Abstract
BACKGROUND AND PURPOSE:
Onyx was developed for embolization of central nervous system AVMs but is increasingly used extracranially because of its unique physical properties. We review our experience and results with the use of Onyx for the treatment of fast-flow extracranial vascular lesions.
MATERIALS AND METHODS:
We retrospectively analyzed clinical and imaging records of 22 patients who underwent 71 extracranial embolizations from March 2007 through January 2010. The diagnoses were the following: cervicofacial AVM (n = 18), traumatic fistula (n = 3), and vessel laceration (n = 1). In 62 of 71 procedures (87%), Onyx was the sole embolic agent; it was delivered transarterially in 67/71 and percutaneously in 4/71 procedures. Clinical goals included amelioration of pain and control of bleeding. The clinical efficacy of embolization was judged by symptom control, and adverse events were assessed by clinical examination and history, both postembolization and 4 weeks postprocedure.
RESULTS:
Cessation of acute bleeding was achieved in 13/14 cases, with 1 case of immediate recurrent massive epistaxis prompting reintubation and further embolization. Control of subacute bleeding episodes and pain was achieved for all patients. Following staged embolization, 7 patients underwent surgical resection without significant blood loss. Surgeons reported high satisfaction with the intraoperative handling properties of Onyx. Transient swelling, local tenderness, or numbness was encountered after 7 procedures. There were no stuck catheters, vessel dissections, or vessel ruptures and no skin discoloration.
CONCLUSIONS:
Staged Onyx embolization was clinically efficacious in managing extracranial fast-flow vascular malformations and lesions, with low associated morbidity.
Ethylene-vinyl alcohol copolymer, dissolved in the organic solvent DMSO and mixed with micronized tantalum powder to provide fluoroscopic visualization (Onyx; ev3, Irvine, California), is a liquid embolic agent that was granted Humanitarian Use Device approval of the FDA for the presurgical treatment of central nervous system arteriovenous malformations in 2005. Its application in the endovascular treatment of intracranial AVMs was first described by Taki et al1 and Terada et al2 in the early 1990s, followed by a larger series reported by Weber et al,3 Mounayer et al,4 Katsaridis et al,5 and Pierot et al.6 Different formulations of Onyx are available, with a lower concentration of the copolymer resulting in increased fluidity and more distal lesional penetration and a higher concentration resulting in a more viscous liquid.3 Onyx potentially combines the positive aspects of other embolic agents, allowing distal penetration, like PVA particles, and providing permanent occlusion similar to that in n-BCA. Because of its unique physical properties, Onyx has gained increasing popularity for use in managing extracranial vascular anomalies in recent years.7–9
The aim of this retrospective study was to present the clinical efficacy and safety of Onyx when used for embolization of various extracranial arteriovenous and arterial lesions, either as the sole embolic agent or in combination with other nonabsorbable particulates and liquids or coils. Most of these extracranial vascular lesions were challenging because of their complexity, requiring multidisciplinary treatment. We report here the largest series to date of patients (n = 22) with extracranial fast-flow vascular anomalies who underwent Onyx embolization.
Materials and Methods
With permission of the institutional review boards of Children's Hospital and Brigham and Women's Hospital, we retrospectively analyzed the clinical and imaging records of 22 patients who underwent 71 embolizations for extracranial vascular anomalies of the head and neck, from March 2007 through January 2010. Indications for embolization included acute hemorrhage (cases 1, 9, 11, 16–18, and 21; n = 14 sessions); recurrent episodic hemorrhage or oozing (cases 2, 5, 8, 9, 11, 12, 15, 16, and 21; n = 35 embolizations); discomfort or pain (cases 3, 4, 6, 7, 10, 13, 14, 19, 20, and 22; n = 22 sessions), either in isolation or related to progressive tissue enlargement; impending necrosis secondary to ischemia or chronic infection; or functional impairment.
The embolization procedures were similar to our protocol for central nervous system AVMs.10 All sessions were performed in a biplane angiographic unit with patients under general anesthesia. After obtaining informed consent, we administered an antibiotic and corticosteroid immediately before the procedure to prevent secondary infection and minimize soft-tissue swelling. Vascular access was obtained through the common femoral artery, and a 5F vascular sheath was introduced. Other than in cases of acute hemorrhage, the patient was heparinized. The sheath, guiding catheter, and microcatheter were continuously flushed via a pressure bag with heparinized saline.
