Abstract
A severe acute respiratory syndrome is an unusual type of contagious pneumonia that is caused by SARS coronavirus. At present, the whole world is trying to combat this coronavirus disease and scientific communities are putting rigorous efforts to develop vaccines. However, there are only a few specific medical treatments for SARS‐CoV‐2. Apart from other public health measures taken to prevent this virus, we can boost our immunity with natural products. In this article, we have highlighted the potential of common spices and herbs as antiviral agents and immunity boosters. A questionnaire‐based online survey has been conducted on home remedies during COVID‐19 among a wide range of peoples (n‐531) of different age groups (13–68 years) from various countries. According to the survey, 71.8% of people are taking kadha for combating infection and boosting immunity. Most people (86.1%) think that there is no side effect of kadha while 13.9% think vice versa. A total of 93.6% of people think that spices are helpful in curing coronavirus or other viral infection as well as boosting immunity. Most people are using tulsi drops, vitamin C, and chyawanprash for boosting their immunity. Therefore, we conclude from the survey and available literature that spices and herbs play a significant role against viral infections.
Keywords: antiviral, bioactive compounds, coronavirus, herbs, immunity boosters, SARS‐CoV‐2, spices
1. INTRODUCTION
In December 2019, the people of Wuhan city of the Hubei province of China were suffered from deadly “SARS‐CoV‐2” like pneumonia which was later named coronavirus disease (COVID‐19) by the World Health Organization (WHO) (Wang, Wang, Ye, & Liu, 2020). The COVID‐19 cases are increasing day by day, and there have been 37,423,660 confirmed cases of COVID‐19 in more than 200 countries, including 1,074,817 deaths up to October 12, 2020. (https://covid19.who.int/). The WHO declared it initially a public health emergency of international concern and later pandemic where the COVID‐19 symptoms include fever, sneezing, diarrhea, dry cough, malaise, respiratory distress, and shortness of breath. This virus (SARS‐CoV‐2) is a member of beta‐coronavirus and is found similar to earlier coronavirus severe acute respiratory syndrome coronavirus (SARS‐CoV) and the Middle East respiratory syndrome coronavirus (MERS‐CoV), in its pathogenicity and clinical spectrum (Gurunathan et al., 2020).
Coronaviruses (CoV) (family: Coronaviridae) are enveloped viruses containing non‐segmented, positive‐stranded genomic RNA. These viruses are pleomorphic particles ranging from 80–220 nm in diameter. The genome size of coronaviruses ranges from 26–32 kilobases (MacLachlan & Dubovi, 2017). It has better genome sequence vis‐à‐vis to the SARS‐CoV compared to MERS‐CoV, but the amino acid sequence is different from the other coronavirus, especially in the region of 1ab polyprotein and S‐protein or surface glycoprotein (Kannan, Ali, Sheeza, & Hemalatha, 2020). Their entire replication cycle takes place in the cytoplasm. Coronaviruses can cause several of diseases, including bronchitis, hepatitis, gastroenteritis, and even death in birds, humans, and other animals (Chafekar & Fielding, 2018). The coronavirus has been found to attack all types of people, especially elderly patients having diabetes, hypertension, cerebral infarction, chronic bronchitis, Parkinson's disease, chronic obstructive pulmonary disease, cardiovascular disease, and cancer (Deng & Peng, 2020; Guan et al., 2020; Huang et al., 2020). Coronaviruses (CoVs) enter into the host cell through interaction between the S protein of the virus species and the receptor of the host cell. It will bind with the angiotensin‐converting enzyme 2 receptor from the host cell to create a suitable habitation for viral replication (Walls et al., 2020).
Natural‐derived compounds constantly become a worthy therapeutic alternative against several diseases, including viral infections, because they are innately better tolerated in the human body. According to a study, from 1940 to 2014, 49% of all small molecules approved by the U.S. Food and Drug Administration (FDA) were natural products or their derivates (Newman & Cragg, 2016).
Herbal exploration is continually performed, also to diminish coronavirus‐related disease (Islam et al., 2020). Spices and herbs have been extensively studied globally due to their high antioxidant and antimicrobial activity in certain spices and their beneficial effects on humans. Spices contain many bioactive compounds that include flavonoids, phenolic compounds, sulfur‐containing compounds, tannins, alkaloids, phenolic diterpenes, and so on (Devi, Umasanker, & Babu, 2012; Panpatil, Tattari, Kota, & Polasa, 2013; Patra, Jana, Mandal, & Bhattacharjee, 2016; Yashin, Yashin, Xia, & Nemzer, 2017). India has the recognized six systems of medicine, namely, Ayurveda, yoga, Unani, Siddha, naturopathy, and homeopathy (Ravishankar & Shukla, 2007). Ayurveda means the science of life, and it is not only considered as an ethnomedicine but also as a complete medical system for maintaining a healthy and happy living. In India, 20,000 plant species have been recorded, which are having medicinal value, but more than 500 traditional communities use only about 800 plant species for treating different diseases (Dev, 1997).
The outbreak of SARS‐CoV‐2 led to catastrophic events, as there was little specific treatment known to date for coronavirus. So there is a global need to search for the agents that can act against SARS‐CoV‐2 as a precautionary measure which boost our immunity during COVID‐19. Ministry of AYUSH, India has released an advisory on Ayurveda's immunity promoting methods for self‐care during the COVID‐19 pandemic, which includes the use of spices such as turmeric, cumin, coriander, and garlic that are recommended in cooking. They have also advised taking drink herbal tea/decoction (kadha) made from basil, cinnamon, black pepper, ginger, and raisin once or twice in a day. Natural sugar or fresh lemon juice can be added to enhance the taste. Half teaspoon turmeric powder can be added to 150 mL hot milk (Golden Milk) which can be taken once or twice a day (https://www.ayush.gov.in/). This article summarizes the scientific studies on the antiviral activities of spices and herbs along with their derivatives, mechanism of action, and prospects for future studies along with the survey based analysis.
2. ANTIVIRAL PROPERTIES OF SPICES AND HERBS
Various medicinal plants/herbs are known as immunity boosters, namely, Allium sativum (garlic), Tinosporacordifolia (Giloy), Ocimumbasilicum (Tulsi), and so on (Singh, Tailang, & Mehta, 2016). Different spices such as clove, cinnamon, ginger, black pepper, and turmeric are known as immunity boosters along with their antiviral property (Sharma, Gupta, & Prasad, 2017; Shrivastava, 2020; Srivastava, Chaurasia, Khan, Dhand, & Verma, 2020). In this article, we have highlighted the antiviral potential of common spices and herbs mainly curcumin, cinnamon, ginger, clove, black pepper, garlic, neem, giloy, basil used during COVID‐19 as depicted in Figure 1. Neem leaves contain various compounds such as zinc, quercetin, vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin C, vitamin E, and so on, which may boost immunity (Garba & Mungadi, 2019).
FIGURE 1.
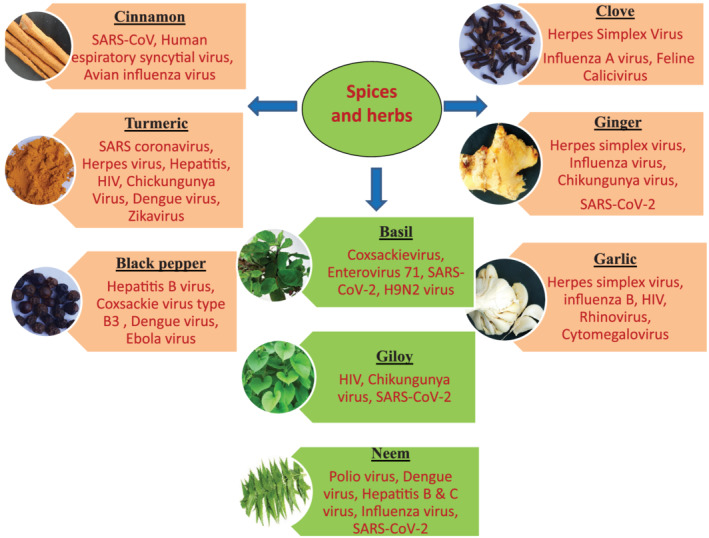
Common spices and herbs with antiviral properties [Colour figure can be viewed at wileyonlinelibrary.com]
2.1. Curcuma longa L. (turmeric)
Turmeric (Curcuma longa L.) belongs to the family of ginger (Zingiberaceae) and natively grows in India and Southeast Asia. Rhizomes of this plant contain several secondary metabolites including curcuminoids, sesquiterpenes, steroids, and polyphenol as major bioactive substances (Omosa, Midiwo, & Kuete, 2017). Curcumin is a natural polyphenol that is isolated for turmeric (Curcuma longa) and has been used from centuries as a traditional medicine in Asian countries to treat various disorders. Several studies have shown that the curcumin possesses some pharmacological properties such as anti‐inflammatory, anti‐angiogenic, and anti‐neoplastic, without toxicity. Food Drug Administration (FDA) categorized it as “Generally Recognized as Safe.” A dose of up to 12 g/day of curcumin was known to be safe for human consumption during the clinical trials without showing any side effects (Gupta, Patchva, & Aggarwal, 2013). Shrivastava (2020) reported that the dose of curcumin from 2,500 to 8,000 mg per day for 3 months showed no toxicity from curcumin. Curcumin is a dynamic antiviral that reduces the replication of viruses.
