Abstract
Background
Currently, the association of nutritional risk screening score with the development of nonventilator hospital-acquired pneumonia (NV-HAP) is unknown. This study investigated whether nutritional risk screening score is an independent predictor of NV-HAP.
Methods
This retrospective cohort study was conducted between September 2017 and June 2020 in a tertiary hospital in China. The tool of Nutritional Risk Screening 2002 (NRS-2002) was used for nutritional risk screening. A total score of ≥3 indicated a patient was “at nutritional risk.” Logistic regression was applied to explore the association between the NRS score and NV-HAP.
Results
A total of 67,280 unique patients were included in the study. The incidence of NV-HAP in the cohort for the NRS < 3 and ≥ 3 NRS group was 0.4% (232/62702) and 2.6% (121/4578), respectively. In a multivariable logistic regression model adjusted for all of the covariates, per 1-point increase in the NRS score was associated with a 30% higher risk of NV-HAP (OR = 1.30; 95%CI:1.19–1.43). Similarly, patients with NRS score ≥ 3 had a higher risk of NV-HAP with an odds ratio (OR) of 2.06 (confidence interval (CI): 1.58–2.70) than those with NRS score < 3. Subgroup analyses indicated that the association between the NRS score and the risk of NV-HAP was similar for most strata. Furthermore, the interaction analyses revealed no interactive role in the association between NRS score and NV-HAP.
Conclusion
NRS score is an independent predictor of NV-HAP, irrespective of the patient’s characteristics. NRS-2002 has the potential as a convenient tool for risk stratification of adult hospitalized patients with different NV-HAP risks.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06014-w.
Keywords: Malnutrition, Screening, Hospital-acquired pneumonia, Aspiration pneumonia, Cohort study
Introduction
Hospital-acquired pneumonia (HAP) is one of the most frequent types of healthcare-associated infections (HAIs) [1]. It includes two distinct subgroups: nonventilator hospital-acquired pneumonia (NV-HAP) and ventilator-associated pneumonia (VAP). Currently, more than two-thirds of HAP cases are of the NV-HAP type [2, 3]. Although both NV-HAP and VAP impose enormous clinical and economic burdens clinical and economic burdens [4–6], evidence suggests that NV-HAP has higher overall medical costs and greater overall mortality than VAP [6]. However, literature concerning NV-HAP is rare. Most studies and prevention strategies targeting HAP have primarily focused on VAP [2]. Studies have revealed that modifiable risk factors, such as swallowing evaluation and oral care, can reduce the risk of NV-HAP [7, 8]. Therefore, the search for additional modifiable risk factors of NV-HAP is urgently needed.
Factors thought to be influencing NV-HAP have been explored in several studies [9, 10], were most patient-related risk factors associated with an increased NV-HAP morbidity cannot be corrected [7, 11]. Malnutrition, as an important risk factor for HAIs [12], is highly prevalent in hospitalized adult patients. The prevalence of malnutrition ranges from 20 to 50% in hospitalized patients [13]. With appropriate nutritional support therapy, malnutrition is potentially reversible. The nutritional support therapy is therefore becoming an appealing target for prevention and management of HAIs, including the NV-HAP [14]. To identify important nutritional targets, the association between nutritional risk and NV-HAP should be explored.
The NRS-2002 is a validated tool for nutritional screening of patients between 18 to 90 years of age who have or are at risk of malnutrition. The tool includes standard screening parameters, such as body mass index (BMI), patient’s age, weight loss, dietary intake, and severity of underlying disease [15]. The NRS-2002 score ranges from 0 to 7, and a total score of ≥3 indicates that a patient is “at nutritional risk”. This tool has been confirmed and validated by several studies worldwide and is widely used for screening hospitalized patients who are nutritionally at risk [16–18]. Several studies have identified the nutritional risk screening (NRS) score as an independent predictor of HAIs [12], such as surgical site infections [19]. However, no longitudinal data concerning the the association of NRS score with the risk of NV-HAP.
Thus, we investigated the relationship between nutritional risk screening scores and NV-HAP.P.
Methods
Data sources and study population
We conducted a retrospective cohort study, including all inpatients admitted between September 1, 2017, through June 30, 2020, at Wenzhou People’s Hospital (a 1500-bed tertiary teaching hospital in Zhejiang, China). Patients who were pregnant, younger than 18 years of age or greater than 90 years of age, length of hospital stay < 48 h, received mechanical ventilation during hospitalization, and lack of nutritional risk screening score were excluded from the analysis. If patients were readmitted during the study period, only their first admission was considered. The study was approved by the Ethics Review Committee of Wenzhou people’s Hospital [approval no. WRY2018070]. Given the retrospective nature of the study, the requirement of informed consent was waived. This paper was reported in line with the STROBE guidelines [20].
Nutritional risk screening (NRS)
All adult patients, except pregnant women, underwent nutritional risk screening. The nutritional risk screening was performed within 24 h after admission by ward nursing staff who were trained to conduct using the NRS-2002 tool.