Diagnostic angiography was performed with a 5F Envoy catheter (Cordis, Miami Lakes, Florida), positioned selectively in the internal, external, and vertebral arteries. Subsequently, a Marathon or UltraFlow microcatheter (ev3) was coaxially advanced over a microwire to the nidus or fistulous point. After the microcatheter was flushed with saline, the microcatheter dead space was filled with DMSO. Under road-mapping, we injected Onyx by using the “plug and push” technique.5 This allowed the formation of a short cast of Onyx 34 around the microcatheter tip in the initial injection, with only a small amount of reflux. Once an adequate plug was formed, Onyx 18 was substituted for Onyx 34 and injected in a controlled fashion to form a lavalike mass, which followed the path of least resistance into the nidal compartments.
For patients with acute severe hemorrhage, embolization continued until the hemorrhage stopped and did not resume after removal of any packing that had been placed for tamponade. Embolization continued until the AVM compartment related to the hemorrhage was thoroughly treated—that is, when control angiography revealed neither further opacification of nidal vessels nor arteriovenous shunting within the target compartment. Given that almost all the lesions were large and multicompartmental, treatment in cases in which the presenting symptom was episodic moderate hemorrhage or pain was staged. As many compartments of the AVM were targeted, before embolization, as could be treated while keeping the total calculated skin entrance dose at ≤1 Gy. When embolization was deemed sufficient, the microcatheter was pulled, and control angiography was performed. All subsequent staged treatments after the first began with angiographic assessment of the interval stability of the previously embolized target. If a small amount of asymptomatic residual AVM was seen in a location particularly vulnerable to loss of normal soft tissue (ear, tip of the nose), further treatment was deferred. Following staged embolization, 7 patients underwent surgical resection.
For reduction of flow in direct AVFs, coils were used, followed by Onyx embolization. In cases with intended coil deployment, an Echelon microcatheter (ev3) was used instead of a Marathon or UltraFlow microcatheter. In immediate preoperative embolizations, PVA particles were used with or without Onyx.
For superficial lesions, in which access to the nidus could not be obtained by transarterial means, Onyx was injected percutaneously. In these cases, transarterial angiography was obtained, as described above. A compartment of the AVM was then cannulated under fluoroscopic guidance by using a 20-ga intravenous catheter/needle system (Angiocath; Becton Dickinson, Sandy, Utah), which was secured with Steri-Strips (3M Nexcare, St. Paul, Minnesota) and attached to continuous flush via a rotating hemostatic valve and extension tubing. Intralesional positioning of the angiocatheter was typically apparent due to vigorous arterial flashback but was documented by means of injection of a small amount (1–2 ml) of dilute contrast material, saved as a roadmap image. A Marathon microcatheter over an X-pedion microwire (ev3) was then advanced through the angiocatheter into the lesion and navigated to an optimal target location, flushed with DMSO, and then used to embolize the AVM with Onyx. The presence of the microcatheter allowed both optimal targeting of the injection site and the operator to stand at some distance from the fluoroscopy tube during potentially prolonged injections. Further description of the embolization technique, with illustrative examples, is described elsewhere.11
After embolization, the patient was allowed to wake up, extubated, and monitored for local pain, swelling, or any deficits overnight. All patients had clinical follow-up by phone within 2 weeks of the embolization and were seen in clinic for a 4-week follow-up visit. If no further embolization was planned, they were seen for follow-up again at 6 months after the last procedure.
Results
Among the 22 patients, there were 9 females (mean age, 21.2 years; median, 15 years; range, 6–43 years) and 13 males (mean age, 28.0 years; median, 26 years; range, 11–65 years), as shown in the On-line Table. Eighteen patients had AVMs: maxillofacial (n = 8), mandibular (n = 5), nasal (n = 3), and scalp (n = 2).
