Abstract
BACKGROUND AND PURPOSE:
Recanalization with the Wingspan stent, which can be deployed rapidly and safely, is an option for treating acute ischemic stroke when intravenous thrombolysis has failed or is contraindicated. This study was performed to evaluate feasibility, efficacy, and safety of recanalization for acute middle cerebral artery occlusion using the Wingspan stent.
MATERIALS AND METHODS:
We collected 10 patients with acute MCA occlusion in whom recanalization was not achieved with a standard intravenous thrombolysis, or who were ineligible for intravenous thrombolysis, or who presented after 3 hours of symptom onset and in whom the stent placement could be completed within 8 hours from symptom onset. We analyzed angiographic and clinical results.
RESULTS:
Successful recanalization with the Wingspan stent was achieved in all patients. The mean NIHSS score on admission was 12.7 points (range 4–21). The occlusion sites were located in the 1st segment (n = 7; 2 left, 5 right) and 2nd segment (n = 3, all right) of the MCA. The mean time interval from stroke symptom onset to stent placement was 344.8 ± 76.3 minutes. No intracranial hemorrhage, vessel perforations, or dissections occurred in any patient. Nine patients improved on the NIHSS at 7 days. One patient did not have a change in the NIHSS score, even though the occluded artery was completely recanalized. At 7 days, the NIHSS score of all patients was 4.4 ± 4.7 (median 4, range 0–13). At discharge, an mRS of ≤3 was achieved in all patients and an mRS of ≤2 was achieved in 7 patients (70%).
CONCLUSIONS:
This small case series demonstrates the feasibility of using the Wingspan stent safely and effectively for MCA occlusions when standard treatments are ineffective or not available.
As a single disease entity, acute ischemic stroke is the leading cause of death in South Korea. Intravenous tPA is effective in treating acute ischemic stroke when given to patients within 3 hours of symptom onset. Complete recanalization is an important factor determining good outcome after stroke. Despite favorable results with tPA in some patients, however, the efficacy of intravenous tPA for complete recanalization is relatively low. The recanalization rate for M1 and M2 of acute MCA occlusions after intravenous tPA are approximately 25% and 30%.1 Intra-arterial thrombolysis alone, or in combination with intravenous thrombolysis, may be more effective. However, the rate of recanalization of chemical thrombolysis through intra-arterial and intravenous routes is still unsatisfactory. For patients with acute ischemic stroke refractory to thrombolysis, or in whom thrombolysis is inappropriate, another endovascular approach is needed to rescue acute stroke patients from a devastating outcome. Recent case series report acceptable safety, good outcomes, and good recanalization rates with self-expanding stents in patients with acute MCA occlusion if other recanalization modalities have failed, or in patients who have been ineligible for intravenous tPA.2–7 We describe recanalization rates, complications, and outcomes in patients treated with the Wingspan stent as a rescue procedure for acute MCA occlusion.
Materials and Methods
Ten patients with acute MCA occlusion underwent Wingspan-assisted recanalization in our stroke center between May 1, 2010, and November 30, 2010. We treated patients in whom recanalization was not achieved with standard intravenous thrombolysis, patients who were ineligible for intravenous thrombolysis, or who presented after 3 hours of symptom onset in whom the stent placement could be completed within 8 hours from stroke symptom onset. DWI, CT, CTP, and CT angiography were performed on admission to assess for arterial occlusion and diffusion/perfusion mismatch. DWI was performed on 9 patients and CTP was performed on 8 patients. We excluded patients whose infarct burden was more than one-third of the middle cerebral artery vascular territory on a CBV map of CTP. We included the patients with acute MCA occlusion, diffusion/perfusion mismatch, and clinical/diffusion mismatch. Clinical charts were reviewed for demographic features, clinical characteristics, location of occlusion, symptom onset to arrival time, arrival to stent placement time, symptom onset to stent placement time, use of adjunctive chemical therapies (urokinase or glycoprotein IIb/IIIa inhibitors), and procedural complications. The NIHSS score was checked before and at 7 days after stent placement. The mRS was also checked at discharge. An mRS of 0–2 was defined as a good neurologic outcome. Recanalization results were evaluated by the TICI score (graded as 0 for absent perfusion, 1 for minimal distal perfusion, 2 for partial perfusion, and 3 for complete perfusion).