Antiviral activity of curcumin was observed against different viruses including hepatitis viruses, SARS coronavirus, influenza viruses, human immunodeficiency virus (HIV), herpes simplex virus, dengue virus, chikungunya virus, and so on, as listed in Table 1. Curcumin's antiviral activities can also be evidenced by its ability to regulate various molecular targets that contribute to various cellular events, such as transcription regulation, and the activation of cellular signaling pathways (Joe, Vijaykumar, & Lokesh, 2004). Curcumin's role in targeting various cellular pathways, further inhibiting the growth, and replication of viruses makes it an ideal candidate as an anti‐viral drug. Utomo, Ikawati, and Meiyanto (2020), based on their molecular docking study, reported that the curcumin binds and inhibits the target receptors including SARS‐CoV‐2 protease, spike glycoprotein‐RBD, and PD‐ACE2, which are involved in virus infection.
TABLE 1.
Antiviral properties and mechanism of action of Curcumin (bioactive compound from turmeric)
| S.No. | Virus | Mechanism of action | References |
|---|---|---|---|
| 1 | SARS coronavirus | Replication and protease activity inhibitor | Wen et al., 2007 |
| 2 | Herpes virus | Gene expression inhibitior | Kutluay, Doroghazi, Roemer, & Triezenberg, 2008 |
| 3 | Hepatitis B virus |
Replication inhibitor cccDNA inhibitor |
Rechtman et al., 2010 Wei et al., 2017 |
| 4 | Hepatitis C virus | Entry inhibitor | Anggakusuma et al., 2014 |
| 5 | Human immunodeficiency virus |
Protease inhibitor Integrase inhibitor Tat protein inhibitor |
Balasubramanyam et al., 2004; Ali and Banerjea, 2016 |
| 6 | Human papilloma virus | Gene expression inhibition |
Maher et al., 2011; Mishra et al., 2015 |
| 7 | Respiratory syncytial virus | Entry inhibitor replication and budding inhibition |
Yang, Li, & Huang, 2016; Yang, Li, Li, Wang, & Huang, 2017 |
| 8 | Chickun gunya virus |
Entry inhibitor |
Rhein et al., 2016 Mounce, Cesaro, Carrau, Vallet, & Vignuzzi, 2017 |
| 9 | Dengue virus |
Entry inhibitor Particle production Inhibition |
Chen et al., 2013 Padilla, Rodríguez, Gonzales, Gallego‐g, & Castaño‐o, 2014 |
| 10 | Zikavirus | Entry inhibitor | Mounce et al., 2017 |
| 11 | Influenza A virus | Inhibitor of virus uptake, replication and particle production |
Dai et al., 2018; Han, Xu, Guo, & Huang, 2018 |
2.2. Zingiber officinale (ginger)
Ginger is one of the important medicinal plants which naturally occur in various countries. Ginger, Zingiber officinale, belongs to family Zingiberaceae and the other famous members of this plant family are turmeric, cardamom, and galangal. The plant is indigenous to Southeast Asia and is cultivated in several countries including India. Ginger (Zingiberofficinale) is known as Sunthi in Ayurveda and the description of the plant appears in the old text like Charaka, Sushruta, Vagbhatta, and Chakra‐dutta (Agrahari, Panda, Verma, Khan, & Darbari, 2015). Zanjabeel (Zingiberofficinale) is a famous herbal drug in the conventional Unani system of medicine (Bashir & Afrin, 2019).
Ginger is a rich source of bioactive compounds such as phenolic groups, alkaloids, and steroids, which have medicinal effect. The chief aromatic agent of the rhizome is the zingiberol with analogues such as the shogoals, paradol, and zingerone. In addition to the main bioactive compounds, ginger also contains other sub‐compounds such as 4‐gingerol, 6‐gingerol, 8‐gingerol, 10‐gingerols, 6‐shogaols, and 14‐shogaols (Ali, Blunde, Tanira, & Nemmar, 2008; US Report, 2013). They are reported to demonstrate antiemetic, antipyretic, analgesic, antiarthritic, and anti‐inflammatory activities.
It has been proven by many studies that the ginger and its bioactive compounds showed effective antiviral activity against SARS‐CoV‐2, Influenza virus, Herpes simplex virus, Human respiratory syncytial virus, Chikungunya virus, and so on as shown in Table 2 (Admas, 2020; Dorra et al., 2019; Imanishi et al., 2006; Sulochana et al., 2020). Antiviral activity of lyophilized juice extracted from Zingiber officinale has been studied on the hepatitis C virus at varying concentrations from 5–200 μg/mL. They found that 100 μg/mL dose was effective, which inhibits virus replication that was monitored by amplification of viral RNA segments (Wahab, Adawi, & Demellawy, 2009).
TABLE 2.
Spices and herbs and their derivatives showing antiviral properties
| Plant parts, extracts and compounds | Virus | Mechanism of action | Reference |
|---|---|---|---|
| Ginger | |||
| ZingiberofficinaleRosc (ZOR) induced conditioned medium | Influenza A/Aichi/2/68 (Aichi) virus | Via macrophage activation leading to production of TNF‐α. | Imanishi et al., 2006 |
| Ginger essential oil | Herpes simplex virus | Disrupts virus envelope | Schnitzler, Koch, & Reichling, 2007 |
| Aquatic extract of fresh ginger | Human respiratory syncytial virus | Blocking viral attachment and stimulate mucosal cells to secrete IFN‐β | Chang, Wanga, Yeh, Shieh, & Chiang, 2013 |
| Hydroethanolic extract of ginger | Influenza virus | — | Dorra, EL‐Barrawy, Sallam, & Mahmoud, 2019 |
| Aquatic extract of ginger | Chikungunya virus | Inhibition of cytopathic effect and cell viability | Sulochana, Jangra, Kundu, Yadav, & Kaushik, 2020 |
| Bioactive compounds of ginger (gingerol, geraniol,shogaol, zingiberene, zingiberenol, zingerone) | SARS‐CoV‐2 | Block the S protein from bindingto the ACE2 receptor or act as an inhibitor for MPro | Ahkam, Hermanto, Alamsyah, Aliyyah, & Fatchiyah, 2020 |
| Cinnamon | |||
| Procyanidins and butanol extract | SARS‐CoV | Interference of clathrin‐dependent endocytosis | Zhuanga et al., 2009 |
| Water extract | Human respiratory syncytial virus | Inhibition of viral attachment and internalization | Yeh, Chang, Wang, Shieh, & Chiang, 2013 |
| Silver nanoparticles of cinnamon bark | Avianinfluenza virus subtype H7N3 | Interaction with viral genome and cellular factors or pathways of host cells required for viral replication | Fatima, Zaidi, Amraiz, & Afzal, 2016 |
| Cinnamaldehyde | T2 bacteriophage | Inhibit the replication of T2 bacteriophage | Goldstein & Shumaker, 2019 |
| Clove | |||
| Eugeniin | Herpes simplex virus 1 and 2 | Inhibiting DNA polymerase | Kurokawa et al., 1998 |
| Influenza A virus | — | ||
| Eugenol | Inhibit viral replication and reducing infection | Reichling, Schnitzler, Suschke, & Saller, 2009 | |
| Clove extract | Feline calicivirus, a surrogate for human Norovirus | — | Aboubakr et al., 2016 |
| Black pepper | |||
| Amide alkaloid | Hepatitis B virus | Unclear | Hao et al., 2012 |
| Extract | Coxsackie virus type B3 | Cytopathic effect inhibition | Mair et al., 2016 |
| Piperine | Dengue virus | Inhibit Methyltransferase | Nag & Chowdhury, 2020 |
| Ebola virus | VP35 interferon inhibitory domain | ||
| Basil | |||
| Ursolic acid | Coxsackievirus | Infection and replication inhibitor | Chiang, Ng, Cheng, Chiang, & Lin, 2005 |
| Enterovirus 71 | |||
| Essential oil and monoterpenes (camphor and 1,8‐cineol) | Bovine viral diarrhoea virus | Viral particle inhibitor | Kubiça, Alves, Weiblen, & Lovato, 2014 |
| Crude extract and terpenoid | H9N2 virus | — | Ghoke et al., 2018 |
| Rosmarinic acid, Oleanolic acid, Ursolic acid and Methyl eugenol | SARS‐CoV‐2 | Main protease | Kumar, 2020 |
| Garlic | |||
| Sulfur constituents | Coxsackie virus species, herpes simplex virus types 1 and 2, influenza B | — | Tsai et al., 1985 |
| Ajoene, Allyl alcohol and diallyl disulfide | HIV | Inhibiting the integrin dependent processes | Tatarintsev et al., 1992 |
| Allicin | Common cold virus (rhinovirus) | Reaction with thiol groups of various enzymes, e.g., alcohol dehydrogenase | Ankri & Mirelman, 1999 |
| Allitridin | Cytomegalovirus | Treg amplification | Zhen et al., 2006 |
| Extract of garlic | Newcastle disease virus | Blocking of attachment of virus to the cell receptors | Harazem, Rahman, & Kenawy, 2019 |
| Neem | |||
| NIM‐76 | Polio virus | Inhibits viral multiplication | Sai Ram et al., 2000 |
| Aqueous extract | Dengue virus type‐2 | Inhibits viral multiplication | Parida, Upadhyay, Pandya, & Jana, 2002 |
| Bark extract | Herpes simplex virus type‐1 | Block HSV‐1 entry into cells | Tiwari, Darmani, Yue, & Shukla, 2009 |
| Water extracted polysaccharides | Bovine herpes virus type‐1 (BoHV‐1) | Inhibits virus adsorption to the cell | Saha et al., 2010 |
| 3‐Deacetyl‐3‐cinnamoyl azadirachtin | Hepatitis C virus (HCV) | Inhibitor against NS3/4A protease | Ashfaq, Jalil, & UlQamar, 2016 |
| Nimbaflavone, Rutin, and Hyperoside | Influenza virus | Interaction with nucleoprotein | Ahmad, Javed, Rao, & Husnain, 2016 |
| Chloroformic leaf extracts | Foot and mouth disease virus | — | Younus et al., 2016 |
| Bark extract | Newcastle disease virus (NDV) | — | Mahmood, Amir, Abbas, Aslam, & Rafique, 2018 |
| Azadirachtin | Hepatitis B virus | Interaction with HBV polymerase | Parvez et al., 2018 |
| Neem terpenoids | SARS‐CoV‐2 | Inhibitor of membrane and envelop | Borkotoky & Banerjee, 2020 |
| Giloy | |||
| Ethanol extract | HIV | HIV protease inhibitors | Rege & Chowdhary, 2014 |
| Silver nanoparticles | Chikungunya virus | Inhibition on cytopathic effect | Sharma et al., 2019 |
| Tinosponone | SARS‐CoV‐2 | Inhibitor of main protease (3CL pro) | Krupanidhi et al., 2020 |
| Tinocordiside | SARS‐CoV‐2 | Inhibitor of main protease | Shree et al., 2020 |
Ahkam et al. (2020) studied the potential of a few bioactive compounds, namely, gingerenone A, gingerol, geraniol, shogaol, zingiberene, zingiberenol, and zingerone from Ginger as anti‐SARS‐CoV‐2 for their interaction to spike and main protease (Mpro) protein based on molecular docking study. They found that the bioactive compounds of ginger block the spike (S) protein from binding to the ACE2 receptor or act as an inhibitor for MPro. The S protein is responsible for SARS‐CoV‐2 entry during the infection which binds with angiotensin‐converting enzyme 2 (ACE2) receptor from the host cell to generate an appropriate environment for viral replication (Walls et al., 2020). Main Protease (MPro) is accountable for processing the poly‐proteins pp1a and pp1ab during viral replication (Hilgenfeld, 2014).