A subset of 5 patients presented with extensive cervicofacial AVMs (cases 8, 10, 11, 12, and 16 in the On-line Table: 3 males and 2 females with a mean age of 21.8 years and a median age of 22 years). All 5 patients had undergone previous embolizations at another institution; 3 also had partial resections. Onyx was used as the endovascular embolization agent in all sessions, with the exception of 1 patient who was treated during her first stage at our institution, before we had ever delivered Onyx percutaneously, with transarterial and percutaneous n-BCA on an emergency basis for massive scalp hemorrhage (case 8); Onyx was used alone in the subsequent 4 staged embolizations. In another patient with an extensive right facial AVM (case 16), Onyx alone was used for the first 6 staged embolizations, while PVA particles were used in the last session for preoperative embolization. In all 5 patients, elimination of episodic bleeding and pain was achieved after a median of 6 staged Onyx embolizations (range, 3–9).
Three patients had posttraumatic AVFs (cases 6, 18, and 19 in the On-line Table), and 1 patient had a facial artery laceration (case 17). Complete embolization, with no angiographic residual and total elimination of symptoms, was achieved in those 4 patients in a single stage of embolization, by using Onyx alone in 2 and Onyx after deployment of detachable coils in 1 patient with an AVF of the right STA. One patient (case 2) with a small premaxillary and upper lip AVM was likewise treated in 1 session alone, with no angiographic residual.
Embolization was nearly complete in 8 patients with AVMs (cases 5, 8, 9, 10, 11, 12, 16, and 21) with significantly reduced arteriovenous shunting on control angiography. The median number of embolizations required to achieve near-complete embolization was 6, ranging from 3 to 9. Five of these patients (cases 5, 8, 11, 12, and 16) underwent surgical resection with reported minor intraoperative blood loss and no need for transfusions. The surgeons also noted satisfaction with the intraoperative visualization and handling properties of Onyx, as well as with the increased visual contrast lent to the AVM by the black color of the Onyx.
Embolization was clinically well-tolerated by all patients, without evidence of systemic toxicity. Transient local swelling, tenderness, or numbness following Onyx embolization of facial AVMs were encountered following 7 procedures in 5 patients (cases 5, 9, 11, 16, and 21). Corticosteroid was administered for several days to 1 patient for pain and swelling and was tapered after resolution of symptoms.
Recurrent massive epistaxis occurred in 1 patient with a very extensive nasal, maxillary, and orbital AVM (case 11, Fig 1) immediately after embolization, prompting rapid reintubation and re-embolization. Recurrent mild epistaxis after staged embolization was encountered in 4 patients (cases 9, 11, 15, and 21) and spotting from the gums, in 1 patient (case 5), albeit with markedly reduced frequency and volume and a marked decrement in severity of pain.
Fig 1.
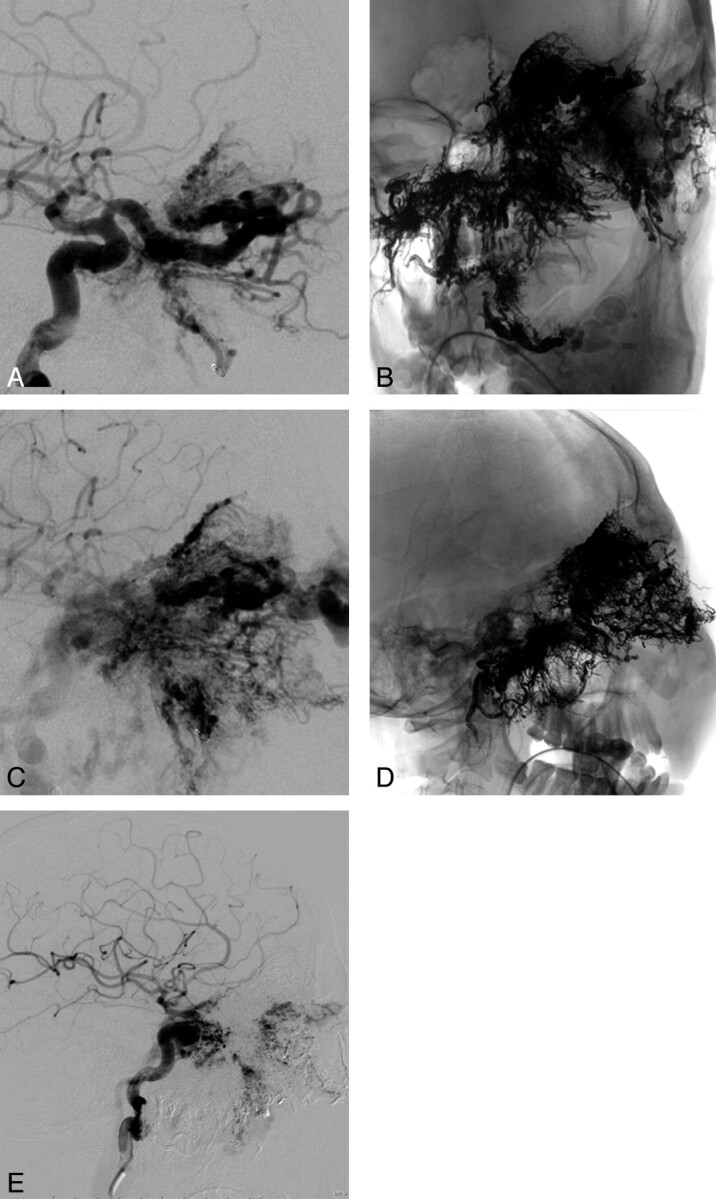
A 22-year-old man (case 11) with a giant left craniofacial AVM, which was initially diagnosed at 3 weeks of age with gum line overgrowth and recurrent epistaxis. The symptoms were controlled with embolizations and partial maxillectomy. The lesion and accompanying epistaxis progressed more aggressively in his late teens. Embolization was performed in 9 stages, via the right ascending pharyngeal artery, the left ophthalmic artery, and the right internal maxillary artery, with markedly decreased nidus and no further epistaxis. Lateral views in early arterial (A) and late arterial (C) phases as well as unsubtracted frontal (B) and lateral (D) views of the Onyx cast and a lateral view of the postembolization appearance in the midarterial phase (E) are shown.