Outcome
The NV-HAP data was obtained from the Xinglin system [21]. This system is a web-based, real-time monitoring system of nosocomial infection, which automatically identify symptoms of infections and clinical data such as fever, positive bacterial culture, and elevated inflammatory response markers for initial diagnoses. Meanwhile, the system is also used to transfer nosocomial infection cases identified by the clinicians to senior infection control practitioner for a definitive diagnosis. In case of a disagreement between the two sides, a consensus was made via discussions. Nonventilator hospital-acquired pneumonia (NV-HAP) is defined as a pneumonia not present or incubating at the time of hospital admission and occurring at least 48 h after admission in patients not receiving invasive mechanical ventilation during hospitalization [22]. The diagnostic criteria used in the present study for NV-HAP strictly adhered to the 2018 version of the Chinese guidelines [22]. The 2018 version of the Chinese guidelines is compatible with the guidelines issued by the American Thoracic Society [23].
Covariates
Admission data collected from the electronic medical record system included age; sex; drinking status; smoking status, comorbidities, admission category, Barthel Index, Morse Fall Scale, and season of admission. The Barthel Index (BI) [24] and the Morse Fall Scale [25] were used to assess the patient’s level of independence and nursing-related complications, respectively. Charlson comorbidity index (CCI) was used to measure the burden of comorbid conditions [26].
Based on the outcome and exposure to the hospital environment, we added a covariate termed “time at risk” into the model. For NV-HAP patients, “time at risk” was calculated as the number of days between the admission day and date of diagnosis of NV-HAP. For non-NV-HAP patients, the “time at risk” corresponded to the total hospital days. We collected information from the Xinglin system concerning clinical procedures (including a central venous catheter, indwelling urinary catheter, surgery, parenteral nutrition, and enteral tube feeding), other nosocomial infections, and the use of specific classes of medications such as antacids, sedatives, nonsteroidal anti-inflammatory drug (NSAID), systemic steroid, inhaled steroid, and anticoagulant during the “time at risk” period.
Statistical analysis
Non-normal continuous variables were presented as medians (Q1-Q3) and compared using Mann-Whitney U test. Categorical variables were presented as numbers (proportion) and compared using Chi-square test or Fisher’s exact test. To avoid bias caused by missing NRS score data, the characteristics of individuals with missing data were compared with those with complete data. As less than 1% of the covariates were missing, the missing data were not dealt with. Logistic regression analyses were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between NRS score and risk of NV-HAP. Firstly, possible collinearity was assessed based on the variance inflation factor (VIF); variables with VIF > 10 were removed from the Model. Secondly, we used four different logistic regression models to examine the associations of nutritional risk screening score and the risk for NV-HAP. The Non-adjusted Model examined the association between NRS score and NV-HAP without adjustment for any covariates. Model I included demographic characteristics (age and sex). Model II made an additional adjustment for variables that, when added to this model, changed in effect estimate of more than 10% [27], included the covariates in Model I plus stroke, Charlson comorbidity index, time of risk, central venous catheter, enteral tube feeding, Barthel Index, Morse Fall Scale. The association of each covariate with NV-HAP is shown in Supplementary Table S2. Model III (the fully adjusted model) included the covariates in Model II plus the other covariates listed in Table 1. Thirdly, these analyses were performed on unique patients, making it possible for a patient with multiple admissions; therefore, risk estimation was also performed using the generalized estimation equation (GEE) method with a logit link and exchangeable correlation matrix while adjusting for the possible dependence in the outcome introduced by repeated admissions. Finally, to assess the homogeneity of effects, subgroup analyses and interaction tests were performed for the covariates shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Demographics | Total (n = 67,280) | NRS score < 3 (n = 62,702) | NRS score ≥ 3 (n = 4578) | P value |
