Abstract
Background
Convalescent plasma is undergoing randomized trials as a potential therapeutic option for COVID‐19 infection. Little empirical evidence exists regarding the determination of donor eligibility and experiences with donor selection.
Study Design and Methods
This prospective study was conducted at a tertiary care hospital in New York to select plasma donors for a randomized, double‐blind, controlled convalescent plasma trial. Clearance for donation required successful completion of an online questionnaire and an in‐person screening visit, which included (a) completion of a Donor Health Questionnaire (DHQ), (b) Immunoglobulin G (IgG) antibody testing using an immunochromatographic anti‐ severe acute respiratory coronavirus 2 (SARS‐CoV‐2) test, (c) Polymerase chain reaction (PCR) testing if <28 days from symptom resolution, and (d) routine blood bank testing.
Results
After receiving 3093 online questionnaires, 521 individuals presented for in‐person screening visits, with 40.1% (n = 209) fully qualifying. Subjects (n = 312) failed to progress due to the following reasons: disqualifying answer from DHQ (n = 30, 9.6%), insufficient antibodies (n = 198, 63.5%), persistent positive PCR tests (n = 14, 4.5%), and blood donation testing labs (n = 70, 22.4%). Importantly, 24.6% and 11.1% of potential donors who reported having PCR‐diagnosed infection had low or undetectable SARS‐CoV‐2 antibody levels, respectively. Surprisingly, 62.9% (56/89) of subjects had positive PCR tests 14–27 days after symptom resolution, with 13 individuals continuing to be PCR positive after 27 days.
Conclusion
It is feasible for a single site to fully qualify a large number of convalescent plasma donors in a short period of time. Among otherwise qualified convalescent plasma donors, we found high rates of low or undetectable antibody levels and many individuals with persistently positive PCR tests.
Keywords: blood component preparations, donors, immunology (other than RBC serology)
1. INTRODUCTION
The global spread of severe acute respiratory coronavirus 2 (SARS‐CoV‐2), coupled with a lack of proven treatment options, has resulted in more than a million deaths worldwide. 1 , 2 , 3 There is historical precedence to suggest that convalescent plasma may be a useful tool in the treatment of some viral illnesses with limited therapeutic alternatives, including a potential reduction in SARS mortality. 4 , 5 Several nonrandomized analyses have suggested that convalescent plasma might be safe and effective. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 However, to date, only three randomized clinical trials for convalescent plasma treatment of COVID‐19 infection have been reported in peer‐reviewed publications. 16 , 17 , 18
While there is enormous interest in the outcome of randomized trials, there has been relatively little scrutiny of the criteria used to select qualified COVID‐19 convalescent plasma donors. 14 , 19 , 20 Early in the pandemic, the Food and Drug Administration (FDA) correctly understood that requiring neutralizing antibody titers, or even any antibody testing at some sites, would not be feasible. These guidelines, however, could have important implications given that there is increasing evidence that some individuals mount a poor immune response to COVID‐19 infection. 21 , 22 Early studies that met only the minimum guidance, which did not require positive serological testing prior to transfusion, 8 , 23 , 24 may therefore have utilized a significant fraction of COVID‐19 convalescent plasma that did not contain adequate anti‐SARS‐CoV‐2 antibodies to serve as an effective therapy.
There have also been revisions to the FDA donor eligibility guidelines regarding whether individuals who have recovered from infection require a negative PCR test prior to plasma donation. While the April 8th guidelines required PCR testing only in patients who were symptom free for fewer than 28 days, guidance after May 1st removed the requirement for negative PCR testing entirely and instead required only that donors be symptom free for at least 14 days prior to donation. 25 While highly likely, more infectivity data will be needed to definitively determine whether this 14‐day period is sufficient to ensure potential donors are no longer shedding intact SARS‐CoV‐2.
Therefore, there are currently limited data on the practical implications of the current testing requirements with respect to both the presence of anti‐SARS‐CoV‐2 and ongoing positive PCR testing. To address these questions, here, we report our experiences selecting qualified COVID‐19 convalescent plasma donors for our double‐blind randomized trial of convalescent plasma at a tertiary care hospital in New York (NCT04344535).
2. METHODS
After review and approval by the FDA (IND #19823) and our Institutional Review Board (IRB2020‐00209), potential convalescent plasma donors were recruited from the community via an online survey between April 8th and May 8th, 2020, with a final follow‐up date of May 13th, 2020. Respondents to the online survey who indicated a contraindication to FDA required donation criteria (e.g. recent blood donation, travel restrictions, history of infectious disease, etc.) were excluded from further consideration. Many of the remaining survey respondents who did not report a contraindication to plasma donation were contacted to schedule an in‐person screening visit as availability permitted, with priority given to those potential donors who reported a laboratory‐confirmed COVID‐19 diagnosis.
Individuals providing written informed consent underwent an in‐person screening process, with final eligibility for plasmapheresis determined by the following steps:
Completion of a standard Blood Bank Donor Health Questionnaire (DHQ), as well as questions related to COVID‐19 infection, for example, dates of symptom onset/offset, symptom types, type of diagnosis (PCR confirmed, clinical only, no diagnosis).