There were no cases of stretched, sheared, or retained catheters or fragments. There were no vessel dissections or ruptures. There were no cases of skin discoloration discernible on clinical examination and none reported by a patient or family member.
During treatment of 1 patient with an extensive left nasal/cheek/periorbital AVM and recurrent episodes of epistaxis, there was stretching of a coil that had been deployed at the site of a fistulous component of the lesion, in preparation for injection of Onyx (case 21). The coil fractured in the main feeder, the infraorbital branch of the left internal maxillary artery, and made further embolization via this route, including injection of Onyx, impossible. The patient was subsequently embolized via other left internal maxillary branches, via branches of the right internal maxillary artery, and via percutaneous cannulation of the AVM.
Discussion
Extensive craniofacial AVMs are especially difficult to manage because of disfigurement, functional impairment, and risk of life-threatening hemorrhage.12–15 It is often impossible to achieve a satisfactory therapeutic result in a single treatment session. Management is often complicated by prior failed treatment attempts, most frequently termination of attempted resection in the past because of uncontrollable intraoperative hemorrhage.16 Because of the rarity of the craniofacial AVMs and the relative lack of understanding of their pathogenesis,15 no standard treatment has been established. As a result, various interventional options and treatment algorithms have been proposed.13 Although there have been reports of embolization therapy alone achieving successful results in selected cases with smaller craniofacial AVMs, the outcome is usually palliation rather than “cure” for large lesions.17,18 Some authors have proposed a management algorithm for large craniofacial AVMs, chiefly involving preoperative embolization with n-BCA and PVA several days before resection,18 resulting in control of large craniofacial AVMs with acceptable esthetic results.
Arat et al8 reported the largest series to date by using Onyx as a primary or preoperative embolization agent. There were 9 patients in their series: 3 with craniofacial AVMs and 6 with AVFs. They were successful in treating all AVFs by using Onyx as the primary embolization agent; however, the outcome was less favorable for the 3 patients with AVMs.
One reported major difficulty encountered was reflux of Onyx over the microcatheter,8 also reported as a significant technical problem for embolization of intracranial AVMs. Onyx reflux results in difficult retrieval of the microcatheter, potentially resulting in vessel dissection or rupture, or shearing of the distal microcatheter segment. In our experience, the difficulty with proximal reflux of Onyx could be ameliorated by using the plug and push injection method. The Onyx plug formed in the initial injection not only acted as a physical barrier to prevent any significant proximal reflux during the subsequent injections but also promoted distal nidal penetration by increased proximal resistance in the feeding artery.
We achieved complete embolization in only 4 patients, with AVFs (n = 2), facial artery laceration (n = 1), and a maxillary AVM (n = 1), with a single stage of Onyx embolization because of a well-defined fistulous point or nidus isolated to 1 single compartment of the head and neck.
