [DOI] [PubMed] [Google Scholar]
- 2. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008; 62: 753– 64 [DOI] [PubMed] [Google Scholar]
- 3. Mayer L. Outcome after language mapping for glioma resection. N Engl J Med 2008; 358: 1750– 51 [DOI] [PubMed] [Google Scholar]
- 4. Mulderink TA, Schaeffer AC, Meyer JR, et al. Simultaneous assessment of motor and language areas with a single functional MR imaging paradigm: feasibility. Radiology 2005; 236: 655– 60 [DOI] [PubMed] [Google Scholar]
- 5. Abou-Khalil B. Methods for determination of language dominance: the Wada test and proposed non-invasive alternatives. Curr Neurol Neurosci Rep 2007; 7: 483– 90 [DOI] [PubMed] [Google Scholar]
- 6. Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 2005; 4: 476– 86 [DOI] [PubMed] [Google Scholar]
- 7. Friston KJ, Frith CD, Frackowiak RS, et al. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 1995; 2: 166– 72 [DOI] [PubMed] [Google Scholar]
- 8. Holodny AI, Schulder M, Liu WC, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000; 21: 1415– 22 [PMC free article] [PubMed] [Google Scholar]
- 9. Tie Y, Whalen S, Suarez RO, et al. Group independent component analysis of language fMRI from word generation tasks. Neuroimage 2008; 42: 1214– 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 2008; 6: 160– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97– 113 [DOI] [PubMed] [Google Scholar]
- 12. Bishop A. Test of Reception of Grammar. Cambridge, United Kingdom: Cambridge University Press; 1983 [Google Scholar]
- 13. Håberg A, Kvistad KA, Unsgård G, et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery 2004; 54: 902– 14 [DOI] [PubMed] [Google Scholar]
- 14. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988 [Google Scholar]
- 15. Boynton GM, Engel SA, Glover GH, et al. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 1996; 16: 4207– 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 1999; 10: 626– 34 [DOI] [PubMed] [Google Scholar]
- 17. Mantini D, Perrucci MG, Del Gratta C, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 2007; 104: 13170– 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Martino F, Gentile F, Esposito F, et al. Classification of fMRI independent components using IC-fingerprints and support vector machine classifiers. Neuroimage 2007; 34: 177– 94 [DOI] [PubMed] [Google Scholar]
- 19. Moritz CH, Rogers BP, Meyerand ME. Power spectrum ranked independent component analysis of a periodic fMRI complex motor paradigm. Hum Brain Mapp 2003; 18: 111– 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zar JH. Biostatistical Analysis. Upper Saddle River, New Jersey: Prentice-Hall; 1999 [Google Scholar]
- 21. Seghier M. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 2008; 26: 594– 601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruff IM, Petrovich Brennan NM, Peck KK, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol 2008; 29: 528– 35. Epub 2008 Jan 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97– 109. Epub 2007 Jul 6. Erratum in: Acta Neuropathol 2007;114:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wada J, Rasmussen T. Intracarotid injection of sodium amytal for lateralization of cerebral speech dominance: 1960. J Neurosurg 2007; 106: 1117– 33 [DOI] [PubMed] [Google Scholar]
- 25. Wada JA. A fateful encounter: sixty years later—reflections on the Wada test. Epilepsia 2008; 49: 715– 20, discussion 720–25 [DOI] [PubMed] [Google Scholar]
- 26. Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery 2003; 52: 1335– 45 [DOI] [PubMed] [Google Scholar]
- 27. Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 2003; 61: 699– 701 [DOI] [PubMed] [Google Scholar]
- 28. Bizzi A, Blasi V, Falini A, et al. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping. Radiology 2008; 248: 579– 89 [DOI] [PubMed] [Google Scholar]
- 29. Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci 2008; 265: 97– 101 [DOI] [PubMed] [Google Scholar]
- 30. Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 2007; 130: 898– 914 [DOI] [PubMed] [Google Scholar]
- 31. Stippich C, Rapps N, Dreyhaupt J, et al. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology 2007; 243: 828– 36 [DOI] [PubMed] [Google Scholar]
- 32. Bonnetblanc F, Desmurget M, Duffau H. Low grade gliomas and cerebral plasticity: fundamental and clinical implications [in French]. Med Sci (Paris) 2006; 22: 389– 94 [DOI] [PubMed] [Google Scholar]
- 33. Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006; 27: 392– 401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu H, Engelien W, Feng H, et al. Mapping transient, randomly occurring neuropsychological events using independent component analysis. Neuroimage 2001; 14: 1432– 43 [DOI] [PubMed] [Google Scholar]
- 35. Wu X, Lu J, Chen K, et al. Multiple neural networks supporting a semantic task: an fMRI study using independent component analysis. Neuroimage 2009; 45: 1347– 58 [DOI] [PubMed] [Google Scholar]
- 36. Karunanayaka P, Schmithorst VJ, Vannest J, et al. A group independent component analysis of covert verb generation in children: a functional magnetic resonance imaging study. Neuroimage 2010; 51: 472– 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol 2000; 21: 1636– 44 [PMC free article] [PubMed] [Google Scholar]
- 38. Quigley MA, Haughton VM, Carew J, et al. Comparison of independent component analysis and conventional hypothesis-driven analysis for clinical functional MR image processing. AJNR Am J Neuroradiol 2002; 23: 49– 58 [PMC free article] [PubMed] [Google Scholar]
- 39. Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 1999; 52: 798– 809 [DOI] [PubMed] [Google Scholar]