Semiquantitative Immunoglobulin M (IgM) and IgG serological testing for anti‐SARS‐CoV‐2 nucleocapsid protein (NP) using the ChemBio DPP COVID‐19 System (ChemBio Diagnostics Inc, Medford, NY). The FDA's initial guidance (March 24, 2020) did not require antibody testing but, if done, suggested a minimum neutralizing antibody titer of 1:80, with >1:320 being optimal. 26 However, in the early months of the pandemic, the use of neutralizing antibody titers as a high‐throughput screening method to identify convalescent plasma donors was unfeasible. Therefore, based on preliminary data from plasma sample dilution series (data not shown), we chose 145 and 300 reflectance units in the IgG NP antibody ChemBio DPP COVID‐19 System to approximate the 1:80 and 1:320 neutralizing antibody titers recommended by the FDA at that time, respectively. Importantly, our group has since demonstrated high neutralizing antibody titers to the spike protein in a random subset of samples obtained using these IgG NP antibody cutoffs (Figure S1). 27 We defined a priori four categories of screening IgG NP antibody levels: undetectable (<25), low/insufficient for donation (25–144), medium/adequate for donation (145–300), and high/adequate for donation (>300).
Per the FDA's April 8th guidance, individuals with a 14–28‐day period between self‐reported symptom resolution and donation were required, in our study, to undergo a laboratory test to rule out active infection, for example, negative PCR nasopharyngeal swab test prior to donation. Any positive PCR test triggered scheduling of additional PCR retesting until a negative result was obtained. Per this same FDA guidance, nasopharyngeal swabs were not required for donors with >28 days between symptom resolution and prospective donation.
Individuals who met our adequate antibody threshold underwent venipuncture for additional testing in accordance with standard plasma donation protocols, for example, transmittable disease testing, human leukocyte antigen (HLA) antibody testing, serum protein electrophoresis, hemoglobin, etc. While minimum plasma donation criteria were maintained at all times, our two‐tiered screening process was designed to prevent donor disqualifications and discards during or after plasmapheresis collection appointments.
Once a potential donor fulfilled the above criteria, he or she was permitted to donate plasma up to one time per week. IgG antinucleocapsid levels were rechecked using the same ChemBio DPP System immediately prior to each donation, and donors were permitted to continue donating as long as their antibodies remained adequate (>145 reflectance units). Of the 209 donors we fully qualified, 128 individuals donated at least one time within the initial 1‐month period reported here. Others were scheduled to donate at a later date.
2.1. Statistics
Data for this study were collected and managed using the REDCap electronic capture tools hosted at Stony Brook University. Data analysis was performed using SAS 9.4 software (Cary, NC) and Python 3.4.0. Most of the analyses are descriptive in nature: categorical variables are reported as numbers (%), whereas continuous variables are reported as medians (interquartile range [IQR]). To examine determinants of having an adequate antibody level on the first in‐person visit, an exploratory analysis was performed using multivariable logistic regression. In brief, the demographic parameters described in Table 1 were selected for inclusion based on univariate screening and final model fit. Model fit was assessed using the c‐index and Hosmer‐Lemeshow goodness‐of‐fit test statistic. The final multivariable logistic regression model was trained to differentiate those with adequate antibody levels (IgG antinucleocapsid levels >145 reflectance units) from those with inadequate antibody levels for convalescent plasma donation.
TABLE 1.
Participant demographics by screening step
| Online survey | Screening visit | Adequate antibodies | Fully qualified | |
|---|---|---|---|---|
| No. (%) | 3093 | 521 | 307 (58.9) | 209 (40.1) |
| Age, median (IQR), y | 47 (35‐57) | 47 (35‐56) | 50 (39‐58) | 49 (36‐57) |
| <40 years old, no. (%) | 1009 (32.6) | 177 (34.0) | 85 (27.7) | 67 (32.1) |
| 40‐60 years old, no. (%) | 1521 (49.2) | 271 (52.0) | 169 (55.5) | 11 (53.1) |
| >60 years old, no. (%) | 467 (15.1) | 73 (14.0) | 53 (17.6) | 31 (14.8) |