In contrast, because of their complexity, craniofacial AVMs require multiple staged embolizations to reduce the risk of excessive exposure to radiation, intravenous contrast, and Onyx, in 1 single session, with the number of stages required depending on the age of the patient and the size and complexity of the lesion. We targeted the most symptomatic region initially and, in almost all instances, waited at least 4 weeks between each session to allow the DMSO to clear. We typically limit the contrast dose to 4 mL/kg body weight in elective cases (≤7 ml/kg for urgent cases), radiation exposure is limited to 1 Gy per session for elective cases, and maximum Onyx dosage is, as recommended by the manufacturer, ∼1 mL per 10 pounds (4.54 kg) of body weight.
Proximal embolization of feeding arteries of any AVM is contraindicated because it promotes recruitment of nearby arteries to supply the lesion and limits any future endovascular access to the nidus.19 It is important to carefully position the microcatheter tip distally to avoid proximal arterial ligation in multistaged embolizations.
Given its dark color, superficial Onyx embolization, and especially subcutaneous extravasation, can cause blackish discoloration of the skin.8 We did not encounter cutaneous necrosis or marked discoloration in any patient. When Onyx is used preoperatively to reduce blood loss, the surgeon should be aware that sparks may result if the material comes in contact with monopolar electrocautery. We have not found this to be problematic, and our surgical colleagues continue to use electrocautery during resection in Onyx cases.
The results of using Onyx for embolization of craniofacial AVMs in a multistaged fashion are promising. Immediate postembolization angiography showed successful reduction in the nidal size. Subsequent angiography demonstrated that areas embolized in the previous stage remained occluded, without recanalization or re-expansion of the lesion.
While some authors have suggested the best therapeutic result is achieved for treatment of a craniofacial AVM by combined preoperative embolization with surgery, a “total cure” for an extensive craniofacial AVM may not be achievable because of the extensive area covered and viable structures involved.14 Instead, in patients in whom a complete cure is not possible, the therapeutic goal should be tailored to the patient's expectations, which may emphasize the best cosmetic results achievable and effective long-term symptomatic control. Multistaged embolization can also serve as presurgical devascularization, whether or not the ultimate goal of resection was a total cure.
Gomes17 described alternate approaches to malformations in which transarterial access is limited due to prior surgical ligation or embolization or in fast-flow lesions with a large venous component.17,20 We used percutaneous cannulation and embolization with Onyx in 4 procedures, with excellent results in all 4 cases, and no evidence of skin discoloration.
We did not encounter nontarget embolization or pulmonary complications following Onyx embolization. In fast-flow shunts, we used coils to form the initial meshwork through which the column of Onyx advanced, thereby lowering the risk of inadvertent venous migration (cases 6, 12, and 20). Local reactions to Onyx, in the form of swelling, local tenderness, or numbness, followed 7 procedures, requiring corticosteroid therapy in 1 patient; all completely resolved within days. As mentioned previously, stretching of a coil occurred in 1 patient; Niemann et al21 reported coil stretching in 0.7% of coil embolizations.
The recurrence rate of AVM symptoms and signs after Onyx embolization is unknown. A recent report by Liu et al22 documents near-universal (98%) recurrence in patients with extracranial AVMs after embolization with agents other than Onyx (with an 81% recurrence rate after resection). Whether Onyx embolization will result in a lower recurrence rate remains to be determined with intermediate and long-term follow-up studies.
In summary, our experience by using Onyx for extracranial vascular anomalies shows promising results, particularly for extensive complex craniofacial AVMs. Staged embolizations with Onyx were safe and clinically effective. The permanence of the results in this cohort remains to be determined.
Conclusions
With knowledge of the angioarchitectural characteristics of extracranial vascular anomalies that are suitable for the treatment with Onyx, symptom control and few complications are possible with a multistaged approach.
Supplementary Material
Abbreviations
- AVF
arteriovenous fistula
- AVM
arteriovenous malformation
- DMSO
dimethyl-sulfoxide
- FDA
US Food and Drug Administration
- LECA
left external carotid artery
- n-BCA
n-butyl cyanoacrylate
- PTEN
phosphatase and tensin homolog
- PVA
polyvinyl alcohol
- STA
superficial temporal artery
Footnotes
Indicates article with supplemental on-line table.
