SUMMARY:
Panitumumab (Vectibix), is a human monoclonal antibody EGFR antagonist indicated as a single agent for the treatment of metastatic colorectal carcinoma with disease progression on or following fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy regimens. This article will present the mechanism of action as well as the clinical role for this monoclonal antibody.
Panitumumab is a human monoclonal antibody antagonist specific to the EGFR (also known as EGF receptor, c-erbB-1, and HER1 in humans).1 It is indicated as a single agent for the treatment of metastatic colorectal carcinoma with disease progression on or following fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy regimen.2–4 Drug approval by the FDA is based on progression-free survival; no data demonstrate an improvement in disease-related symptoms or increased survival with panitumumab.5 Panitumumab is manufactured by Amgen (Thousand Oaks, California) and is marketed as Vectibix.
Mechanism of Action
EGFR is a transmembrane glycoprotein that is a member of a subfamily of type I receptor tyrosine kinases including HER2, HER3, and HER4.6 EGFR is constitutively expressed in normal epithelial tissues, including the skin and hair follicles. EGFR is overexpressed in certain human cancers, including colon and rectal cancer. Panitumumab works by binding to the extracellular domain of the EGFR preventing its activation.7 This results in halting of the cascade of intracellular signals dependent on this receptor (Fig 1).
Fig 1.
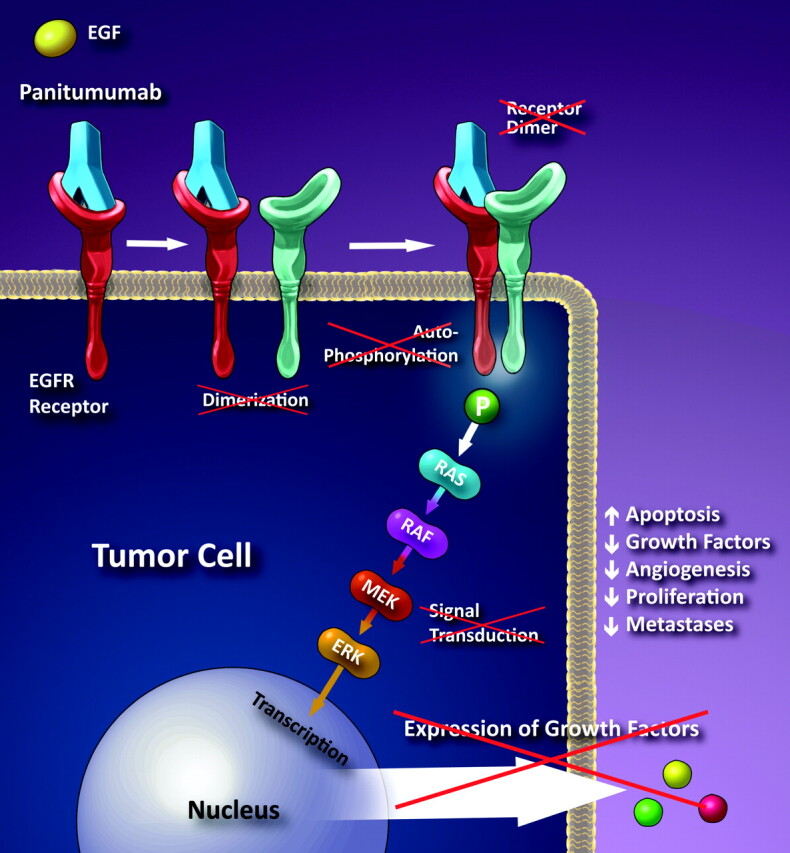
Schematic illustration of the proposed mechanism of panitumumab. Panitumumab is a monoclonal antibody that binds to the extracellular portion of the EGFR preventing dimerization and the cascade that leads to the expression of growth factors. (Illustration courtesy of Carolyn Nowak.)
Clinical Indication
The FDA approved panitumumab for the first time in September 2006, for the treatment of EGFR-expressing metastatic colorectal cancer with disease progression despite prior treatment. Panitumumab was approved by the European Medicines Agency in 2007 and by Health Canada in 2008 for the treatment of refractory EGFR-expressing metastatic colorectal cancer with nonmutated (wild-type) KRAS. Panitumumab was the first monoclonal antibody to demonstrate the use of KRAS as a predictive biomarker.8
Administration and Side Effects
Panitumumab is administered at 6 mg/kg every 14 days as an intravenous infusion for 60 minutes (≤1000 mg) or 90 minutes (>1000 mg). Panitumumab administered as a single agent exhibits nonlinear pharmacokinetics. The elimination half-life is approximately 7.5 days (range, 3.6–10.9 days). No formal pharmacokinetic studies of panitumumab have been conducted in patients with renal or hepatic impairment, to our knowledge.
The most common adverse reactions (≥20%) are skin toxicities (ie, erythema, dermatitis acneiform, pruritus, exfoliation, rash, and fissures), paronychia, hypomagnesemia, fatigue, abdominal pain, nausea, diarrhea, and constipation. The most serious adverse events with the administration of panitumumab include pulmonary fibrosis, pulmonary embolism, and severe dermatologic toxicity. Electrolyte levels should be monitored along with clinical signs of dermatologic and ocular toxicity.9,10
Economic Issues
Like other genetically engineered monoclonal antibodies, panitumumab is quite expensive, and the costs can vary widely from country to country. Panitumumab costs approximately $3000 per treatment in the United States based on an average healthy male weight of 70 kg.
Abbreviations
- c-erbB-1
EGFR gene
- EGFR
epidermal growth factor receptor
- FDA
US Food and Drug Administration
- HER
human epidermal growth factor receptor
- KRAS
Kristen rat sarcoma viral oncogene homolog
Footnotes
DISCLOSURES: Suresh K. Mukherji, Consultant: Philips Healthcare.
References
- 1. Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer 2003; 2: 246– 51 [DOI] [PubMed] [Google Scholar]
- 2. Kim GP, Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics 2008; 2: 223– 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keating GM. Spotlight on panitumumab in metastatic colorectal cancer. BioDrugs 2010; 1 24: 275– 78 [DOI] [PubMed] [Google Scholar]
- 4. Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 28 2010; 70: 1059– 78 [DOI] [PubMed] [Google Scholar]
- 5. Hoda D, Simon GR, Garrett CR. Targeting colorectal cancer with anti-epidermal growth factor receptor antibodies: focus on panitumumab. Ther Clin Risk Manag 2008; 4: 1221– 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinelli E, De Palma R, Orditura M, et al. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol 2009; 158: 1– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois EA, Cohen AF. Panitumumab. Br J Clin Pharmacol 2009; 68: 482– 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berardi R, Onofri A, Pistelli M, et al. Panitumumab: the evidence for its use in the treatment of metastatic colorectal cancer. Core Evid 2010; 5: 61– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vectibix (panitumumab) [package insert]. Thousand Oaks, California: Amgen Inc; 2006 [Google Scholar]
- 10. Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician 2008; 77: 311– 19 [PubMed] [Google Scholar]