2.3. Cinnamomum cassia (cinnanon)
Cinnamomum cassia is an aromatic tree species belonging to the Lauraceae family. Cinnamon has been prominently used in traditional Chinese, Indian, Persian, and Unani medicine for a long time. Cinnamon has been used as a popular spice by different countries around the world for thousands of years. Cinnamon is obtained from the bark of its young branches which is widely used all around the world as a daily condiment. It can be also used as a material for medical products and has high economic value. It is used for several conditions such as; flatulence, amenorrhea, diarrhea, toothache, fever, leukorrhea, common cold, and headache. It has also been reported that the regular use of cinnamon averts throat infections (Hajimonfarednejad et al., 2018).
Ojagh, Rezaei, Razavi, and Hosseini (2012) reported that the bark of cinnamon contains 21 chemical compounds, which include cinnamaldehyde (60.41%) and eugenol (3.19%), which have an antibacterial effect. Several scientific studies have shown the antimicrobial, antiviral, antifungal, antioxidant, antihypertensive, antidiabetic, antitumor, gastroprotective, and immunomodulatory effects of cinnamon (Shen et al., 2012). According to a study, a higher dose of cinnamon (100 mg/kg) drastically increased the phagocytic index, serum immunoglobulin levels, and antibody titer, while its low dose (10 mg/kg) improved serum immunoglobulin levels only. So, the higher dose increases both cell‐mediated and humoral immunity, whereas the low dose showed an effect only on humoral immunity (Niphade, Asad, Chandrakala, Toppo, & Deshmukh, 2009).
Moshaverinia, Rastegarfar, Moattari, and Lavaee (2020) studied the effect of hydro alcoholic extract of cinnamon on herpes simplex virus‐1. They found that the hydroalcoholic extract of cinnamon was effective in reducing the viral titer of HSV‐1 by prevention of viral attachment to cells.
2.4. Syzygium aromaticum (clove)
Clove (Syzygiumaromaticum), belonging to the family Myrtaceae, is globally used in medicine as an antiseptic against contagious diseases due to the antimicrobial activities against oral bacteria. Clove is also used in the food industry due to its antimicrobial activities for increasing shelf‐life. FDA has confirmed the safety of clove buds, clove oil, eugenol, and oleoresins as a food supplement (Vijayasteltar, Nair, Maliakel, Kuttan, & Krishnakumar, 2016). The WHO has given the acceptable daily uptake of clove in humans is 2.5 mg/kg body weight (Ogunwande et al., 2005).
Clove has main phenolic compounds such as flavonoids, hidroxicinamic acids, hidroxibenzoic acids, and hidroxiphenylpropens. The main bioactive component of clove is eugenol (Neveu et al., 2010). Eugenol exhibits broad antimicrobial activities against both Gram‐positive, Gram‐negative, and acid‐fact bacteria, as well as fungi. Cloves are well known also for their antiemetic (relieves nausea and vomiting) and carminative properties. Eugeniin, a compound isolated from the herbal extracts of S. aromaticum, and Geum japonicum, was identified as anti‐Herpes Simplex Virus compound at 5 μg/mL concentration. The inhibitory action of eugeniin is on the viral DNA synthesis by acting as a selective inhibitor of the HSV‐1 DNA polymerase and eugenol on viral replication and reducing infection (Kurokawa et al., 1998; Reichling et al., 2009).
2.5. Piper nigrum (black pepper)
Piper is a member of family Piperaceae and famous as the king of spices due to its pungent smell. Black pepper is grown in many tropical regions like Brazil, Indonesia, and India. Piper nigrum has significant biological properties and its bioactive compounds are used medicine, preservative, and perfumery. Piperine, a dynamic alkaloid of black pepper, is widely used in the as conventional system of medicine (Ayurveda, Siddha, Unani, and Tibetan). It contains major pungent alkaloid piperine (1‐peperoyl piperidine) which is known to possess many interesting pharmacological properties such as antihypertensive, anti‐Alzheimer's, antidepressant, antiplatelets, anti‐inflammatory, antioxidant, antipyretic, antitumor, antiasthmatic, analgesic, antimicrobial, and so on (Damanhouri & Ahmad, 2014; Jafri et al., 2019; Tiwari, Mahadik, & Gabhe, 2020; Yoo et al., 2019).
Priya and Saravana (2017) evaluated the antiviral activity of Piper nigrumin chloroform and methanolic extracts against vesicular stomatitis virus (an enteric virus) and human parainfluenza virus on human cell lines. They found that the anti‐viral activity of Piper nigrum is higher in chloroform extract due to the presence of higher content ofalkaloids. According to molecular docking based study, it has been found that piperine could inhibit methyltransferase of Dengue virus and VP35 interferon inhibitory domain of Ebola virus comparative to commercial antiviral Ribavirin (Nag & Chowdhury, 2020). Rajagopal, Byran, Jupudi, and Vadivelan (2020) in a docking based study reported that the bioactive compounds from black pepper such as piperdardiine and piperanine are considerably active against COVID‐19, which can be further used for its treatment.
2.6. Ocimum basilicum L. (basil)
Ocimum basilicum L. (OB) is a popular medicinal herb of the family Labiatae which is also known as Sweet basil. The essential oils of these plant materials have been used extensively in food, perfumery, dental and oral products for many years. Basil is a natural spice that possesses antimicrobial activities as many studies have reported. The essential oils of OB have been reported to show activity against a wide range of bacteria, fungi, and parasites. The different components of OB are used as remedies for treating disorders such as viral ocular, respiratory, and hepatic infections. Ocimum basilicum has been reported to contain several of interesting compounds, such as monoterpenoids (carvone, cineole, fenchone, geraniol, linalool, myrcene, and thujone), sesquiterpenoids (caryophyllene and farnesol), triterpenoid (ursolic acid), and flavonoid (apigenin) (Chiang et al., 2005).
Numerous studies showed that the aqueous and methanol extract of leaf and seed oil of basil enhances immune response by increasing T‐helper and natural killer cells, lymphocyte count, phagocytic activity, neutrophil count, antibody titer, and so on against the variety of infection as a defense mechanism (Jamshidi & Cohen, 2017; Pattanayak, Behera, Das, & Panda, 2010; Vasudevan, Kashyap, & Sharma, 1999).
Ursolic acid has been reported to inhibit viral infections of herpes simplex virus (HSV)‐1 and human immunodeficiency virus (HIV), as well as tumor growth (Nonotny, Vachalkova, & Biggs, 2001). Extracts and selected purified components of OB showed a broad spectrum of anti‐DNA and RNA virus activities also. Three phytochemical compounds of tulsi, namely, vicenin, sorientin 4'‐O‐glucoside 2”‐O‐p‐hydroxy‐benzoagte, and ursolic acid showed inhibition of main protease of SARS‐CoV‐2 in a molecular docking study (Shree et al., 2020).
2.7. Allium sativum L. (garlic)
Allium sativum L. (Garlic) family Liliaceae is originally from Asia but it is also cultivated in other countries, namely, China, North Africa (Egypt), Europe, and Mexico. It has been used as a medicinal agent from thousands of years. This plant is a bulb growing to 25–70 cm with flowers used as a spice and flavoring agent for foods. Garlic is having high nutritive value, improves taste of food, and also helps indigestion. Garlic is having a wide range of pharmacological effects with low toxicity such as anthelmintics, anti‐inflammatory, antioxidant, antifungal, and so on (Alam, Hoq, & Uddin, 2016).