References
- 1. Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol 1990; 11: 163– 68 [PMC free article] [PubMed] [Google Scholar]
- 2. Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer: report of three cases. J Neurosurg 1991; 75: 655– 60 [DOI] [PubMed] [Google Scholar]
- 3. Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol 2007; 28: 371– 77 [PMC free article] [PubMed] [Google Scholar]
- 4. Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol 2007; 28: 518– 23 [PMC free article] [PubMed] [Google Scholar]
- 5. Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology 2008; 50: 589– 97 [DOI] [PubMed] [Google Scholar]
- 6. Pierot L, Januel AC, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using Onyx: results of a prospective, multicenter study. J Neuroradiol 2009; 36: 147– 52 [DOI] [PubMed] [Google Scholar]
- 7. Gobin YP, Murayama Y, Milanese K, et al. Head and neck hypervascular lesions: embolization with ethylene vinyl alcohol copolymer—laboratory evaluation in Swine and clinical evaluation in humans. Radiology 2001; 221: 309–17 [DOI] [PubMed] [Google Scholar]
- 8. Arat A, Cil BE, Vargel I, et al. Embolization of high-flow craniofacial vascular malformations with Onyx. AJNR Am J Neuroradiol 2007; 28: 1409– 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiteside OJ, Monksfield P, Steventon NB, et al. Endovascular embolization of a traumatic arteriovenous fistula of the superficial temporal artery. J Laryngol Otol 2005; 119: 322– 24 [DOI] [PubMed] [Google Scholar]
- 10. Thiex R, Williams A, Smith E, et al. The use of Onyx for embolization of central nervous system arteriovenous lesions in pediatric patients. AJNR Am J Neuroradiol 2010; 31: 112– 20. Epub 2009 Sep 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu IC, Orbach DB. Neurointerventional management of high-flow vascular malformations of the head and neck. Neuroimaging Clin N Am 2009; 19: 219– 40. [DOI] [PubMed] [Google Scholar]
- 12. Jeong HS, Baek CH, Son YI, et al. Treatment for extracranial arteriovenous malformations of the head and neck. Acta Otolaryngol 2006; 126: 295– 300 [DOI] [PubMed] [Google Scholar]
- 13. Kohout MP, Hansen M, Pribaz JJ, et al. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg 1998; 102: 643–54 [DOI] [PubMed] [Google Scholar]
- 14. Bradley JP, Zide BM, Berenstein A, et al. Large arteriovenous malformations of the face: aesthetic results with recurrence control. Plast Reconstr Surg 1999; 103: 351–61 [DOI] [PubMed] [Google Scholar]
- 15. Lopez-Gutierrez JC, Ros Z, Perez-Higueras A. Giant vascular malformation of the face in a premature infant: complete resolution by embolization. J Pediatr Surg 1995; 30: 1519– 20 [DOI] [PubMed] [Google Scholar]
- 16. Jackson IT, Keskin M, Yavuzer R, et al. Compartmentalization of massive vascular malformations. Plast Reconstr Surg 2005; 115: 10– 21 [PubMed] [Google Scholar]
- 17. Gomes AS. Embolization therapy of congenital arteriovenous malformations: use of alternate approaches. Radiology 1994; 190: 191– 98 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman SL, Kumar AA, Roland JM, et al. Transcatheter embolization in the management of congenital arteriovenous malformations. Radiology 1980; 137: 21–29 [DOI] [PubMed] [Google Scholar]
- 19. Turowski B, Zanella FE. Interventional neuroradiology of the head and neck. Neuroimaging Clin N Am 2003; 13: 619– 45 [DOI] [PubMed] [Google Scholar]
- 20. Firat M, Dincer C, Cerezci I, et al. Treatment of a giant orbitofacial vascular malformation and ophthalmic artery aneurysms with intralesion glue injections: case report. AJNR Am J Neuroradiol 2001; 22: 1419– 23 [PMC free article] [PubMed] [Google Scholar]
- 21. Niemann D, Aviv R, Cowsill C, et al. Anatomically conformable, three-dimensional, detachable platinum microcoil system for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2004; 25: 813– 18 [PMC free article] [PubMed] [Google Scholar]
- 22. Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg 2010; 125: 1185– 94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