All patients were fully awake to receive a neurologic examination during the procedure. A 6F guiding catheter (Envoy; Cordis, Miami Lakes, Florida) was positioned in the distal cervical or petrous ICA. After obtaining a preliminary angiogram, the catheter was connected to a continuous saline flush. Preprocedural angiographic images were then obtained in orthogonal planes. Via the guiding catheter, a microcatheter (Echelon 10; ev3, Irvine, California) was used to cross the occlusion segment of the MCA over a 0.014-inch microwire (Transend; Boston Scientific, Natick, Massachusetts) and placed distal to the lesion. A 300-cm exchange-length 0.014-inch microwire with distal floppy tip (Transend) was placed via the microcatheter, and the latter was removed. The stent diameter was sized to exceed the diameter of the normal parent vessel by 0.5–1.0 mm. We used the longest stent possible, as it is difficult to know a true length of a lesion before the deployment of the stent. If the length of the occlusion segment was longer than that of a stent, an additional stent was used. The diameter of the parent artery proximal to the lesion was automatically and manually measured by biplane digital subtraction angiography (Axiom Artis; Siemens, Erlangen, Germany). The diameter of the contralateral corresponding artery was used as a reference diameter for the size of the artery distal to the site of occlusion. The Wingspan over-the-wire self-expandable stent system (Boston Scientific) was advanced over the exchange microwire and positioned across the lesion using the road-mapping imaging and external stent markings. The correct position of the stent was angiographically confirmed. We then began to deploy slowly by fixing the stabilizer and unsheathing the stent under the road-mapping image. We did not use prestenting balloon angioplasty because of the increased risk of thrombosis migration. If an in-stent stenosis was found on poststenting angiography, a stent-delivery system was removed, a Gateway balloon (Boston Scientific) was placed in the stenotic lesion, and a balloon angioplasty was performed within the stent, with slow balloon inflation to prevent vascular dissection or rupture. If a residual thrombosis was found on poststenting angiography, a microcatheter was navigated into the thrombotic arterial segment over the exchange microwire, and then immediate thrombolysis using urokinase was given to dissolve the thrombosis. If recurrent thrombosis or reocclusion was identified on poststenting angiography, intra-arterial injection of 6 mg of abciximab (ReoPro) through a guiding catheter was administered, followed by repeat angiography after 10 minutes to identify residual thrombosis. Repeated 2-mg abciximab bolus was administered until a complete thrombolysis was achieved or when the cumulated dose reached the maximal recommended weight-adjusted dose. The complete lysis of the distal thrombosis or acute in-stent thrombosis was established when the lumen normalized on angiography. Repeat angiography was performed to check possible complications, including contrast extravasation or reocclusion of the vessel, for 30 minutes. After the procedure, hemostasis of the femoral artery was achieved using an occlusion device (Perclose Proglide; Abbott Vascular Devices, Redwood City, California). Immediately after the procedure, a complete neurologic examination was performed on all patients by a vascular neurologist, and all patients underwent nonenhanced brain CT for evaluation of possible hemorrhagic complications. MR diffusion imaging was performed on the first postprocedural day. After the procedure, daily aspirin (100 mg) and clopidogrel (75 mg) were administered; 2850 IU of low-molecular-weight nadroparin calcium (Fraxiparine) was also administered subcutaneously 2 or 3 times a day for at least 3 days. Postprocedural intravenous infusion of abciximab was not used because of a risk of cerebral hemorrhage. All patients were seen for follow-up 90 days after stent placement.