| Not reported, no. (%) | 96 (3.1) | ‐ | ‐ | ‐ |
| Sex | ||||
| Male, no. (%) | 1165 (37.7) | 307 (58.9) | 196 (63.8) | 151 (72.3) |
| Female, no. (%) | 1913 (61.9) | 214 (41.1) | 111 (36.2) | 58 (27.8) |
| Not reported, no. (%) | 15 (0.5%) | ‐ | ‐ | ‐ |
| Weight, median (IQR), kg | 170 (145‐200) | 185 (155‐210) | 191 (165‐218) | 199 (170‐222) |
| Survey self‐reported COVID‐19 diagnosis method | ||||
| Positive PCR test reported, no. (%) | 1667 (53.9) | 446 (85.6) | 283 (92.2) | 194 (92.8) |
| Clinical diagnosis by health care provider, no. (%) | 326 (10.5) | 62 (11.9) | 17 (5.5) | 11 (5.63 |
| None, no. (%) | 1095 (35.4) | 12 (2.3) | 6 (2.0) | 4 (1.9) |
| Not reported, no. (%) | 5 (0.2) | 1 (0.2) | 1 (0.33) | ‐ |
| Symptom onset to first Ab test, median (IQR), days | ‐ | 38 (32–44) | 37 (33‐43) | 38 (33‐44) |
| Symptom duration, median (IQR), days | 15 (11‐21) | 12 (7‐17) | 13 (8‐16) | 12 (7‐16) |
| Total symptoms reported | 5 (3‐6) | 5 (3‐6) | 5 (3‐6) | 5 (3‐6) |
| Symptoms | ||||
| Fever | ‐ | 387 (74.3) | 249 (81.1) | 166 (79.4) |
| Myalgias | ‐ | 248 (47.6) | 164 (53.4) | 111 (53.1) |
| Unproductive cough | ‐ | 256 (49.1) | 153 (49.8) | 103 (49.3) |
| Fatigue | ‐ | 243 (46.6) | 149 (48.5) | 100 (47.9) |
| Headache | ‐ | 209 (40.1) | 132 (43.0) | 92 (44.0) |
| Loss of taste or smell | ‐ | 215 (41.3) | 122 (39.7) | 84 (40.2) |
| Shortness of breath | ‐ | 148 (28.4) | 87 (28.3) | 63 (30.1) |
| Gastrointestinal symptoms a | ‐ | 131 (25.1) | 92 (30.0) | 67 (32.1) |
| Chills | ‐ | 132 (25.3) | 85 (27.7) | 63 (30.1) |
| Cold symptoms b | ‐ | 136 (26.1) | 62 (20.2) | 41 (19.6) |
| Chest pain or pressure | ‐ | 83 (15.9) | 46 (15.0) | 30 (14.4) |
| Other | 67 (12.9) | 45 (14.7) | 30 (14.4) | |
| Anorexia | ‐ | 45 (8.6) | 33 (10.8) | 25 (12.0) |
| Vertigo or dizziness | ‐ | 24 (4.6) | 18 (5.9) | 12 (5.7) |
| Sweating or night sweats | ‐ | 25 (4.8) | 15 (4.9) | 8 (8.3) |
| Altered mental status | ‐ | 7 (1.3) | 4 (1.3) | 3 (1.4) |
| Not assessed | ‐ | 6 (1.2) | 1 (0.3) | 0 (0) |
| None | ‐ | 3 (0.6) | 2 (0.65) | 1 (0.5) |
Abbreviations: IQR, interquartile range.
Note: Most subjects had more than 1 symptom, so total % symptoms is greater than 100%.
Gastrointestinal symptoms defined as nausea, vomiting, or diarrhea.
Cold symptoms defined as productive cough or rhinorrhea.
PCR defined as polymerase chain reaction
3. RESULTS
We received 3093 responses to our online questionnaire. As it was not feasible to invite all potentially eligible individuals for an in‐person screening visit in such a short period of time, we prioritized those individuals who denied contraindications to plasma donation and who reported a laboratory‐confirmed COVID‐19 diagnosis. After scheduling 567 individuals for a screening visit (on or before May 8th), 521 (92%) completed the in‐person screening visit (Figure 1). Of note, 11 additional individuals qualified for a screening visit, but 8 declined to schedule the visit because they were no longer interested, and 3 declined due to lack of transportation. As shown in Table 1, the median age of participants was 47 years, with 58.9% being male and 85.6% reporting a prior positive PCR test for COVID‐19. Median time from symptom onset to initial antibody test was 38 days (IQR 32–44). The most reported COVID‐19 symptoms for this group were fever (74.3%), myalgias (47.6%), unproductive cough (49.1%), and fatigue (46.6%).
FIGURE 1.
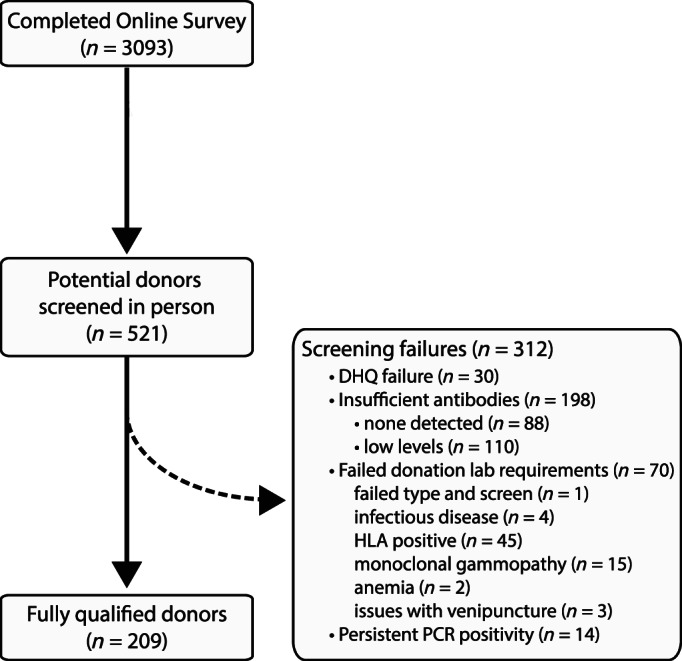
Convalescent plasma donor eligibility screening steps (CONSORT). Shows screening steps from online survey through full qualification of donors
In 505 individuals, antibody levels were measured a median of 38 days from symptom onset (Table 1). Classifying these individuals according to our prespecified categories, we found that 17.4% had no detectable antibodies, 23.4% had low/insufficient antibodies, and 33.3% had “sufficient” but not high antibodies at this initial screening visit (Figure 2). Only 25.9% of all screened potential donors had antibody levels meeting our prespecified high/ideal range (Figure 2). In the subgroup of subjects who reported having a PCR‐documented COVID‐19 infection, the prevalence of no or low antibodies at the initial visit was lower than in the overall population (above). However, even in this self‐reported PCR‐positive group, 24.6% and 11.1% of subjects had low/insufficient or undetectable anti‐SARS‐CoV‐2 levels on initial testing, respectively.