Allicin (diallyl‐dithiosulfinate), which is produced by the garlic enzyme alliinase from the alliin, has been known for wide‐antifungal and antiviral activities. The decreasing order of the compounds having virucidal activity in garlic was ajoene, allicin, allyl methyl thiosulfanate, and methyl allyl thiosulfanate (Gebreyohannes & Gebreyohannes, 2013). Antiviral activity of garlic extract has been studied against influenza virus A/H1N1 in cell culture and it was found that it inhibits the virus penetration and proliferation in cell culture (Mehrbod, Amini, & Tavassoti‐Kheiri, 2009). The garlic extract showed inhibitory activity on infectious bronchitis virus (IBV‐a coronavirus) in the chicken embryo (Shojai, Langeroudi, Karimi, Barin, & Sadri, 2016).
2.8. Azadirachta indica (neem)
The neem tree botanically referred to as Azadirachta indica is a fast‐growing evergreen herb belonging to the family Meliaceae. The Indian origin traditional medicinal plant neem has been used to treat several acute and chronic diseases in different parts of Asia and Africa from the ancient period. All parts of the neem tree such as seeds, roots, leaves, flowers, and bark have been used in traditional medicine as household remedies against various human ailments. They exhibit insecticidal, antimicrobial, larvicidal, antimalarial, antibacterial, antiviral, and spermicidal effects (Gupta et al., 2013).
Different terpenoids isolated from the bark of this herb include nimbin, nimbidin, nimbolide, limonoids, β‐sistosterol, 6‐desacetylnimbinene, nimbione, margocin, quercetin, and so on (Alzohairy, 2016). A compound from the extract of neem leaves called “hyperoside” possesses showed potential as a universal drug against influenza strains due to its free radical scavenging property. Hyperoside compound from neem leaf extract along with the chemical drugs LGH, Naproxen, BMS‐885838, and BMS‐883559 showed best results with conserved residues of nucleoprotein of influenza virus (Ahmad et al., 2016). The neem is an extraordinary plant and United Nations has declared neem as the “tree of the 21st century” (United Nations Environment Programme, 2012).
Due to its already proven antiviral properties and effectiveness, many scientists have started research on neem for discovering drugs against SARS‐COV‐2. Natural bioactive compounds, namely, methyl eugenol, oleanolic acid, and ursolic acid extracted from tulsi and neem act as inhibitors against SARS‐CoV‐2. These bioactive compounds function as effective inhibitors of SARS‐CoV‐2 by binding to the spike glycoprotein, RNA polymerase, and/or its protease which results in the prevention of both viral attachment and replication (Kumar, 2020). Approximately 20 compounds isolated from neem leaves extract showed high binding affinity against COVID‐19 main protease protein which is the key protein for viral replication (Subramanian, 2020). Muralikumar, Ramakrishnamacharya, and Seshachalam (2020) screened ligands from Nimba and Amrita (A. indica and T. cordifolia) known as Nimbamritam in silico to evaluate anti‐SARS‐CoV‐2 activity. They found that the ligand interacted and inhibited the residues of spike protease or Mpro protease of SARS‐CoV‐2.
2.9. Tinospora cordifolia (giloy)
Tinospora cordifolia (giloy) is a member of the family of Menispermaceae and is usually found in Asian counties like India, Sri Lanka, Myanmar, and China. It is a medicinal plant native to India commonly called Guduchiand used in Ayurvedic formulations as a medicine to treat several diseases. Due to its medicinal importance, T. cordifolia has been highly exploited for commercial purposes and used as an effective medicine for therapies against several diseases such as jaundice, urinary disorder, skin diseases, diabetes, anemia, inflammation, allergic condition, and so on (Kumar, 2020; Sonkamble & Kamble, 2015). Different parts of T. cordifolia, such as leaves, stem, root, flower, seed, and so on, have all the above mentioned pharmacological activities. This plant is also used in Ayurvedic “Rasayanas” to improve the immune system and the body's resistance against infections.
Pruthvish and Gopinatha (2018) reported that the crude extract of dry stem of T. cordifolia showed antiviral activity against herpes simplex virus which was evaluated by MTT assay. Chowdhury (2020) evaluated the five phytoconstituents of T. cordifolia (giloy), namely, berberine, b‐sitosterol, coline, tetrahydropalmatine, and octacosanol using molecular dynamics approach. She found that berberine can regulate 3CLpro protein's function by inhibition and subsequently control viral replication. Tinocordiside, one of the phytochemicals of giloy, showed inhibition of main protease of SARS‐CoV‐2 in a molecular docking study (Shree et al., 2020). Berberine, Isocolumbin, Magnoflorine, and Tinocordiside compounds isolated from Giloy showed high binding efficacy against all the four key SARS‐CoV‐2 target surface glycoprotein (6VSB), receptor‐binding domain (6M0J), RNA dependent RNA polymerase (6M71), and main protease (6Y84) involved in virus attachment and replication (Sagar & Kumar, 2020).
3. METHODOLOGY
A questionnaire based online survey has been conducted on home remedies during COVID‐19 among people (n‐531) of different age groups varying from 13–68 years from countries namely India, United Kingdom, and United States. This survey has covered 17 states (Maharashtra, Tamil Nadu, Rajasthan, Arunachal Pradesh, Gujarat, Uttar Pradesh, Madhya Pradesh, Bihar, Himachal Pradesh, Haryana, Telangana, Assam, Kerala, Punjab, Uttarakhand, Chhatisgarh, and Manipur) and two Union territories of India (Delhi and Chandigarh) which include overall 124 cities.
4. RESULTS
Out of 531 people who have participated in the survey, 26.6% of people were tested for COVID‐19 in which 7.8% of people were found positive as shown in Figure 2a. In the survey, we found that people are boosting their immunity in various ways apart from using sanitizers and wearing masks. Most people (93.6%) think that Indian spices and home remedies are helpful in the treatment of coronavirus or other viral infection as well as boosting immunity.
FIGURE 2.
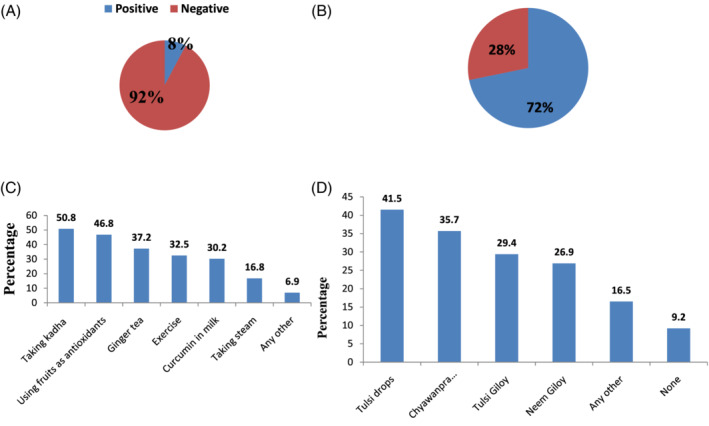
Survey Analysis on home remedies during COVID‐19. (a) Coronavirus positive cases. (b) Percentage of people taking kadha. (c) Methods of boosting immunity. (d) Natural immunity boosting products [Colour figure can be viewed at wileyonlinelibrary.com]
According to the survey, 71.8% of people are taking kadha (basil, cinnamon, black pepper, ginger, and raisin) prescribed by Ayush Ministry, India as shown in Figure 2b. Many people (52.4%) are taking kadha only one time in a day while 24.1% of people are taking kadha two times in a day. People (68.8%) are using ginger, clove, dalchini, black pepper, and tulsi in their kadha. Mostly people (86.1%) think that there is no side effect of kadha, while 13.9% think and experience the side effects of kadha, that is, acidity in the stomach, heartburn, constipation, diarrhea ulcers in mouth, and high blood pressure (especially in senior citizens). According to Ayurveda, if we take kadha in excess, then it can create problems; otherwise there are no side effects.
People (83.1%) are boosting immunity by taking Amla/lemon or other fruits as a rich source of vitamin C. Vitamin C is an antioxidant that serves as an enzyme cofactor for various biochemical and physiological processes in humans (Ngo, Ripper, Cantley, & Yun, 2019). Mostly people are using tulsi drops, chyawanprash, tulsigiloy, and neemgiloy (41.5, 35.7, 29.4, and 26.9%, respectively) for boosting their immunity. Chyawanprash is an Indian Ayurvedic health supplement that is prepared by approximately 50 herbs and spices showing immunity boosting as well as antioxidant properties (Sharma et al., 2019).
5. DISCUSSION
The coronavirus disease is highly transmittable with no effective antiviral therapy to combat the infection (Guan et al., 2020). However, in our study, we highlighted the role of spices and herbs in the treatment of COVID‐19. The survey has been conducted to identify the various home remedies used during COVID‐19, which include many spices and herbs.