Results
From May 2010 until November 2010, 10 patients (7 men and 3 women, mean age 62.6 ± 14.7 years) were included in this study. The mean patient NIHSS score on admission was 12.7 points, with a standard deviation of 6.3 points (range 4–21). In all 10 patients, the occlusion sites were located in the M1 (n = 7; 2 left, 5 right) and M2 (n = 3; all right) segments of the MCA. The mean time interval from stroke onset to arrival at the emergency department was 120.4 ± 83 minutes (Table 1). Six patients received standard intravenous tPA (0.9 mg/kg body weight). Two patients (patients 8 and 9) arrived in our hospital beyond the 3-hour time window. Another 2 patients were not able to receive rtPA, as 1 (patient 5) had gastric polypectomy 12 days before he was admitted to the emergency department and the other patient (patient 10) was taking warfarin for atrial fibrillation. Wingspan stents were used in all patients. Successful stent deployment and recanalization were achieved in all patients (TICI 2a: 1, TICI 2b: 3, TICI 3: 6) (Figs 1–3). The mean time interval from stroke symptom onset to stent placement was 344.8 ± 76.3 minutes. In 6 patients (60%), an in-stent stenosis was found on poststenting angiography and was dilated with balloon angioplasty using a Gateway balloon. Additional poststenting intra-arterial chemical thrombolysis was used in 7 patients. These included glycoprotein IIb/IIIa inhibitor (n = 2, 10 mg) and urokinase (n = 6; mean dose, 270,000U). We had no device-related complications, such as perforations or dissections at the target artery, in any of the patients. None of the patients had intracranial hemorrhage visible on the control CT. Table 2 shows the lesion location, the stent used, the angiographic results, and the complications of Wingspan stent placement. Neurologic improvement on the NIHSS (mean 8.3 points) at 7 days after stent placement occurred in 9 patients. Patient 7 had no change in NIHSS at 7 days, but his NIHSS improved to 4 points at discharge. At 7 days, patient NIHSS score was 4.4 ± 4.7 (median 4; range 0–13). At discharge, an mRS of ≤3 was achieved in all 10 patients and an mRS of ≤2 was achieved in 7 patients (70%). No ischemic events occurred within 90 days after stent placement.
Table 1.
Patient characteristics and clinical outcomes of thrombolysis using stenting for acute MCA occlusion
| Patient | Sex | Age | Time (minutes) |
NIHSS Score |
mRS at Discharge | |||
|---|---|---|---|---|---|---|---|---|
| Sx Onset to Arrival | Arrival to Stenting | Sx Onset to Stenting | Initial | 7 Days (Change) | ||||
| 1 | M | 40 | 141 | 161 | 302 | 9 | 5 (4↓) | 2 |
| 2 | M | 71 | 94 | 196 | 290 | 24 | 13 (8↓) | 3 |
| 3 | F | 40 | 204 | 91 | 295 | 18 | 5 (13↓) | 3 |
| 4 | M | 56 | 44 | 273 | 317 | 12 | 9 (3↓) | 3 |
| 5 | M | 67 | 114 | 286 | 400 | 9 | 3 (6↓) | 1 |
| 6 | F | 76 | 28 | 178 | 206 | 19 | 0 (19↓) | 1 |
| 7 | M | 80 | 47 | 404 | 451 | 9 | 9 (→) | 2 |
| 8 | M | 55 | 207 | 175 | 382 | 4 | 0 (4↓) | 0 |
| 9 | M | 63 | 300 | 66 | 366 | 20 | 0 (20↓) | 0 |
| 10 | F | 78 | 139 | 300 | 439 | 6 | 0 (6↓) | 0 |
Note:—Sx indicates symptom.
Fig 1.