|---|---|---|---|---|
| Age (years), median (Q1-Q3) | 51 (37–65) | 50 (37–64) | 68 (43–78) | < 0.001 |
| Male, n (%) | 28,684 (42.6) | 26,499 (42.3) | 2185 (47.7) | < 0.001 |
| Drinking status, n (%) | < 0.001 | |||
| Never drinker | 57,117 (84.9) | 53,246 (84.9) | 3871 (84.5) | |
| Current drinker | 7802 (11.6) | 7346 (11.7) | 456 (10.0) | |
| Former drinker | 2119 (3.1) | 1890 (3.0) | 229 (5.0) | |
| Missing | 242(0.4) | 220 (0.4) | 22 (0.5) | |
| Smoking status, n (%) | < 0.001 | |||
| never smoker | 55,266 (82.1) | 51,584 (82.3) | 3682 (80.4) | |
| Current smoker | 8634 (12.9) | 8092 (12.9) | 542 (11.8) | |
| Former smoker | 3230 (4.8) | 2886 (4.6) | 344 (7.6) | |
| Missing | 150(0.2) | 140 (0.2) | 10 (0.2) | |
| Comorbidities, n (%) | ||||
| COPD | 803 (1.2) | 651 (1.0) | 152 (3.3) | < 0.001 |
| Swallow disability | 126 (0.2) | 63 (0.1) | 63 (1.4) | < 0.001 |
| Stroke | 7237 (10.8) | 6072 (9.7) | 1165 (25.4) | < 0.001 |
| Diabetes mellitus | 9612 (14.3) | 8805 (14.0) | 807 (17.6) | < 0.001 |
| Peptic ulcer disease | 2236 (3.3) | 2128 (3.4) | 108 (2.4) | < 0.001 |
| Moderate or severe renal disease | 2944 (4.4) | 2733 (4.4) | 211 (4.6) | 0.424 |
| Liver disease | 11,993 (17.8) | 11,560 (18.4) | 433 (9.5) | < 0.001 |
| Congestive heart failure | 328 (0.5) | 284 (0.5) | 44 (1.0) | < 0.001 |
| Solid tumour | 4962 (7.4) | 4272 (6.8) | 690 (15.1) | < 0.001 |
| CCI (points), median (Q1- Q3) | 1 (0–3) | 1 (0–3) | 4 (1–5) | < 0.001 |
| Time of risk (days), median (Q1- Q3) | 7 (4–10) | 7 (4–10) | 10 (6–16) | < 0.001 |
| Admission category, n (%) | < 0.001 | |||
| Internal medicine | 27,769 (41.3) | 25,487 (40.6) | 2282 (49.8) | |
| Surgery | 19,556 (29.1) | 18,110 (28.9) | 1446 (31.6) | |
| Gynaecology | 15,548 (23.1) | 15,084 (24.1) | 464 (10.1) | |
| Emergency department | 3172 (4.7) | 2902 (4.6) | 270 (5.9) | |
| ICU | 203 (0.3) | 137 (0.2) | 66 (1.4) | |
| Others | 1032 (1.5) | 982 (1.6) | 50 (1.1) | |
| Clinical procedure, n (%) | ||||
| Central venous catheter | 1762 (2.6) | 1294 (2.1) | 468 (10.2) | < 0.001 |
| Indwelling urinary catheter | 13,823 (20.5) | 12,972 (20.7) | 851 (18.6) | < 0.001 |
| Surgery | 20,979 (31.2) | 20,161 (32.2) | 818 (17.9) | < 0.001 |
| Parenteral nutrition | 1479 (2.2) | 1077 (1.7) | 402 (8.8) | < 0.001 |
| Enteral tube feeding | 5698 (8.5) | 4849 (7.7) | 849 (18.5) | < 0.001 |
| Barthel Index, n (%) | < 0.001 | |||
| Independent | 43,273 (64.3) | 41,706 (66.5) | 1567 (34.2) | |
| Slight dependency | 6057 (9.0) | 5601 (8.9) | 456 (10.0) | |
| Moderate dependency | 10,085 (15.0) | 9109 (14.5) | 976 (21.3) | |
| Severe dependency | 6495 (9.7) | 5461 (8.7) | 1034 (22.6) | |
| Total dependency | 1370 (2.0) | 825 (1.3) | 545 (11.9) | |
| Morse Fall Scale, n (%) | < 0.001 | |||
| No Risk | 41,008 (61.0) | 39,220 (62.5) | 1788 (39.1) | |
| Low Risk | 21,697 (32.2) | 19,805 (31.6) | 1892 (41.3) | |
| High Risk | 4575 (6.8) | 3677 (5.9) | 898 (19.6) | |
| Other nosocomial infections, n (%) | 1137 (1.7) | 983 (1.6) | 154 (3.4) | < 0.001 |
| Season of admission, n (%) | < 0.001 | |||
| Spring | 16,431 (24.4) | 15,356 (24.5) | 1075 (23.5) | |
| Summer | 18,652 (27.7) | 17,451 (27.8) | 1201 (26.2) | |
| Fall | 14,497 (21.5) | 13,390 (21.4) | 1107 (24.2) | |
| Winter | 17,700 (26.3) | 16,505 (26.3) | 1195 (26.1) | |
| In-hospital medications, n (%) | ||||
| Antacids | 37,406 (55.6) | 34,396 (54.9) | 3010 (65.7) | < 0.001 |
| Sedatives | 6615 (9.8) | 5889 (9.4) | 726 (15.9) | < 0.001 |
| NSAID | 6503 (9.7) | 5879 (9.4) | 624 (13.6) | < 0.001 |
| Systemic steroid | 13,231 (19.7) | 12,445 (19.8) | 786 (17.2) | < 0.001 |
| Inhaled steroid | 4281 (6.4) | 3760 (6.0) | 521 (11.4) | < 0.001 |
| Anticoagulant | 8972 (13.3) | 8229 (13.1) | 743 (16.2) | < 0.001 |
| NV-HAP | 353(0.5) | 232(0.4) | 121(2.6) | < 0.001 |
Abbreviations: NRS Nutritional risk screening, Q1 First quartile, Q3 Third quartile, COPD Chronic obstructive pulmonary disease, CCI Charlson comorbidity index, ICU Intensive care unit, NSAID Nonsteroidal anti-inflammatory drug, NV-HAP Nonventilator hospital-acquired pneumonia
Statistical analyses were performed with the R software (version 3.4.3; http://www.R-project.org) and EmpowerStats software (www.empowerstats.com, X&Y solutions, Inc. Boston MA). A two-tailed P-value of ≤0.05 was considered to be statistically significant.