FIGURE 2.
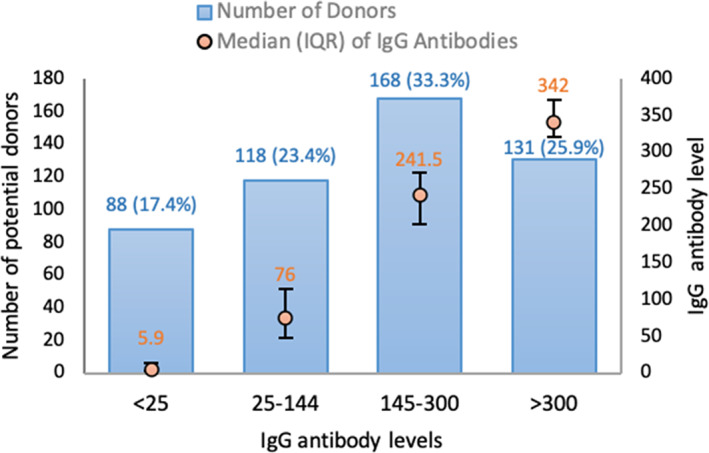
Distribution of immunoglobulin G (IgG) antibody levels to severe acute respiratory coronavirus 2 on initial screening visit. Potential donors (n = 505) were categorized by IgG nucleocapsid protein antibody level on the initial screening visit into four groups using criteria defined a priori: undetectable (<25 reflectance units, n = 88), low/insufficient (25‐144, n = 118), medium/adequate (145‐300, n = 168), and high/adequate (>300, n = 131) groups. IgG antibody levels are shown as median (orange circle) with interquartile range for each group [Color figure can be viewed at wileyonlinelibrary.com]
A total of 61 individuals with marginal levels were asked to return for a retest, but only 34 completed this retest. Of these 34 individuals who returned for retesting, only 8 achieved our prespecified criteria for adequate antibodies. It should be noted that we did not ask all individuals to come back for testing if their initial antibody levels were marginal (25–144). At the beginning of the pandemic, we were more eager to ask individuals to return for repeat testing. This included some individuals who had lower marginal IgG antibodies (e.g., <100). Several weeks into the trial, however, we were collecting enough plasma; therefore, we did not need to request those with lower marginal antibodies to return for repeat testing.
In summary, 299 subjects had adequate antibodies on the initial screening visit (Figure 2) and 8 additional subjects had adequate antibodies on repeat screening, giving a total of 307 with adequate antibodies (Table 1, column 3). Some individuals (n = 23) had repeated IgG antibody levels across a total of 72 screening and apheresis visits (Figure S2). A clinically relevant decline (i.e., one that would preclude donors from donating) was not observed between IgG antinucleocapsid levels and time from symptom onset.
To better understand the characteristics of individuals who had adequate antibodies at their initial screening visit, we performed an exploratory multivariable logistic regression using features reported in Table 1. Characteristics were selected for inclusion based on initial univariate screening and final model fit. After adjustment, older age, including both age > 60 versus <40 years old (odds ratio [OR] (95% confidence intervals), 3.0 (1.5, 6.0)) and age 40–60 versus <40 (OR 2.0 [1.3, 3.1]); self‐reported prior PCR (OR 4.0 [2.2, 7.3]); fever (OR 2.3 [1.4, 3.6]); and gastrointestinal symptoms (OR 1.66 [1.0, 2.7]) were significantly associated with adequate antibodies. Interestingly, cold symptoms were significantly associated with having inadequate antibodies (OR 0.5 [0.3–0.8]) (Figure 3).
FIGURE 3.