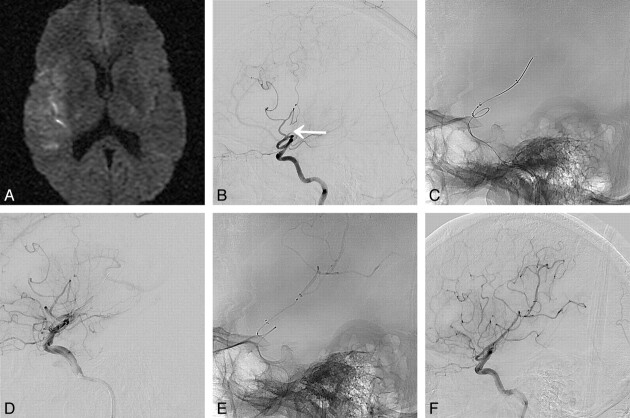
A 63-year-old man (patient 9) presented with acute infarction in the right MCA territory. A, DWI shows acute infarction in the right basal ganglia and frontal lobe (NIHSS score is 20 points). B, CT perfusion images show perfusion defect with large area of diffusion/perfusion mismatching in the right MCA territory. C, Right ICA angiogram shows complete occlusion of proximal M1 segment. On ICA angiogram after a Wingspan stent deployment, anteroposterior view (D) shows moderate to severe stenosis (arrows) within stent, and lateral view (E) reveals thrombosis (arrow) at inferior M2 segment distal to the stent and no recanalization of the superior M2 segment. F, Balloon angioplasty (arrow) is performed for the stenosis. The thrombosis distal to stent is solved by intra-arterial urokinase injection (not shown). G, Immediate postprocedural angiogram reveals complete recanalization of the M1 and inferior M2 segments (TICI grade 2a with sufficient collateral flow). H, MRA performed at 9 days postprocedure shows additional recanalization of the right superior M2 segment (arrow) without reocclusion (NIHSS score is 0 at 7 days postprocedure).
Fig 3.
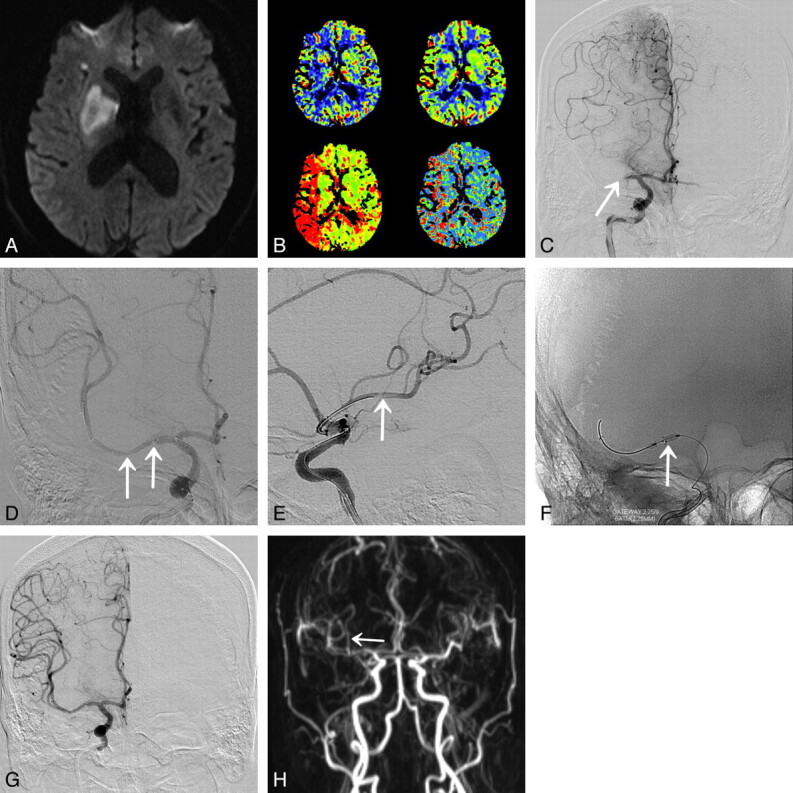
A 40-year-old woman (patient 3) presented with acute infarction in the right MCA territory. A, DWI shows acute infarction in the right MCA territory (NIHSS score is 18 points). B, Lateral view of the right ICA angiogram shows complete occlusion of the inferior M2 segment (arrow). C and D, Lateral radiograph and angiogram after Wingspan stent deployment reveal partial recanalization with residual thrombosis of inferior M2 segment. E, Urokinase is administered for remnant thrombus in cortical arteries after Wingspan deployment. F, Final angiogram reveals near-complete recanalization (TICI grade 2b with sufficient collateral flow) of the MCA (NIHSS is improved up to 5 points at 7 days postprocedure).