As per our survey data, most people are taking kadha only one time a day and they are using ginger, clove, cinnamon, black pepper, and tulsi as main ingredients in kadha. We have analyzed that cinnamon, black pepper, tulsi, and turmeric play vital role against SARS‐CoV‐2 (COVID‐19) as well as other viral infections, which was also supported by some other recent studies mentioned in Tables 1 and 2. Our findings were also well supported by Rastogi, Pandey, and Singh (2020), who proposed the use of Tinospora cordifolia (Giloy), Zingiber officinale (Ginger), Curcuma longa (Curcumin), and Ocimum sanctum (Tulsi) due to their antiviral property. Shrivastava (2020) reported that tulsi leaves increase the level of helper T cells as well as natural killer cells, which helps fight against viral infection. Tulsi is being used for curing pain, pneumonia, diarrhea, cough, and fever of ancient times, which are the common symptoms of COVID‐19 (Goothy et al., 2020). Black pepper provides relaxation from sinusitis and nasal congestion, which are the most common symptoms of COVID‐19 (Pathak & Khandelwal, 2007). Quercetin, a flavonoid present in black pepper, improves the body's immunity constantly due to its antiviral properties (Yao et al., 2017). Our findings were also well supported by Rajagopal et al. (2020) who recommended the consumption of black pepper and ginger in a daily diet, as it may be helpful in the prevention of coronavirus. According to our survey, people (83.1%) are boosting immunity by taking Amla/lemon or other fruits as a rich source of vitamin C for boosting their immunity. Arandomized controlled trial to carry out in the USA in 167 patients with sepsis‐related ARDS indicated that uptake of ~15 g/day of vitamin C for 4 days may decrease mortality in these patients (Flower et al., 2020). A randomized, controlled clinical trial was also performed on patients with confirmed SARS‐CoV‐2 infection in the ICU at three hospitals in Hubei, China. They have given high‐dose intravenous vitamin C, that is, 12 g of vitamin C/50 mL every 12 h for 7 days and found that the high‐dose intravenous vitamin C may provide a defensive effect without any side effects in critically COVID‐19 patients (Zhang et al., 2020). Utomo et al. (2020) reported that the Citrus sp. exhibits the best prospective as an inhibitor to the development of the SARS‐CoV‐2.
According to ASSOCHAM, India dipstick study spices export from India went up by 23% during COVID‐19 (June 2020) compared with the same month of 2019. Major Indian spices that are transported abroad include pepper, ginger, turmeric, coriander, cumin, fennel, fenugreek, nutmeg, spice oils cardamom, and mint products. The main countries where the spices are being imported include the United States, United Kingdom, Germany, France, Italy, Canada, Australia, UAE, Iran, Singapore, China, and Bangladesh, which shows that the world is benefitted by the magical spices of India.
6. CONCLUSIONS
In the current pandemic scenario, precautions and boosting immunity are one of the best choices to get away from COVID‐19 infection. As per our study, we conclude that the uses of spices and herbs may play a significant role against viral infections. We have analyzed that cinnamon, black pepper, basil, and turmeric play a vital role against SARS‐CoV‐2 (COVID‐19) as well as other viral infections, which was also supported by some other recent studies. In India, people are using spices as well as herbs from ancient times due to their taste, antiviral, antimicrobial, antioxidant, and immunity‐boosting properties. Since ages Indians are habitual of taking these natural products that have conferred immunity in the Indian population, which probably is the major cause for low mortality in India. However, the excessive use of spices and herbs may cause various side effects, namely, acidity in the stomach, heartburn, constipation, diarrhea ulcers in the mouth, high blood pressure, and so on. Therefore, detailed studies about the bioactive compounds present in common Indian herb and spices and their effectiveness and mode of action against lethal viruses need to be explored.
CONFLICT OF INTEREST
The authors do not have a conflict of interest.
AUTHOR CONTRIBUTIONS
Dr. Namita Ashish Singh conceived, conducted, and analyzed the questionnaire results of the survey. Dr. Pradeep Kumar was involved in questionnaire analysis, review and editing. The original draft was written by Jyoti, while Dr. Naresh Kumar was involved in the review and editing of the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the higher authorities of respective university and institutions for the support in conducting and writing this manuscript. The authors are also grateful to the people who have participated in the COVID‐19 questionnaire survey.
Singh NA, Kumar P, Jyoti, Kumar N. Spices and herbs: Potential antiviral preventives and immunity boosters during COVID‐19. Phytotherapy Research. 2021;35:2745–2757. 10.1002/ptr.7019
REFRENCES
- Aboubakr, H. A. , Nauertz, A. , Luong, N. T. , Agarwal, S. , El‐Sohaimy, S. , Youssef, M. M. , & Goyal, S. M. (2016). In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. Journal of Food Protection, 79(6), 1001–1012. 10.4315/0362-028X.JFP-15-593 [DOI] [PubMed] [Google Scholar]
- Admas, C. (2020). Ginger fights multiple viral infections. The Journal of Plant Medicines https://plantmedicines.org/ginger-fights-multiple-virus-infections [Google Scholar]
- Agrahari, P. , Panda, P. , Verma, N. K. , Khan, W. U. , & Darbari, S. (2015). A brief study on Zingiber officinale‐A review. Journal of Drug Discovery and Therapeutics, 3(28), 20–27. [Google Scholar]
- Ahkam, A. H. , Hermanto, F. E. , Alamsyah, A. , Aliyyah, I. H. , & Fatchiyah, F. (2020). Virtual prediction of antiviral potential of ginger (Zingiber officinale) bioactive compounds against spike and MPro of SARS‐CoV2 protein. Journal of Biological Researches, 25(2), 52–57. [Google Scholar]
- Ahmad, A. , Javed, M. R. , Rao, A. Q. , & Husnain, T. (2016). Designing and screening of universal drug from neem (Azadirachta indica) and standard drug chemicals against influenza virus nucleoprotein. BMC Complementary and Alternative Medicine, 16, 519. 10.1186/s12906-016-1,469-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, K. , Hoq, O. , & Uddin, S. (2016). Medicinal plant Allium sativum‐A review. Journal of Medicinal Plants Studies, 4(6), 72–79. [Google Scholar]
- Ali, B. H. , Blunde, G. , Tanira, M. O. , & Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food and Chemical Toxicology, 46, 409–420. 10.1016/j.fct.2007.09.085 [DOI] [PubMed] [Google Scholar]
- Ali, A. , Banerjea, A. C. (2016). Curcumin inhibits HIV‐1 by promoting Tat protein degradation. Scientific Reports, 6(1). 10.1038/srep27539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzohairy, M. A. (2016). Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence‐Based Complementary and Alternative Medicine, 2016, 7382506. 10.1155/2016/7382506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggakusuma, C. C. C. , Schang, L. M. , Rachmawati, H. , Frentzen, A. , Pfaender, S. , Behrendt, P. , … Steinmann, E. (2014). Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut, 63, 1137–1149. 10.1136/gutjnl-2012-304299 [DOI] [PubMed] [Google Scholar]
- Ankri, S. , & Mirelman, D. (1999). Antimicrobial properties of allicin from garlic. Microbes and Infection, 1(2), 125–129. 10.1016/s1286-4579(99)80003-3 [DOI] [PubMed] [Google Scholar]
- Ashfaq, U. A. , Jalil, A. , & UlQamar, M. T. (2016). Antiviral phytochemicals identification from Azadirachta indica leaves against HCV NS3 protease: An in silico approach. Natural Product Research, 30(16), 1866–1869. 10.1080/14786419.2015.1075527 [DOI] [PubMed] [Google Scholar]
- Balasubramanyam, K. , Varier, R. A. , Altaf, M. , Swaminathan, V. , Siddappa, N. B. , Ranga, U. , & Kundu, T. K. (2004). Curcumin, a novel p300/CREB‐binding protein‐specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase‐dependent chromatin transcription. Journal of Biological Chemistry, 279, 51163–51171. 10.1074/jbc.M409024200 [DOI] [PubMed] [Google Scholar]
- Bashir, F. , & Afrin, Z. (2019). Zanjabeel (Zingiber offcinale) transformation of culinary spice to amulti‐functional medicine. Journal of Drug Delivery and Therapeutics, 9, 721–725. [Google Scholar]
- Borkotoky, S. , & Banerjee, M. (2020). A computational prediction of SARS‐CoV‐2 structural protein inhibitors from Azadirachta indica (Neem). Journal of Biomolecular Structure and Dynamics, 1–17. 10.1080/07391102.2020.1774419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafekar, A. , & Fielding, B. C. (2018). MERS‐CoV: Understanding the latest human coronavirus threat. Viruses, 10(2), 93. 10.3390/v10020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. S. , Wanga, K. C. , Yeh, C. F. , Shieh, D. E. , & Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti‐viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology, 145, 146–151. [DOI] [PubMed] [Google Scholar]