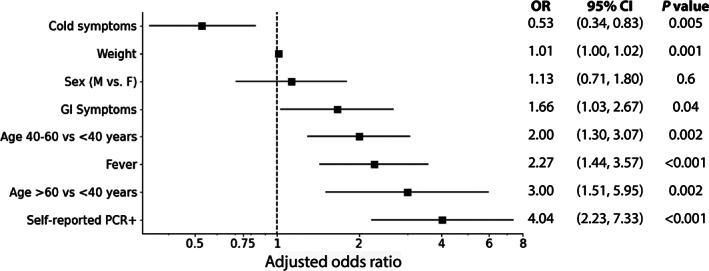
Associations between screening characteristics and adequate antibody levels. An exploratory multivariable logistic regression model was trained to differentiate those donors with adequate antibody levels (defined as an immunoglobulin G antinucleocapsid level ≥ 145) from those with inadequate antibody levels at the initial testing. Characteristics from Table 1 were selected for inclusion based on univariate screening and final model fit. All final model variables and adjusted results are presented in the figure above. Final c‐Index = 0.753, Hosmer‐Lemeshow = 0.42, indicating a good model fit
Per the FDA's April 8th guidance, we required individuals with fewer than 28 days between substantive symptom resolution and donation to have a negative nasopharyngeal SARS‐CoV‐2 PCR (n = 113). A negative PCR test was initially obtained in 45 of 113 subjects (39.8%), regardless of the time from symptom resolution to PCR testing. Of the remaining 68 subjects, 28 have not completed a follow‐up PCR test, or their follow‐up test result is pending. Next, we examined persistent PCR positivity in the remaining 40 subjects with an initially positive PCR result and at least one follow‐up test result (Figure 4 and Figure S3). Of note, we observed PCR positivity in 13 of these subjects who had not had any symptoms for at least 28 days. Indeed, one subject was PCR positive 48 days following symptom resolution and 65 days after symptom onset. Finally, when we considered only those individuals with an initial PCR test performed between 14 and 27 days after substantive symptom resolution, as recommended by the April 8th FDA guidelines, we found that 56 of 89 (62.9%) individuals were positive for SARS‐CoV‐2.
FIGURE 4.
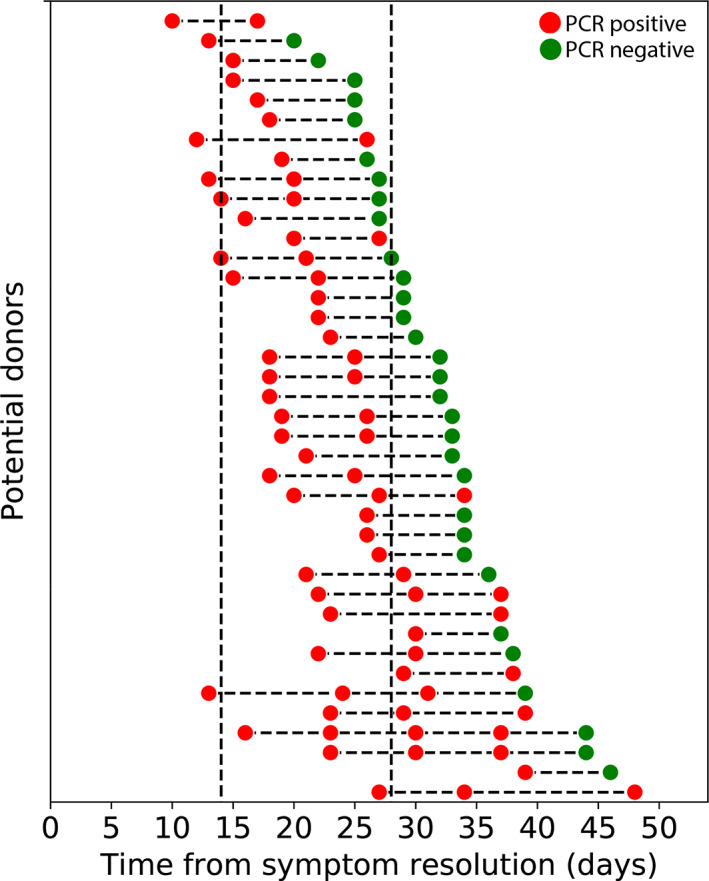
Persistent polymerase chain reaction (PCR) positivity as a function of time since symptom resolution. Persistent PCR positivity is shown for the 40 potential donors who had a positive initial PCR test fewer than 28 days following substantive symptom resolution and at least one additional test result prior to the May 13th follow‐up date (database lock). Once a potential donor tested positive (red circle), he or she was required to have a negative PCR (green circle) prior to donation, regardless of the time since symptom resolution. Additional follow‐up testing is still required for nine potential donors who have not yet had a negative PCR test. Current Food and Drug Administration (FDA) guidance requires only that potential donors be symptom free for at least 14 days (dashed line, left) and does not require any PCR testing prior to donation. The April 8th FDA guidance previously required PCR testing for potential donors who were symptom free for fewer than 28 days (dashed line, right) [Color figure can be viewed at wileyonlinelibrary.com]
Individuals who met our adequate antibody threshold underwent blood screening in accordance with standard blood bank donation protocols, with additional testing of males for HLA antigen antibodies, which is usually not required. We excluded 70 individuals on routine blood bank laboratory testing, including HLA antigen antibodies (n = 45), monoclonal gammopathy (n = 15), blood‐borne infectious disease (n = 4), anemia (n = 2), positive antibody screen (n = 1), and other reasons—for example, could not obtain blood (n = 3).