Results
Study participants and baseline characteristics
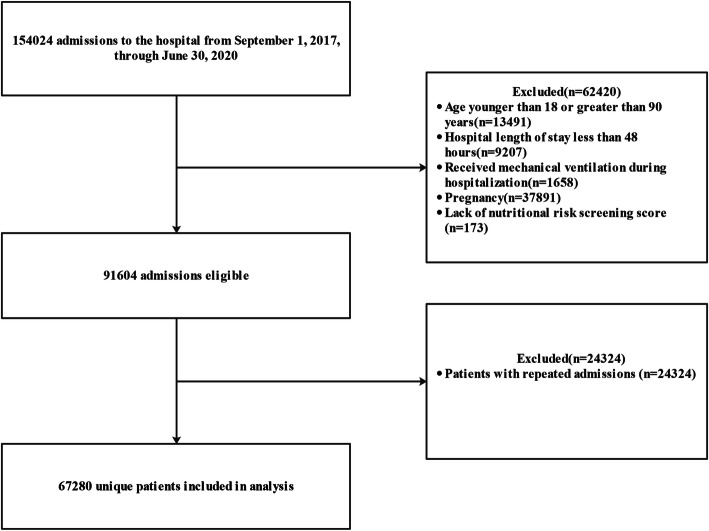
There were 154,024 admissions to the medical centre from September 1, 2017, through June 30, 2020. After excluding admissions with younger than 18 years of age or greater than 90 years of age (n = 13,491), length of hospital stay < 48 h (n = 9207), received mechanical ventilation during hospitalization (n = 1658), pregnancy (n = 37,891), lack of nutritional risk screening score (n = 173), and repeated admissions (n = 24,324), a total of 67,280 unique patients were included in the final analysis (Fig. 1). Baseline characteristics of study participants by NRS score are listed in Table 1. In the present study, 4578 (6.8%) patients were at nutritional risk (NRS-2002 ≥ 3). There were significant differences in baseline characteristics between patients with NRS score < 3 and those with NRS score ≥ 3 (Table 1).
Fig. 1.
Flow chart of the study population
The incidence of NV-HAP according to NRS scores
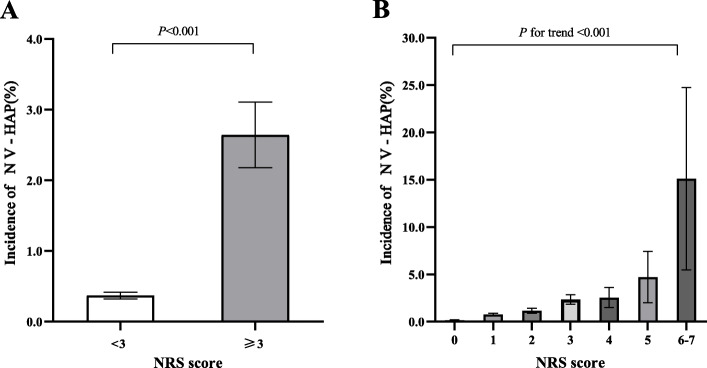
The incidence of NV-HAP in the cohort for the NRS < 3 group and NRS score ≥ 3 group was 0.4% (232/62702) and 2.6% (121/4578), respectively (Table 1). The proportion of patients with NV-HAP was significantly higher in the NRS ≥3 groups (Fig. 2a). The incidence of NV-HAP showed an NRS score-dependent increase (P for trend< 0.001). The incidence of NV-HAP was 0.2, 0.8, 1.1, 2.3, 2.5, 4.7, and 15.1% for NRS scores of 0, 1, 2, 3, 4,5, and ≥ 6, respectively (Fig. 2b).
Fig. 2.
Incidence of NV-HAP among Chinese adults for the NRS < 3 group and NRS score ≥ 3 group (A), and for NRS score of 0, 1, 2, 3, 4,5, and ≥ 6, respectively (B). Abbreviations: NRS, nutritional risk screening; NV-HAP, nonventilator hospital-acquired pneumonia. Note: patients with NRS scores of 6 and 7 were combined for analysis due to small sample size; a total score of ≥3 indicated a patient is “at nutritional risk”
Relationship between NRS score and NV-HAP
Results of VIF analysis for variables showed that there was no collinearity bias (Table S1 in the Supplementary Appendix). The unadjusted and multivariate-adjusted analyses of the relationship between NRS score and NV-HAP are shown in Table 2. NRS score, whether considered a categorical or continuous variable, was independently associated with the risk of NV-HAP in different multivariate logistic regression models. Patients with NRS score ≥ 3 were at a higher risk of NV-HAP (OR = 7.31; 95%CI: 5.86, 9.31) than those with NRS score was < 3. After adjusting for age and sex (Model I), the association remained unchanged (OR = 4.39;95% CI: 3.47, 5.55).
Table 2.
Association between nutritional risk screening score and nonventilator hospital-acquired pneumonia in multivariate logistic regression model
| NRS score | Non-adjusted Model | Model I | Model II | Model III | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Continuous, per 1-point increment | 2.05(1.92–2.19) | < 0.001 | 1.74(1.62–1.87) | < 0.001 | 1.28 (1.18, 1.40) | < 0.001 | 1.30(1.19–1.43) | < 0.001 |
| Categories | ||||||||
| < 3 | Ref | Ref | Ref | Ref | ||||
| ≥ 3 | 7.31(5.86–9.31) | < 0.001 | 4.39(3.47–5.55) | < 0.001 | 1.97 (1.52–2.56) | < 0.001 | 2.06(1.58–2.70) | < 0.001 |
Abbreviations: OR Odds ratio, CI Confidence interval
Model I: Adjusted for age and sex
Model II: Adjust for variables that, when added to this model, changed in effect estimate of more than 10%, included the covariates in Model I plus stroke, CCI, time of risk, central venous catheter, enteral tube feeding, Barthel Index, and Morse Fall Scale
Model III: Adjust for all of these variables, included the covariates in Model II plus adjusted for drinking status, smoking status, COPD, swallow disability, diabetes mellitus, peptic ulcer disease, moderate or severe renal disease, liver disease, congestive heart failure, solid tumor, admission category, indwelling urinary catheter, surgery, parenteral nutrition, other nosocomial infections, season of admission, antacids, sedatives, NSAID, systemic steroid, Inhaled steroid, and anticoagulant
Note: a total score of≥3 indicated a patient is “at nutritional risk”
After additional adjustment for stroke, CCI, time of risk, central venous catheter, enteral tube feeding, Barthel Index, and Morse Fall Scale (Model II), the association did not change (OR = 1.97;95% CI: 1.52, 2.56). Finally, in the fully adjusted model (Model III), the OR was 2.06 (95% CI: 1.58, 2.70). Similarly, per 1-point increase in the NRS score was associated with a 105,74,28, and 30% higher risk of NV-HAP in different multivariate logistic regression models.