- Chen, T. Y. , Chen, D. Y. , Wen, H. W. , Ou, J. L. , Chiou, S. S. , Chen, J. M. , … Hsu, W. L. (2013). Inhibition of enveloped viruses infectivity by curcumin. PLoS One, 8, e62482. 10.1371/journal.pone.0062482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, L. C. , Ng, L. , Cheng, P. , Chiang, W. , & Lin, C. (2005). Antiviral activities of extracts and selected pure constituents of Ocimum basilicum . Clinical and Experimental Pharmacology and Physiology, 32, 811–816. 10.1111/j.1440-1681.2005.04270.x [DOI] [PubMed] [Google Scholar]
- Chowdhury, P. (2020). Insilico investigation of phytoconstituents from Indian medicinal herb “Tinosporacordifolia (giloy)” against SARS‐CoV‐2 (COVID‐19) by molecular dynamics approach. Journal of Biomolecular Structure and Dynamics, 1–18. 10.1080/07391102.2020.1803968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J. , Gu, L. , Su, Y. , Wang, Q. , Zhao, Y. , Chen, X. , … Li, K. (2018). Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF‐κB pathways. International Immunopharmacology, 54, 177–187. 10.1016/j.intimp.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Damanhouri, Z. A. , & Ahmad, A. (2014). A review on therapeutic potential of Piper nigrum L. (black pepper): The king of spices. Medicial Aromatic Plants, 3, 161. 10.4172/2167-0412.1000161 [DOI] [Google Scholar]
- Deng, S. Q. , & Peng, H. J. (2020). Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. Journal of Clinical Medicine, 9, 575. 10.3390/jcm9020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev, S . (1997). Ethnotherapeutic and modern drug development: The potential of Ayurveda. Current Science, 73(11), 909–928. [Google Scholar]
- Devi, S. A. , Umasanker, S. , & Babu, M. E. (2012). A comparative study of antioxidant properties in common Indian spices. International Research Journal of Pharmacy, 3, 465–468. [Google Scholar]
- Dorra, N. H. , EL‐Barrawy, M. A. , Sallam, S. M. , & Mahmoud, R. S. (2019). Evaluation of antiviral and antioxidant activity of selected herbal extracts. Journal of High Institute of Public Health, 49(1), 36–40. [Google Scholar]
- Fatima, M. , Zaidi, N. S. , Amraiz, D. , & Afzal, F. (2016). In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 Influenza A Virus. Journal of Microbiology and Biotechnology, 26(1), 151–159. 10.4014/jmb.1508.08024 [DOI] [PubMed] [Google Scholar]
- Fowler, A. A. , Truwit, J. D. , Hite, R. D. , Morris, P. E. , DeWilde, C. , Priday, A. , … Halquist, M. (2019). Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure. JAMA, 322(13). 1261. 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garba, S. , & Mungadi, H. U. (2019). Quantitative chemical compositions of neem (Azadirachta indica) leaf aqueous extracts in Sokoto, Nigeria. International Journal of Research and Scientific Innovation, 6(7), 2,321–2,705. [Google Scholar]
- Gebreyohannes, G. , & Gebreyohannes, M. (2013). Medicinal values of garlic: A review. International Journal of Medicine and Medical Sciences, 5(9), 401–408. 10.5897/IJMMS2013.0960 [DOI] [Google Scholar]
- Ghoke, S. S. , Sood, R. , Kumar, N. , Pateriya, A. K. , Bhatia, S. , Mishra, A. , … Singh, V. P. (2018). Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complementary and Alternative Medicine, 18, 174. 10.1186/s12906-018-2238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, G. , & Shumaker, A. G. (2019). Cinnamon extract and cinnamaldehyde inhibit the replication of t2 bacteriophage in E. coli: Potential for use in antiviral and anticancer therapy. Research & Reviews: A Journal of Biotechnology, 9(3), 8–17. [Google Scholar]
- Goothy, S. S. K. , Goothy, S. , Choudhary, A. , Potey, G. G. , Chakraborty, H. , Kumar, A. H. S. , & Mahadik, V. K. (2020). Ayurveda's holistic lifestyle approach for the management of coronavirus disease (COVID‐19): Possible role of tulsi. International Journal of Research in Pharmaceutical Sciences, 11(SPL1), 16–18. 10.26452/ijrps.v11iSPL1.1976 [DOI] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. C. , Patchva, S. , & Aggarwal, B. B. (2013). Therapeutic roles of curcumin: Lessons learned from clinical trials. The American Association of Pharmaceutical Scientists Journal, 15(1), 195–218. 10.1208/s12248-012-9,432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan, S. , Qasim, M. , Choi, Y. , Do, J. T. , Park, C. , Hong, K. , … Song, H. (2020). Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses. Nanomaterials, 10, 1645. 10.3390/nano10091645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajimonfarednejad, M. , Ostovar, M. , Raee, M. J. , Hashempur, M. H. , Mayer, J. G. , & Heydari, M. (2018). Cinnamon: A systematic review of adverse events. Clinical Nutrition, 38, 594–602. 10.1016/j.clnu.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Han, S. , Xu, J. , Guo, X. , & Huang, M. (2018). Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clinical and Experimental Pharmacology and Physiology, 45, 84–93. 10.1111/1440-1681.12848 [DOI] [PubMed] [Google Scholar]
- Hao, B. J. , Wu, Y. H. , Wang, J. G. , Hu, S. Q. , Keil, D. J. , Hu, H. J. , … Zhao, Y. (2012). Hepatoprotective and antiviral properties of isochlorogenic acid A from Laggeraalata against hepatitis B virus infection. Journal of Ethnopharmacology, 144, 190–194. [DOI] [PubMed] [Google Scholar]
- Harazem, R. , Rahman, S. A. , & Kenawy, A. (2019). Evaluation of antiviral activity of Allium cepa and Allium sativum extracts against Newcastle disease virus. Alexandria Journal of Veterinary Sciences, 61(1), 108. 10.5455/ajvs.29663 [DOI] [Google Scholar]
- Hilgenfeld, R. (2014). From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. The FEBS Journal, 281(18), 4085–4096. 10.1111/febs.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Gu, X. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi, N. , Andoh, T. , Mantani, N. , Sakai, S. , Terasawa, K. , Shimada, Y. , … Ochiai, H. (2006). Macrophage‐mediated inhibitory effect of Zingiber officinale rosc, a traditional oriental herbal medicine, on the growth of Influenza A/Aichi/2/68 Virus. The American Journal of Chinese Medicine, 34(01), 157–169. 10.1142/S0192415X06003722 [DOI] [PubMed] [Google Scholar]
- Islam, M. T. , Sarkar, C. , El‐Kersh, D. M. , Jamaddar, S. , Uddin, S. J. , Shilpi, J. A. , & Mubarak, M. S. (2020). Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytotherapy Research, 34, 2471–2492. 10.1002/ptr.6700 [DOI] [PubMed] [Google Scholar]
- Jafri, A. , Siddiqui, S. , Rais, J. , Ahmad, M. S. , Kumar, S. , Jafar, T. , … Arshad, M. (2019). Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase‐3 activation. EXCLI Journal, 18, 154–164. 10.17179/excli2018-1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi, N. , & Cohen, M. M. (2017). The clinical efficacy and safety of Tulsi in humans: A systematic review of the literature. Evidence‐Based Complementary and Alternative Medicine, 13, 9217567. 10.1155/2017/9217567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe, B. , Vijaykumar, M. , & Lokesh, B. R. (2004). Biological properties of curcumin‐cellular and molecular mechanisms of action. Critical Reviews in Food Science and Nutrition, 44(2), 97–111. 10.1080/10408690490424702 [DOI] [PubMed] [Google Scholar]
- Kannan, S. , Ali, P. S. S. , Sheeza, A. , & Hemalatha, K. (2020). COVID‐19 (novel coronavirus 2019)–recent trends. European Review for Medical and Pharmacological Sciences, 24, 2006–2011. [DOI] [PubMed] [Google Scholar]
- Krupanidhi, S. , Peele, K. A. , Venkateswarulu, T. C. , Ayyagari, V. S. , Bobby, M. N. , Babu, D. J. , … Aishwarya, G. (2020). Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS‐CoV‐2: An in silico study. Journal of Biomolecular Structure and Dynamics, 1–5. 10.1080/07391102.2020.1787226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiça, T. F. , Alves, S. H. , Weiblen, R. , & Lovato, L. T. (2014). In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Brazilian Journal of Microbiology, 45(1), 209–214. 10.1590/S1517-83822014005000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. (2020). Molecular docking of natural compounds from Tulsi (Ocimum sanctum) and neem (Azadirachta indica) against SARS‐CoV‐2 protein targets . Preprints. 10.21203/rs.3.rs-27151/v1. [DOI]
- Kurokawa, M. , Hozumi, T. , Basnet, P. , Nakano, M. , Kadota, S. , Namba, T. , … Shiraki,K. (1998). Purification and characterization of eugeniin as an antiherpesvirus compound from Geum japonicum and Syzygium aromaticum . The Journal of Pharmacology and Experimental Therapeutics, 284(2), 728–735. [PubMed] [Google Scholar]
- Kutluay, S. B. , Doroghazi, J. , Roemer, M. E. , & Triezenberg, S. J. (2008). Curcumin inhibits herpes simplex virus immediate‐early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology, 373, 239–247. 10.1016/j.virol.2007.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan, N. J. , & Dubovi, E. J. (2017). Coronaviridae In: Fenner's Veterinary Virology, 5th Edition, Academic Press, USA pp.435–461. ISBN 978‐0‐12‐800946‐8 10.1016/B978-0-12-800946-8.00024-6 [DOI] [Google Scholar]