After the above screening process, 209 individuals were fully qualified to donate plasma as of our May 13, 2020 database lock date (Figure 1, Table 1).
4. DISCUSSION
The therapeutic potential of COVID‐19 convalescent plasma is dependent upon the identification of plasma donors who can provide safe and effective convalescent plasma. However, results from previous studies regarding the seroprevalence of anti‐SARS‐CoV‐2 following COVID‐19 resolution have produced conflicting results. Up to 30% of convalescent patients were found to have low neutralizing antibody titers in one study of 175 hospitalized patients, 22 while other studies have estimated that nearly 100% of individuals have anti‐SARS‐CoV‐2 within 20 days of symptom onset. 28 In the context of identifying convalescent plasma donors, one recent study reported that nearly all (621/624) participants who self‐reported a positive nasopharyngeal swab had high antibody titers on serological testing at 1 month, while only 37% of individuals without laboratory‐confirmed COVID‐19 infection seroconverted. 29 The ability of the current donor eligibility guidelines to ensure that all convalescent plasma donors have sufficient anti‐SARS‐CoV‐2 is therefore uncertain. To address this question, here, we have reported our experiences in screening 521 potential donors.
Overall, we were successful at fully qualifying 209 convalescent plasma donors (40.1% of the 521 individuals undergoing in‐person screening). This was deemed to be a reasonable success rate, especially as it was achieved over only 1 month (April 8‐May 8, 2020); however, we are not aware of how this compares to success rates at other centers. A total of 312 individuals (59.9%) who presented for in‐person screening did not qualify to donate convalescent plasma. The most common reason was low or inadequate antibodies to SARS‐CoV‐2 observed in 198 individuals. Notably, we found that 24.6% and 11.1% of potential donors who reported having PCR‐diagnosed infection had low or undetectable SARS‐CoV‐2 NP antibody levels on initial testing, respectively. Thus, 35.7% of subjects self‐reporting a prior positive PCR result did not have adequate antibody levels. Our screening process was therefore crucial in preventing these individuals with undetectable or low/insufficient SARS‐CoV‐2 antibody levels from donating convalescent plasma.
The second most common reason for screen failures included abnormal routine blood bank donation laboratory testing (e.g., HLA+, transmissible disease testing, monoclonal gammopathy), which occurred in 70 individuals. The incidental finding of monoclonal gammopathy in 15 of 293 (5.1%) convalescent COVID‐19 patients who underwent the required blood donation testing is interesting given that previous studies have suggested that monoclonal gammopathy of undetermined significance may be associated with an increased risk of developing viral infections, potentially including COVID‐19. 30 Other common reasons for failing our convalescent plasma‐screening process included 30 individuals who reported contraindications to donation on their DHQ and 14 subjects with persistently positive PCR tests as of our database lock date.
Our results highlight an aspect of donor selection that has undergone revision by the FDA, specifically the need to rule out those with active infections prior to donation. We found that 63% (56/89) of subjects had positive PCR swabs 14–27 days after symptom resolution, with 13 of these individuals continuing to have positive PCRs after 27 days. Indeed, one subject was PCR positive 48 days following symptom resolution and 65 days after symptom onset. These findings are consistent with previous reports of positive PCR swabs up to 4–6 weeks after disease onset, 31 , 32 , 33 although persistent PCR positivity does not appear to be correlated with clinical history or anti‐COVID‐19 serological testing results. 34 Importantly, previous studies have shown that, despite persistent PCR positivity, the recovery of replication‐competent SARS‐CoV‐2 is unlikely more than 10 days following symptom onset. 35 , 36 , 37 The lack of SARS‐CoV‐2 infectivity in individuals more than 6 days after symptom onset has been further supported by large contact‐tracing studies. 38 Combined with anecdotal data from our site indicating that most individuals with persistent positive PCRs have low viral counts, it is therefore highly likely that the detected RNA from persistently positive PCRs is from a nonviable virus and does not represent active viral shedding.