Sensitivity and subgroup analyses
A total of 174 patients with missing NRS score data were found. After excluding 40 patients with repeated admissions, 134 patients with missing NRS score data were retained, accounting for 0.2% of all analysed patients (134/ 67,280). There was no significant difference in most baseline characteristics, including the Charlson comorbidity index, between the two groups (Table S3 in the Supplementary Appendix).
In the sensitivity analyses, similar results were observed in analyses using a GEE method with a logit link and exchangeable correlation matrix (Table S4 in the Supplementary Appendix).
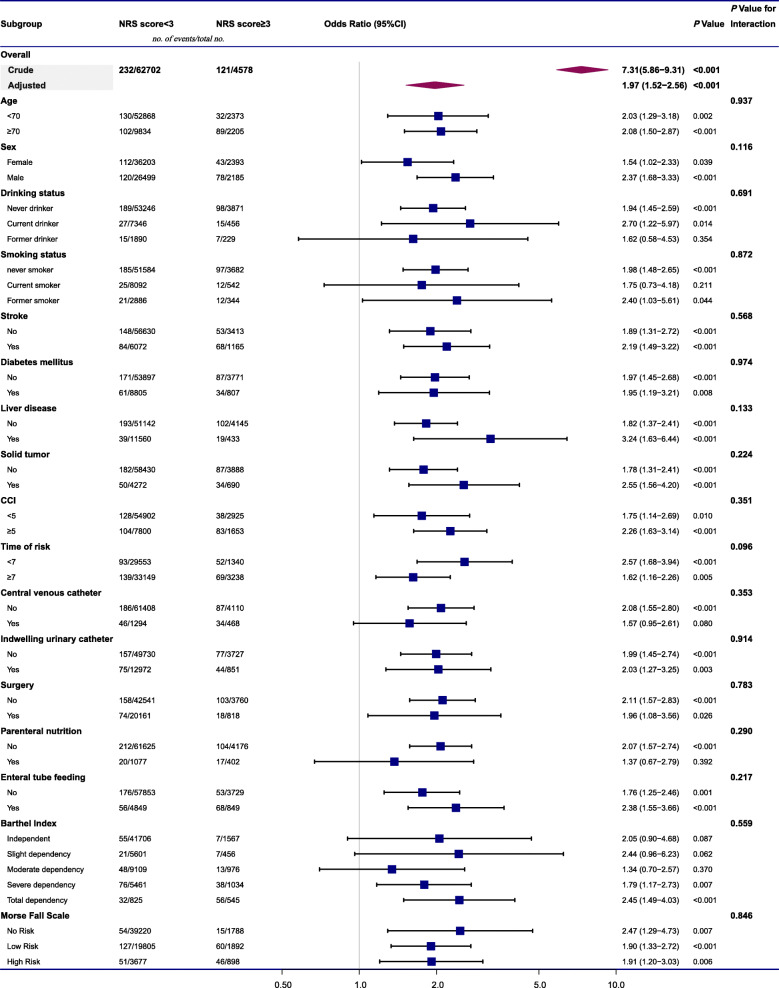
We further performed stratified and interaction analyses to assess the effect of the NRS score (< 3/≥3) on NV-HAP in various subgroups (Fig. 3). In the subgroup analyses, the NRS score was associated with a greater risk of NV-HAP in most subgroups. The difference was not statistically significant in some subgroups, probably due to the limited sample size. Tests for interaction showed no statistically significant differences for effect modification by other covariates (all Pvalue for interaction > 0.05).
Fig. 3.
The association between NRS score (≥3/< 3) and the risk of NV-HAP in various subgroups. Values were adjusted for age, sex, stroke, CCI, time of risk, central venous catheter, enteral tube feeding, Barthel Index, and Morse Fall Scale. Abbreviations: NRS, nutritional risk screening; NV-HAP, nonventilator hospital-acquired pneumonia; CCI, charlson comorbidity index; CI, confidence interval. Note: The p value for interaction represents the likelihood of interaction between the variable and the NRS score
Discussion
Given the alarming increasing burden of NV-HAP, there is an urgent need to accurately identify high-risk patients with NV-HAP. To our knowledge, this is the first study to demonstrate the usefulness of NRS score in predicting NV-HAP. The main finding of this study is that NRS score is independently associated with the development of NV-HAP. This association was also robust in different regression models and different subgroups. Furthermore, no significant interactive effects of the NRS score and NV-HAP were found, suggesting that the association between the NRS score and NV-HAP is consistent and stable, regardless of the patient’s characteristics. These findings suggest that the NRS score may assist risk stratification, to identify specific subgroups of patients at a higher risk of developing NV-HAP. In addition, these results provide ideas for developing strategies to reduce the incidence of NV-HAP based on nutritional.