- Maher, D. M. , Bell, M. C. , O'donnell, E. A. , Gupta, B. K. , Jaggi, M. , & Chauhan, S. C. (2011). Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzoapyrene‐induced upregulation of HPV E7. Molecular Carcinogenesis, 50, 47–57. 10.1002/mc.20695 [DOI] [PubMed] [Google Scholar]
- Mahmood, M. S. , Amir, H. W. , Abbas, R. Z. , Aslam, B. , & Rafique, A. (2018). Evaluation of antiviral activity of Azadirachta indica (Neem) bark extract against Newcastle disease virus. Pakistan Veterinary Journal, 38(1), 25–28. 10.29261/pakvetj/2018.005 [DOI] [Google Scholar]
- Mair, C. E. , Liu, R. , Atanasov, A. G. , Schmidtke, M. , Dirsch, V. M. , & Rollinger, J. M. (2016). Antiviral and anti‐proliferative in vitro activities of piperamides from black pepper. Planta Medica, 82, P807. 10.1055/s-0036-1596830 [DOI] [Google Scholar]
- Mehrbod, P. , Amini, E. , & Tavassoti‐Kheiri, M. (2009). Antiviral activity of garlic extract on influenza virus. Iranian Journal of Virology, 3(1), 19–23. [Google Scholar]
- Mishra, A. , Kumar, R. , Tyagi, A. , Kohaar, I. , Hedau, S. , Bharti, A. C. , … Das, B. (2015). Curcumin modulates cellular AP‐1, NF‐kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience, 9, 525. 10.3332/ecancer.2015.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshaverinia, M. , Rastegarfar, M. , Moattari, A. , & Lavaee, F. (2020). Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus‐1. Dental Research Journal (Isfahan), 17(2), 114–119. [PMC free article] [PubMed] [Google Scholar]
- Mounce, B. C. , Cesaro, T. , Carrau, L. , Vallet, T. , & Vignuzzi, M. (2017). Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Research, 142, 148–157. 10.1016/j.antiviral.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Muralikumar, V. , Ramakrishnamacharya, C. , & Seshachalam, C. (2020). Inhibitory effect of phytochemicals from Azadirachta indica a juss. and Tinospora cordifolia (thunb.) miers against SARS‐COV‐2 mpro and spike protease—An in silico analysis. International Journal of Engineering Applied Sciences and Technology, 5(3), 303–319. [Google Scholar]
- Nag, A. , & Chowdhury, R. R. (2020). Piperine, an alkaloid of black pepper seeds can effectively inhibit the antiviral enzymes of Dengue and Ebola viruses, an in silico molecular docking study. Virus Disease, 31(3), 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu, V. , Perez‐Jiménez, J. , Vos, F. , Crespy, V. , du Chaffaut, L. , Mennen, L. , … Scalbert, A. (2010). Phenol‐Explorer: An online comprehensive database on polyphenol contents in foods. Database (Oxford). 10.1093/database/bap024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, D. J. , & Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79, 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Ngo, B. , Ripper, J. M. V. , Cantley, L. C. , & Yun, J. (2019). Targeting cancer vulnerabilities with high‐dose vitamin C. Nature Reviews Cancer, 19(5), 271–282. 10.1038/s41568-019-0135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphade, S. R. , Asad, M. , Chandrakala, G. K. , Toppo, E. , & Deshmukh, P. (2009). Immunomodulatory activity of Cinnamomum zeylanicum Bark. Pharmaceutical Biology, 47, 1168e73. 10.3109/13880200903019234 [DOI] [Google Scholar]
- Nonotny, L. , Vachalkova, A. , & Biggs, D. (2001). Ursolic acid: Anti‐tumorigenic and chemopreventive activity. Neoplasma, 48, 241–246. [PubMed] [Google Scholar]
- Ogunwande, I. , Olawore, N. , Ekundayo, O. , Walker, T. M. , Schmidt, J. M. , & Setzer, W. N. (2005). Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. International Journal of Aromatherapy, 15, 147–152. [Google Scholar]
- Ojagh, S. M. , Rezaei, M. , Razavi, S. H. , & Hosseini, S. M. (2012). Investigation of antibacterial activity cinnamon bark essential oil (Cinnamomum zeylanicum) in vitro antibacterial activity against five food spoilage bacteria. Journal of Food Science and Technology, 9, 67–76. [Google Scholar]
- Omosa, L. K. , Midiwo, J. O. , & Kuete, V. (2017). Curcuma longa. In Kuete V. (Ed.), Therapeutic potential against metabolic, inflammatory, infectious and systemic diseases (pp. 425–435). Cambridge, MA: Academic Press; 10.1016/B978-0-12-809286-6.00019-4 [DOI] [Google Scholar]
- Padilla, L. , Rodríguez, A. , Gonzales, M. M. , Gallego‐g, J. C. , & Castaño‐o, J. C. (2014). Inhibitory effects of curcumin on dengue virus type 2‐infected cells in vitro. Archives of Virology, 159, 573–579. 10.1007/s00705-013-1849-6 [DOI] [PubMed] [Google Scholar]
- Panpatil, V. V. , Tattari, S. , Kota, N. , & Polasa, K. (2013). In vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. Journal of Pharmacognosy and Phytochemistry, 2, 143–148. [Google Scholar]
- Parida, M. M. , Upadhyay, C. , Pandya, G. , & Jana, A. M. (2002). Inhibitory potential of neem (Azadirachta indica Juss) leaves on Dengue virus type‐2 replication. Journal of Ethnopharmacology, 79(2), 273–278. [DOI] [PubMed] [Google Scholar]
- Parvez, M. K. , Rehman, M. T. , Alam, P. , Al‐Dosari, M. S. , Alqasoumi, S. I. , & Alajmi, M. F. (2019). Plant‐derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharmaceutical Journal, 27(3), 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak, N. , & Khandelwal, S. (2007). Cytoprotective and immunomodulating properties of piperine on murine splenocytes: An in vitro study. European Journal of Pharmacology, 576, 160–170. [DOI] [PubMed] [Google Scholar]
- Patra, K. , Jana, K. , Mandal, D. P. , & Bhattacharjee, S. (2016). Evaluation of the antioxidant activity of extracts and active principles of commonly consumed Indian spices. Journal of Environmental Pathology, Toxicology and Oncology, 35, 299–315. [DOI] [PubMed] [Google Scholar]
- Pattanayak, P. , Behera, P. , Das, D. , & Panda, S. (2010). Ocimum sanctum Linn. A reservoir plant for therapeutic applications: an overview. Pharmacognosy Reviews, 4, 95e105. 10.4103/0973-7847.65323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya, N. C. , & Saravana, K. (2017). Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. International Journal of Pharmaceutical Sciences Review and Research, 44(1), 197–202. [Google Scholar]
- Pruthvish, R. , & Gopinatha, S. M. (2018). Antiviral prospective of Tinospora cordifolia on HSV‐1. International Journal of Current Microbiology and Applied Sciences, 7(01), 3617–3624. 10.20546/ijcmas.2018.701.425 [DOI] [Google Scholar]
- Rajagopal, K. , Byran, G. , Jupudi, S. , & Vadivelan, R. (2020). Activity of phytochemicalconstituents of black pepper, ginger, and garlic againstcoronavirus (COVID‐19): An in silico approach. International Journal of Health and Allied Sciences, 9, S43–S50. [Google Scholar]
- Rastogi, S. , Pandey, D. N. , & Singh, R. H. (2020). COVID‐19 pandemic: A pragmatic plan for ayurveda intervention. Journal of Ayurveda and Integrative Medicine. 10.1016/j.jaim.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar, B. , & Shukla, V. J. (2007). Indian systems of medicine: A brief profile. African Journal of Traditional, Complementary and Alternative Medicines, 4, 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtman, M. M. , Har‐Noy, O. , Bar‐Yishay, I. , Fishman, S. , Adamovich, Y. , Shaul, Y. , … Shlomai, A. (2010). Curcumin inhibits hepatitis B virus via down‐regulation of the metabolic coactivator PGC‐1a. FEBS Letters, 584, 2485–2490. 10.1016/j.febslet.2010.04.067 [DOI] [PubMed] [Google Scholar]
- Rege, A. A. , & Chowdhary, A. (2014). Evaluation of Ocimum sanctum and Tinosporacordifolia as probable HIV‐protease inhibitors. International Journal of Pharmaceutical Sciences Review and Research, 25(1), 315–318. [Google Scholar]
- Reichling, J. , Schnitzler, P. , Suschke, U. , & Saller, R. (2009). Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties–an overview. Forsch Komplementmed, 16, 79–90. [DOI] [PubMed] [Google Scholar]
- Rhein, C. , Weiner, T. , Henß, L. , Martin, J. , Weber, C. , Sliva, K. , & Schnierle, B. S. (2016). Curcumin and Boswelliaserrata gum resin extract inhibit chikungunya and vesicular stomatitis virus infections in vitro. Antiviral Research, 125, 51–57. 10.1016/j.antiviral.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Sagar, V. K. , & Kumar, A. H. S . (2020). Efficacy of natural compounds from Tinosporacordifolia against SARS‐CoV‐2 protease, surface glycoprotein and RNA polymerase . Preprint. 10.21203/rs.3.rs-27375/v1 [DOI]
- Saha, S. , Galhardi, L. C. , Yamamoto, K. A. , Linhares, R. E. , Bandyopadhyay, S. S. , Sinha, S. , … Ray, B. (2010). Water extracted polysaccharides from Azadirachta indica leaves: Structural features, chemical modification and the anti‐bovine herpesvirus type 1 (BoHV‐1) activity. International Journal of Biological Macromolecules, 47(5), 640–645. [DOI] [PubMed] [Google Scholar]
- Sai Ram, M. , Ilavazhagan, G. , Sharma, S. K. , Dhanraj, S. A. , Suresh, B. , Parida, M. M. , … Selvamurthy, W. (2000). Anti‐microbial activity of a new vaginal contraceptive NIM‐76 from neem oil (Azadirachta indica). Journal of Ethnopharmacology, 71(3), 377–382. 10.1016/s0378-8,741(99)00211-1 [DOI] [PubMed] [Google Scholar]