Our study has several limitations. It was conducted at a single hospital, so the results may not be generalizable to other centers. The FDA's initial guidance (March 24, 2020) did not require antibody testing but, if done, suggested a minimum neutralizing antibody titer of 1:80, with >1:320 being optimal. 26 Similar to other centers at the time, we were unable to measure neutralizing antibody titers as part of our initial donor screening given that measuring neutralizing antibody titers requires specialized equipment, is very labor intensive, and requires Biosafety level‐3 containment if the plaque reduction assay is performed utilizing live SARS‐CoV‐2 virus. Consequently, we and other centers employed surrogate measure of antibodies to SARS‐CoV‐2 to screen potential donors. We specifically used a semiquantitative test to measure IgG nucleocapsid antibody levels, classifying donors into four a priori specified categories: undetectable (IgG <25 reflectance units), low/insufficient for donation (25–144 reflectance units), medium/adequate for donation (145–300 reflectance units, with 145 chosen to approximate the FDA's recommended minimum neutralizing antibody titer of 1:80), and high/adequate (>300 reflectance units, with 300 chosen to approximate the FDA's initial optimal neutralizing antibody titer of 1:320). Crucially, our group has recently confirmed the reliability of these nucleocapsid IgG antibody levels, with our data demonstrating a strong correlation between our prespecified IgG antibody to NP thresholds (145 and 300 reflectance units) and neutralizing antibody titers to the spike protein. 27 In this related study, all randomly selected convalescent plasma units with an nucleocapsid IgG level > 145 reflectance units exceeded the FDA's minimum 1:80 neutralizing antibody titer (Figure S1). 27
Our study was completed before the FDA raised concerns on June 16, 2020 regarding the suboptimal sensitivity and specificity of ChemBio DPP system for diagnosing COVID‐19 infection (cutoff of 25 reflectance density units). These concerns were not relevant to our screening process, where we only selected donors who had very strong readings, that is, minimum of 145 and ideally over 300. As described above, our neutralizing antibody experiments confirm that the use of this IgG antibody test for NP was able to identify convalescent plasma donors (Figure S1). 27
In summary, we show that is it feasible for a single site to fully qualify a large number of convalescent plasma donors in a short period of time. Our experiences may be instructive for other sites where COVID‐19 case counts continue to rise and, most importantly, strongly support the establishment of minimum antibody titers for the donation of COVID‐19 convalescent plasma. Finally, our observations regarding persistent positive PCR tests in a substantial subset of potential donors, even those >4 weeks after symptom onset and resolution, are interesting, but the clinical relevance of this currently remains unclear.
AUTHOR CONTRIBUTIONS
Jason A Carter*: Literature search, figures, study design, data collection, data analysis, data interpretation, and writing (Contributed Equally).
Alex T Freedenberg*: Literature search, figures, study design, data collection, data analysis, data interpretation, and writing (Contributed Equally).
Jamie L Romeiser: Literature search, figures, data collection, data analysis, data interpretation, and writing.
Lillian R Talbot: Literature search, data collection, data interpretation, and writing.
Nicholas J Browne: Literature search, data collection, data interpretation, and writing.
Megan E Cosgrove: Literature search, data collection, data interpretation, and writing.
Margaret E Shevik: Literature search, data collection, data interpretation, and writing.
Laura M Generale: Literature search, data collection, data interpretation, and writing.
Molly G Rago: Data collection and data interpretation.
Giuseppina A Caravella: Data collection and data interpretation.
Tahmeena Ahmed: Data interpretation and study design.
Linda J Mamone: Data collection and data interpretation.
Elliott Bennett‐Guerrero: Literature search, figures, study design, data collection, data analysis, data interpretation, and writing.
STONY BROOK MEDICINE COVID PLASMA TRIAL GROUP
Investigators.
Elliott Bennett‐Guerrero (PI), Tahmeena Ahmed, Bettina C. Fries, Sharon Nachman, Jamie Romeiser, Huda Salman, Lisa Senzel, Eric Spitzer.
Team 1 (Online Survey/In Person Scheduling).
Giuseppina Caravella (Team Leader), Laura Harper, Diana Kaell, Melanie Keister, David Komatsu, Jessica Lamb, Deidre Lee, Jane O'Keefe, Ajish Pallai, Elizabeth Roemer, William Scherl, Sandra Skinner, Leah Smith‐McAllister.
Team 2 (In Person Screening Visits).
Molly Rago (Team Leader), Margaret Brand, Andrew Bryan, Lauren Festa, Susan Fiore, Shannen Harbourne, Audrey Hecker‐Crawford, Ann Lavorna, Caryn McKenna, Robert Repetti, Curtis Roggemann, Haseena Sahib, Margaret Shevik, Sunitha Singh, Ruth Stein, Kathy Vivas.
Team 3 (Patient/Recipient Screening and Plasma Administration).
Margaret Andrew (Team Leader), Audrey Anderson, Joan Arata, Marlene Baumeister, Susan Boudreau, Patricia Brill, Noelle Daley, Christine Gearwar, Laura Generale, Darcy Halper, Coleen Letscher, Dawn Madigan, Katherine Markarian‐Askinazi, Ana Mavarez‐Martinez, Sebastian Munoz, Christine Pol, Grace Propper.
Team M (Antibody testing/Randomization).
Lillian Talbot (Team Leader), Nicholas Browne, Jason Carter, Megan Cosgrove, Alex Freedenberg, Andrew Sisti.
Regulatory (IND and IRB Support).
Suman Grewal, Caterina Vacchi‐Suzzi, Angie Wong.
FUNDING/SUPPORT
This investigator‐initiated study was sponsored/funded by Stony Brook Medicine. There was no external funding/support.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Figure S1 Neutralizing Antibody Titers in Plasma
Figure S2 Anti‐SARS‐CoV‐2 Levels From Symptom Onset
Figure S3 Persistent PCR Positivity As A Function of Time Since Symptom Onset
ACKNOWLEDGMENTS
We thank the Apheresis/Blood Bank staff and Stony Brook Medicine Information Technology for their significant assistance in helping us to operationalize this program.
We also thank the leadership of Stony Brook Medicine for financially supporting this research.