There is no clear and unified criteria for the diagnosis of malnutrition [28]. Over the years, several screening tools have been developed to identify malnutrition risk [29, 30], such as the NRS-2002, the Malnutrition Universal Screening Tool (MUST), Mini Nutritional Assessment (MNA), and Subjective Global Assessment (SGA). The NRS-2002 has been tested and used in hundreds of randomized controlled trials [15] and was found to be an accurate and reliable screening tool if applied by trained staff [30]. In contrast to SGA and MNA, NRS 2002 takes much less time to perform and requires less rigorous examiner training [31, 32]. In addition, the NRS-2002 can more accurately identify individuals at high nutritional risk or have poor nutritional status compared to MUST [31]. For these reasons, our hospital chose the NRS-2002 as the screening tool.
Malnutrition is a significant issue closely related to infection as it can impair normal immune system development and cause severe damages to mucosal epithelial barriers in the mucosal tissues [33]. Several studies have linked nutritional parameters with the development and progression of pneumonia. A recent study reported that age and early postoperative hypoalbuminemia were independent risk factors for postoperative pneumonia in patients undergoing hip fracture surgery [34]. Another study revealed that in patients under the age of 65, age, serum cholinesterase and total cholesterol levels were associated with both the severity of pneumococcal pneumonia and length of hospital stay [35]. Besides, several studies have shown that nutritional risk scores using these tools associated with HAIs. A recent cross-sectional study observed a strong positive association between the MUST score and the prevalence of HAIs [12]. Similar findings were obtained in a longitudinal study of hospitalized elderly patients by Gamaletsou et al. [36] Several studies have demonstrated the predictive value of preoperative nutrition risk in various surgical site infections [37–41]. Although the association between the nutrition risk and the risk of HAIs, especially in surgical site infection, has been reported, the role of the nutritional risk screening score in NV-HAP remains poorly undefined.
Scientific evidence about identifying modifiable risk factors or predictive factors of NV-HAP is meager and of limited quality. A case-control study of 132 patients showed that age, the use of antacids, and central nervous system disease were independent risk factors of NV-HAP, but the poor nutritional status was not [9]. However, this conclusion should be interpreted with caution due to the small sample size of the study. In a systematic review and meta-analysis that included 144 studies, Schreiber et al. assessed the proportion of HAIs prevented by multifaceted interventions and only two of the studies involved NV-HAP [42]. To the best of our knowledge, no study has assessed the effectiveness of nutritional control measures on a large scale of patients with NV-HAP. Only one study proposed a procedure of healthy control as a preventive measure of postoperative pneumonia.
Hiramatsu et al. [43] conducted a historical case-control study in which they examined the influence of a preoperative care bundle, including a procedure of nutritional control, three breathing exercise procedures, two oral care procedures, and smoking cessation, on the incidence of postoperative pneumonia among esophageal cancer patients. The results indicated that the risk of postoperative pneumonia was reduced by 84% (OR = 0.16; 95%CI: 0.01, 0.94) after implementation of the care bundle. However, the independent role of nutritional control procedure in the successful implementation of practice measures has not been clarified. Thus, understanding what works, why, and for whom is pivotal to the effective management of patients at risk of postoperative pneumonia [44]. The present findings provide a new theoretical perspective to the role of NRS score as a predictor of nonventilator hospital-acquired pneumonia.
The strengths of this study include inclusion of a large patient cohort, collection of detailed covariate data, adjusted patients’ covariates exposure time to the hospital environment, and implementation of sensitivity analysis to test the robustness of the results. The study also conformed to the recommended diagnostic criteria instead of the International Classification of Diseases codes to diagnose NV-HAP [10] as a critical stronghold. Also, misclassification bias was minimized as each case of NV-HAP was rigorously reviewed and co-confirmed by a clinician and a senior infection control practitioner.
Nonetheless, there are also some limitations that are worth mentioning. First, as with any observational study, we cannot rule out the possibility of residual confounders despite controlling for 31 variables in our models. Furthermore, the use of retrospective data may introduce selection biases. Therefore, in the sensitivity analyses, we tested consecutive patients by the GEE method to minimize selection bias. Secondly, there were likely possible NRS score measurement errors. Although the ward nursing staff were well-trained to conduct nutritional risk screening, there still could be measurement bias as the screening was carried out by different nurses. Finally, the numbers of patients in some subgroups were small, yielding limited statistical power as this may conceal some meaningful results in both stratified and interaction analyses.
In summary, this study shows that the NRS score is an independent predictor of NV-HAP, irrespective of the patient’s characteristics. NRS-2002 as a simple and rapid tool for nutritional risk screening, has the potential to be applied as a convenient tool for risk stratification of adult hospitalized patients with different NV-HAP risks.
Supplementary Information
Additional file 1 :Table S1. Variance inflation factor test for covariates. Table S2.Associations of covariates with NV-HAP.Table S3. Differences in baseline characteristics between missing and non-missing NRS score data patients. Table S4. Association between nutritional risk screening score and NV-HAP in GEE regression model (N = 91,604).
Acknowledgments
None.
Authors’ contributions
ZHC collected data, analysed and wrote the manuscript. HMW, SCG, LC and HHW were involved in the conception and design of the study. JHJ and KX participated in data collection and analysis. XYL revised the manuscript. All authors critically read, revised, and approved the final manuscript, and agreed to be fully accountable for the integrity and accuracy of the work.