- Schnitzler, P. , Koch, C. , & Reichling, J. (2007). Susceptibility of drug‐resistant clinical herpes simplex virus type 1 strain to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrobial Agents and Chemotherapy, 51(5), 1859–1862. 10.1128/AAC.00426-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M. , Gupta, A. , & Prasad, R. (2017). A review on herbs, spices and functional food used in diseases. International Journal of Research & Review, 4(1), 103–108. [Google Scholar]
- Sharma, R. , Martins, N. , Kuca, K. , Chaudhary, A. , Kabra, A. , Rao, M. M. , & Prajapati, P. K. (2019). Chyawanprash: A traditional Indian bioactive health supplement. Biomolecules, 9, 161. 10.3390/biom9050161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, V. , Kaushik, S. , Pandit, P. , Dhull, D. , Yadav, J. P. , & Kaushik, S. (2019). Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Applied Microbiology and Biotechnology, 103(2), 881–891. 10.1007/s00253-018-9488-1 [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Jia, L. , Honma, N. , Hosono, T. , Ariga, T. , & Seki, T. (2012). Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects – A review. Journal of Traditional and Complementary Medicine, 2(1), 27–32. 10.1016/s2225-4110(16)30067-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojai, T. M. , Langeroudi, A. G. , Karimi, V. , Barin, A. , & Sadri, N. (2016). The effect of Allium sativum (garlic) extract on infectious bronchitis virus in specific pathogen free embryonic egg. Avicenna Journal of Phytomedicine, 6, 458–467. [PMC free article] [PubMed] [Google Scholar]
- Shree, P. , Mishra, P. , Selvaraj, C. , Singh, S. K. , Chaube, R. , Garg, N. , & Bhusan Tripathi, Y. (2020). Targeting COVID‐19 (SARS‐CoV‐2) main protease through active phytochemicals of ayurvedic medicinal plants – Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) – A molecular docking study. Journal of Biomolecular Structure and Dynamics, 1–14. 10.1080/07391102.2020.1810778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava, R. (2020). Immunity boosters: Solutions from nature – Herbs and spices. Journal of Renal Nutrition Metabolism, 6, 35–37. [Google Scholar]
- Singh, N. , Tailang, M. , & Mehta, S. C. (2016). Review on herbal plants as immunomodulators. International Journal of Pharmaceutical Sciences and Research, 7(9), 3602–3610. [Google Scholar]
- Sonkamble, V. V. , & Kamble, L. H. (2015). Antidiabetic potential and identification of phytochemicals from Tinosporacordifolia . American Journal of Phytomedicine and Clinical Therapeutics, 3, 97–110. [Google Scholar]
- Srivastava, A. K. , Chaurasia, J. P. , Khan, R. , Dhand, C. , & Verma, S. (2020). Role of medicinal plants of traditional use in recuperating devastating COVID‐19 situation. Medicinal Aromatic Plants (Los Angeles), 9, 359. 10.35248/2167-0412.20.9.359 [DOI] [Google Scholar]
- Subramanian, S. (2020). Some Compounds from Neem leaves extract exhibit binding affinity as high as‐14.3 kcal/mol against COVID‐19 Main Protease(Mpro): A Molecular Docking Study . Preprint. 10.21203/rs.3.rs-25649/v1 [DOI]
- Sulochana, K. , Jangra, G. , Kundu, V. , Yadav, J. P. , & Kaushik, S. (2020). Anti‐viral activity of Zingiber officinale (ginger) ingredients against the Chikungunya virus. Virus Disease, 31, 270–276. 10.1007/s13337-020-00584-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarintsev, A. V. , Vrzhets, P. V. , Ershov, D. E. , Shchegolev, A. A. , Turgiev, A. S. , Karamov, E. V. , … Varfolomeev, S. D. (1992). The ajoene blockade of integrin‐dependent processes in an HIV infected cell system. Vestnik Rossiiskoi akademii meditsinskikh nauk, 12, 6–10. [PubMed] [Google Scholar]
- Tiwari, A. , Mahadik, K. R. , & Gabhe, S. Y. (2020). Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Medicine in Drug Discovery, 7, 100027. [Google Scholar]
- Tiwari, V. , Darmani, N. A. , Yue, B. Y. J. T. , & Shukla, D. (2009). In vitroantiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type‐1 infection. Phytotherapy Research, 24(8), 1132–1140. 10.1002/ptr.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, Y. , Cole, L. L. , Davis, L. E. , Lockwood, S. J. , Simmons, V. , & Wild, G. C. (1985). Antiviral properties of garlic: in vitro effects on influenza B, herpes simplex and coxsackie viruses. Planta Medica, 5, 460–461. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme . (2012). United Nations Environment Programme Neem: The UN's tree of the 21st Century. Nairobi: United Nations Environment Programme. [Google Scholar]
- United States Department of Agriculture . (2013). National Nutrient Database for Standard Reference Release 26 Full Report (All Nutrients). Nutrient data for Spices. Ginger.
- Utomo, R. Y. , Ikawati, M , & Meiyanto, E. (2020). Revealing the potency of citrus and galangal constituents to Halt SARS‐CoV‐2 infection . Preprints. 2020030214. 10.20944/preprints202003.0214.v1 [DOI]
- Vasudevan, P. , Kashyap, S. , & Sharma, S. (1999). Bioactive botanicals from basil (Ocimum sp.). Journal of Scientific and Industrial Research, 58, 332e8 http://nopr.niscair.res.in/handle/123456789/17820 [Google Scholar]
- Vijayasteltar, L. , Nair, G. G. , Maliakel, B. , Kuttan, R. , & Krishnakumar, I. M. (2016). Safety assessment of a standardized polyphenolic extract of clove buds: Subchronic toxicity and mutagenicity studies. Toxicology Reports, 3, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab, A. A. , Adawi, H. , & Demellawy, M. E. (2009). In vitro study of the antiviral activity of Zingiber officinale . Planta Medica, 75, PF7. 10.1055/s-0029-1234649 [DOI] [Google Scholar]
- Walls, A. C. , Park, Y.‐J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181(2), 281–292. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wang, Y. , Ye, D. , & Liu, Q. (2020). Review of the 2019 novel coronavirus (SARS‐CoV‐2) based on current evidence. International Journal of Antimicrobial Agents, 55, 105948. 10.1016/j.ijantimicag.2020.105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. Q. , Zhang, Y.‐H. , Ke, C.‐Z. , Chen, H.‐X. , Ren, P. , He, Y.‐L. , … Meng, Z. J. (2017). Curcumin inhibits hepatitis B virus infection by down‐regulating cccDNA bound histone acetylation. World Journal of Gastroenterol, 23, 6252–6260. 10.3748/wjg.v23.i34.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, C.‐C. , Kuo, Y.‐H. , Jan, J.‐T. , Liang, P.‐H. , Wang, S.‐Y. , Liu, H.‐G. , … Yang, N.‐S. (2007). Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. Journal of Medicinal Chemistry, 50(17), 4087–4095. 10.1021/jm070295s [DOI] [PubMed] [Google Scholar]
- Yao, L. , Yao, J. , Han, C. , Yang, J , Chaudhry, M. , Wang, S. , … Yin, Y. (2016). Quercetin, inflammation and immunity. Nutrients, 8(3), 167. 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. X. , Li, C. M. , & Huang, C. Z. (2016). Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale, 8, 3040–3048. 10.1039/c5nr07918g [DOI] [PubMed] [Google Scholar]
- Yang, X. X. , Li, C. M. , Li, Y. F. , Wang, J. , & Huang, C. Z. (2017). Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale, 9, 16086–16092. 10.1039/c7nr06520e [DOI] [PubMed] [Google Scholar]
- Yashin, A. , Yashin, Y. , Xia, X. , & Nemzer, B. (2017). Antioxidant activity of spices and their impact on human health: A review. Antioxidants, 6, 70. 10.3390/antiox6030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, C. F. , Chang, J. S. , Wang, K. C. , Shieh, D. E. , & Chiang, L. C. (2013). Water extract of Cinnamomum cassia Blume inhibited human respiratory syncytial virus by preventing viral attachment, internalization, and syncytium formation. Journal of Ethnopharmacology, 147(2), 321–326. 10.1016/j.jep.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Yoo, E. S. , Choo, G. S. , Kim, S. H. , Woo, J. S. , Kim, H. J. , Park, Y. S. , … Jung, J. Y. (2019). Antitumor and apoptosis‐inducing effects of piperine on human melanoma cells. Anticancer Research, 39(4), 1883–1892. 10.21873/anticanres.13296 [DOI] [PubMed] [Google Scholar]
- Younus, I. , Siddiq, A. , Ishaq, H. , Anwer, L. , Badar, S. , & Ashraf, M. (2016). Evaluation of antiviral activity of plant extracts against foot and mouth disease virus in vitro. Pakistan Journal of Pharmaceutical Sciences., 29(4), 1263–1268. [PubMed] [Google Scholar]
- Zhang, J. , Rao, X. , Li, Y. , Zhu, Y. , Liu, F. , Guo, G. , …, Peng, Z . (2020). High‐dose vitamin C infusion for the treatment of critically ill COVID‐19 . Preprints. 10.21203/rs.3.rs-52778/v1 [DOI]
- Zhen, H. , Fang, F. , Ye, D. Y. , Shu, S. N. , Zhou, Y. F. , Dong, Y. S. , … Li, G. (2006). Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate‐early genes. Antiviral Research, 72, 68–74. 10.1016/j.antiviral.2006.03.01 [DOI] [PubMed] [Google Scholar]
- Zhuanga, M. , Jiang, H. , Yasuhiro, S. , Li, X. , Xiao, P. , Tanakad, T. , … Hattori, T. (2009). Procyanidins and butanol extract of Cinnamomi cortex inhibit SARS‐CoV infection. Antiviral Research, 82(1), 73–81. 10.1016/j.antiviral.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