Carter JA, Freedenberg AT, Romeiser JL, et al. Impact of serological and PCR testing requirements on the selection of COVID‐19 convalescent plasma donors. Transfusion. 2021;61:1461–1470. 10.1111/trf.16293
Jason A. Carter and Alex T. Freedenberg are Equal contributors.
Principal Investigator: Elliott Bennett‐Guerrero, M.D.
Trial registration: Clinicaltrials.gov NCT04344535
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533–4. 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): A review. JAMA. 2020;323:1824–1836. 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni Z‐Y, Hu Y, Liang W‐H, Ou C‐Q, He J‐H, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw F‐M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2015;14:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin, C. , Gu, J. , Yuan, Y. & Long, Q . Treatment of 6 COVID‐19 patients with convalescent plasma. medRxiv, 2020.2005.2021.20109512 (2020).
- 8. Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of coronavirus disease 2019 (COVID‐19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323:1582–1589. 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92:1890–1901. 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu STH, Lin H‐M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: A propensity score‐matched control study. Nature Med. 2020;26:1708–1713. 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 12. Rogers R, Shehadeh F, Mylona EK, Rich J, Neill M, Touzard‐Romo F, et al. Convalescent plasma for patients with severe COVID‐19: A matched cohort study. Clin Infect Dis. 2020;ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salazar E, Christensen PA, Graviss EA, Ngygen DT, Castillo B, Chen J, et al. Treatment of COVID‐19 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;191:90–107. 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joyner, M. , Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: initial three‐ month experience. medRxiv. 10.1101/2020.08.12.20169359 (2020). [DOI]
- 16. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. A randomized clinical trial. JAMA. 2020;324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. Convalescent plasma in the management of moderate COVID‐19 in India: An open‐label parallel‐arm phase II multicentre randomized controlled trial (PLACID trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130:1545–8. 10.1172/jci138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Tong X, Chen H, He R, Lv Q, Yang R, et al. Characteristics and serological patterns of COVID‐19 convalescent plasma donors: Optimal donors and timing of donaiton. Transfusion. 2020;60:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harvala, H. , Robb, M. L. , Watkins, N. , Ijaz, S. & Dicks, S . Convalescent plasma therapy for the treatment of patients with COVID‐19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. medRxiv, 2020.2005.2020.20091694 (2020). [DOI] [PMC free article] [PubMed]
- 22. Wu, F. , Wang A, Liu M Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv, 2020.2003.2030.20047365 (2020).
- 23. Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K. Early safety indicators of COVID‐19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130:4791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheridan C. Convalescent serum lines up as first‐choice treatment for coronavirus. Nat Biotechnol. 2020;38:655–8. 10.1038/d41587-020-00011-1. [DOI] [PubMed] [Google Scholar]
- 25. CBER . https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (2020).
- 26. Budhai A, Wu AA, Hall L, Strauss D, Paradiso S. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;70:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freedenberg AT, Pan C‐H, Diehl WH, Romeiser JL, Hwang G‐R, Leiton CV, et al. Neutralizing activity to SARS‐CoV‐2 of convalescent and control plasma used in a randomized controlled trial. Transfusion. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845–8. 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 29. Wajnberg A, Mansour M, Leven E, Bouvier NM, Patel G, Firpo A, et al. Humoral response and PCR positivity in patients with COVID‐19 in the New York City region, USA: An observational study. Lancet Microb. 2020;1:e283–9. 10.1101/2020.04.30.20085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain A, Ramasamy K. Potential ‘significance’ of monoclonal gammopathy of ‘undetermined significance’ during COVID‐19 pandemic. Blood Cells Mol Dis. 2020;85:102481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465–9. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 32. Xiao AT, Tong YX, Zhang S. Profile of RT‐PCR for SARS‐CoV‐2: A preliminary study from 56 COVID‐19 patients. Clin Infect Dis. 2020;71:2249–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikegami S, Benirschke R, Flanagan T, Tanna N, Klein T, Elue R, et al. Persistence of SARS‐CoV‐2 nasopharyngeal swab PCR positivity in COVID‐19 convalescent plasma donors. Transfusion. 2020;60:2962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu J, Peng J, Xiong Q, Liu Z, Lin H, Tan X, et al. Clinical, immunological and virological characterization of COVID‐19 patients that test re‐positive for SARS‐CoV‐2 by RT‐PCR. EBioMedicine. 2020;59:102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Kampen, J. J. A. , van de Vijver, D. A. M. C. , Fraaij, P. L. A. , Haagmans, B. L. & Lamers, M. M . Hedding of infectious virus in hospitalized patients with coronavirus disease‐2019 (COVID‐19): duration and key determinants. medRxiv, 2020.2006.2008.20125310 (2020). [DOI] [PMC free article] [PubMed]
- 38. Cheng H, Jian S‐W, Liu D‐P, Ng T‐C, Huang W‐T, Lin H‐H, et al. Contact tracing assessment of COVID‐19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Neutralizing Antibody Titers in Plasma
Figure S2 Anti‐SARS‐CoV‐2 Levels From Symptom Onset
Figure S3 Persistent PCR Positivity As A Function of Time Since Symptom Onset