Funding
This work was supported by Medicine and Health Science and Technology Project of Wenzhou Health Bureau [grant numbers 2018B01]. The funder was not involved in the design of the study, collection, analysis, and interpretation of data or in writing the manuscript.
Availability of data and materials
The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval for the study was obtained from the Wenzhou people’s Hospital Ethics Review Committee [approval no. WRY2018070]. The requirement for informed consent was waived by the Ethics Review Committee of Wenzhou people’s Hospital, given the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker D, Quinn B. Hospital acquired pneumonia prevention Initiative-2: incidence of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(1):2–7. doi: 10.1016/j.ajic.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H, Adeyemi AO, Rascati KL. Direct medical costs and utilization of health care services to treat pneumonia in the united states: an analysis of the 2007–2011 medical expenditure panel survey. Clin Ther. 2015;37(7):1466–1476.e1. doi: 10.1016/j.clinthera.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Micek ST, Chew B, Hampton N, Kollef MH. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest. 2016;150(5):1008–1014. doi: 10.1016/j.chest.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 7.de Assis MCS, Macedo ABT, Martins C, Konkewicz LR, Viana LV, Tavares JP, et al. The impact of a bundle to prevent hospital-acquired pneumonia in a cohort of nonventilated patients on enteral nutrition. Infect Control Hosp Epidemiol. 2021;42(1):100–102. doi: 10.1017/ice.2020.374. [DOI] [PubMed] [Google Scholar]
- 8.Lacerna CC, Patey D, Block L, Naik S, Kevorkova Y, Galin J, Parker M, Betts R, Parodi S, Witt D. A successful program preventing nonventilator hospital-acquired pneumonia in a large hospital system. Infect Control Hosp Epidemiol. 2020;41(5):547–552. doi: 10.1017/ice.2019.368. [DOI] [PubMed] [Google Scholar]
- 9.Fortaleza CM, Abati PA, Batista MR, Dias A. Risk factors for hospital-acquired pneumonia in nonventilated adults. Braz J Infect Dis. 2009;13(4):284–288. doi: 10.1590/s1413-86702009000400009. [DOI] [PubMed] [Google Scholar]
- 10.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. Jama. 2009;301(20):2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 11.Klompas M. Hospital-acquired pneumonia in nonventilated patients: the next frontier. Infect Control Hosp Epidemiol. 2016;37(7):825–826. doi: 10.1017/ice.2016.101. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick F, Skally M, O’Hanlon C, Foley M, Houlihan J, Gaughan L, et al. Food for thought. Malnutrition risk associated with increased risk of healthcare-associated infection. J Hosp Infect. 2019;101(3):300–304. doi: 10.1016/j.jhin.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8(2):514–527. doi: 10.3390/ijerph8020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Wang Y, Jiang ZM, Kondrup J, Fang H, Andrews M, Nolan MT, Mu SY, Zhang J, Yu K, Lu Q, Kang WM. Impact of nutrition support on clinical outcome and cost-effectiveness analysis in patients at nutritional risk: a prospective cohort study with propensity score matching. Nutrition. 2017;37:53–59. doi: 10.1016/j.nut.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Kondrup J. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 16.Patel C, Omer E, Diamond SJ, McClave SA. Can nutritional assessment tools predict response to nutritional therapy? Curr Gastroenterol Rep. 2016;18(4):15. doi: 10.1007/s11894-016-0488-y. [DOI] [PubMed] [Google Scholar]
- 17.Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brändle M, Benz C, Henzen C, Mattmann S, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donzé J, Stanga Z, Mueller B. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Zhou Y, He T, Zhuang X. Effect of preoperative nutritional risk screening and enteral nutrition support in accelerated recovery after resection for esophageal cancer. Nutr Cancer. 2020;1:1–6. doi: 10.1080/01635581.2020.1764981. [DOI] [PubMed] [Google Scholar]
- 19.Kwag SJ, Kim JG, Kang WK, Lee JK, Oh ST. The nutritional risk is a independent factor for postoperative morbidity in surgery for colorectal cancer. Ann Surg Treat Res. 2014;86(4):206–11. 10.4174/astr.2014.86.4.206. [DOI] [PMC free article] [PubMed]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed]
- 21.Du M, Xing Y, Suo J, Liu B, Jia N, Huo R, et al. Real-time automatic hospital-wide surveillance of nosocomial infections and outbreaks in a large Chinese tertiary hospital. BMC Med Inform Decis Mak. 2014;14(1):9. 10.1186/1472-6947-14-9. [DOI] [PMC free article] [PubMed]
- 22.Shi Y, Huang Y, Zhang T-T, Cao B, Wang H, Zhuo C, et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition). J Thorac Dis. 2019;11(6):2581–616. 10.21037/jtd.2019.06.09. [DOI] [PMC free article] [PubMed]
- 23.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–111. 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed]
- 24.Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–5. [PubMed]
- 25.Chow SK, Lai CK, Wong TK, Suen LK, Kong SK, Chan CK, et al. Evaluation of the Morse fall scale: applicability in Chinese hospital populations. Int J Nurs Stud. 2007;44(4):556–65. 10.1016/j.ijnurstu.2005.12.003. [DOI] [PubMed]
- 26.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. 10.1016/0895-4356(94)90129-5. [DOI] [PubMed]
- 27.Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. Bmj. 2014;348(jan23 1):g14. 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed]
- 28.Rasheed S, Woods RT. Malnutrition and quality of life in older people: a systematic review and meta-analysis. Ageing Res Rev. 2013;12(2):561–6. 10.1016/j.arr.2012.11.003. [DOI] [PubMed]
- 29.Dent E, Hoogendijk EO, Visvanathan R, Wright ORL. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. 2019;23(5):431–41. 10.1007/s12603-019-1176-z. [DOI] [PubMed]
- 30.Reber E, Gomes F, Vasiloglou MF, Schuetz P, Stanga Z. Nutritional risk screening and assessment. J Clin Med. 2019;8(7):1065. 10.3390/jcm8071065. [DOI] [PMC free article] [PubMed]
- 31.Kyle UG, Kossovsky MP, Karsegard VL, Pichard C. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr. 2006;25(3):409–17. 10.1016/j.clnu.2005.11.001. [DOI] [PubMed]
- 32.Raslan M, Gonzalez MC, Dias MC, Nascimento M, Castro M, Marques P, et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. 2010;26(7–8):721–6. 10.1016/j.nut.2009.07.010. [DOI] [PubMed]
- 33.Rodriguez L, Cervantes E, Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health. 2011;8(4):1174–205. 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed]
- 34.Shin KH, Kim JJ, Son SW, Hwang KS, Han SB. Early postoperative Hypoalbuminaemia as a risk factor for postoperative pneumonia following hip fracture surgery. Clin Interv Aging. 2020;15:1907–15. 10.2147/CIA.S272610. [DOI] [PMC free article] [PubMed]
- 35.Akuzawa N, Naito H. Nutritional parameters affecting severity of pneumonia and length of hospital stay in patients with pneumococcal pneumonia: a retrospective cross-sectional study. BMC Pulm Med. 2015;15(1):149. 10.1186/s12890-015-0143-7. [DOI] [PMC free article] [PubMed]
- 36.Gamaletsou MN, Poulia KA, Karageorgou D, Yannakoulia M, Ziakas PD, Zampelas A, et al. Nutritional risk as predictor for healthcare-associated infection among hospitalized elderly patients in the acute care setting. J Hosp Infect. 2012;80(2):168–72. 10.1016/j.jhin.2011.08.020. [DOI] [PubMed]
- 37.Shinkawa H, Takemura S, Uenishi T, Sakae M, Ohata K, Urata Y, et al. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today. 2012;43(3):276–83. 10.1007/s00595-012-0350-2. [DOI] [PubMed]
- 38.Skeie E, Koch AM, Harthug S, Fosse U, Sygnestveit K, Nilsen RM, et al. A positive association between nutritional risk and the incidence of surgical site infections: a hospital-based register study. PLoS One. 2018;13(5):e0197344. 10.1371/journal.pone.0197344. [DOI] [PMC free article] [PubMed]
- 39.Hu Q, Wang G, Ren J, Ren H, Li G, Wu X, et al. Preoperative prognostic nutritional index predicts postoperative surgical site infections in gastrointestinal fistula patients undergoing bowel resections. Med. 2016;95(27):e4084. 10.1097/MD.0000000000004084. [DOI] [PMC free article] [PubMed]
- 40.Son HJ, Roh JL, Choi SH, Nam SY, Kim SY. Nutritional and hematologic markers as predictors of risk of surgical site infection in patients with head and neck cancer undergoing major oncologic surgery. Head Neck. 2018;40(3):596–604. 10.1002/hed.25031. [DOI] [PubMed]
- 41.Tsantes AG, Papadopoulos DV, Lytras T, Tsantes AE, Mavrogenis AF, Koulouvaris P, et al. Association of malnutrition with surgical site infection following spinal surgery: systematic review and meta-analysis. J Hosp Infect. 2020;104(1):111–9. 10.1016/j.jhin.2019.09.015. [DOI] [PubMed]
- 42.Schreiber PW, Sax H, Wolfensberger A, Clack L, Kuster SP. Swissnoso. The preventable proportion of healthcare-associated infections 2005-2016: systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2018;39(11):1277–95. 10.1017/ice.2018.183. [DOI] [PubMed]
- 43.Hiramatsu T, Sugiyama M, Kuwabara S, Tachimori Y, Nishioka M. Effectiveness of an outpatient preoperative care bundle in preventing postoperative pneumonia among esophageal cancer patients. Am J Infect Control. 2014;42(4):385–8. 10.1016/j.ajic.2013.11.017. [DOI] [PubMed]
- 44.Wolfensberger A, Clack L, Stefanie VF, Kusejko K, Hesse MF, Jakob W, et al. Implementation and evaluation of a care bundle for prevention of non-ventilator-associated hospital-acquired pneumonia (nvHAP) - a mixed-methods study protocol for a hybrid type 2 effectiveness-implementation trial. BMC Infect Dis. 2020;20(1):603. 10.1186/s12879-020-05271-5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 :Table S1. Variance inflation factor test for covariates. Table S2.Associations of covariates with NV-HAP.Table S3. Differences in baseline characteristics between missing and non-missing NRS score data patients. Table S4. Association between nutritional risk screening score and NV-HAP in GEE regression model (N = 91,604).
Data Availability Statement
The datasets used and analysed during this study are available from the corresponding author on reasonable request.