Table 2.
Lesions characteristics, stents used, complications, and angiographic results of thrombolysis using stenting for acute MCA occlusion
| Patient | Occlusion Segment of MCA | IV tPA | Stent Used (mm) | PTA for Stenosis | IA UK (Unit) | IA ReoPro | Procedural Complications | Symptomatic ICH | TICI Grade |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M1 | Done | 3.0/20 | Done | 300 000 | None | None | 3 | |
| 2 | M1 | Done | 3.0/20 | Done | 200 000 | None | None | 3 | |
| 3 | M2 | Done | 2.5/20 | No | 300 000 | None | None | 2b | |
| 4 | M1 | Done | 3.0/20 | Done | None | None | 2b | ||
| 5 | M1 | No | 3.0/15 | No | None | None | 3 | ||
| 6 | M2 | Done | 2.5/20 | No | 500 000 | 10 mg | None | None | 2b |
| 7 | M1 | Done | 3.0/9 & 3.5/9 | Done | None | None | 3 | ||
| 9 | M1 | No | 3.0/20 | No | 220 000 | None | None | 3 | |
| 9 | M1 | No | 3.0/20 | Done | 100 000 | None | None | 2a | |
| 10 | M2 | No | 3.5/20 & 3.5/20 | Done | 10 mg | None | None | 3 |
Note:—IA indicates intra-arterial; ICH, intracranial hemorrhage; PTA, percutaneous balloon angioplasty; UK, urokinase.
Fig 2.
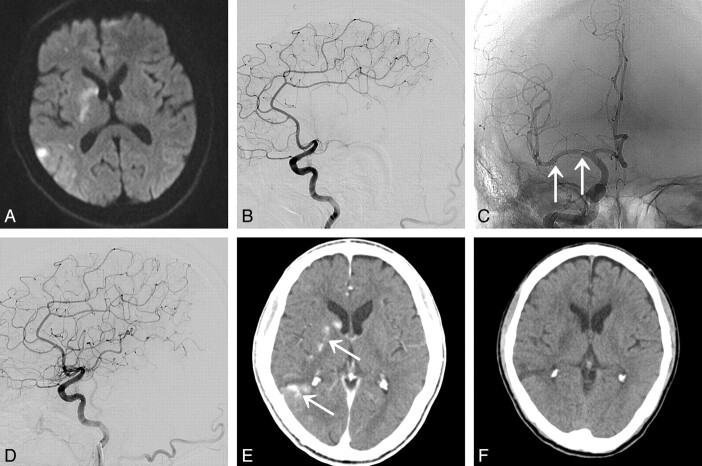
A 67-year-old man (patient 5) presented with acute infarction in the right MCA territory. A, DWI shows acute infarction in the right basal ganglia and frontal and parietal lobes (NIHSS score is 9 points). B, Lateral view of the right ICA angiogram shows complete occlusion of M1 segment. C, Poststenting angiogram after Wingspan stent deployment (arrows) reveals complete recanalization of MCA. D, Immediate postprocedural angiogram reveals complete recanalization without luminal thrombosis (TICI grade 3) of the MCA. E, Multiple lesions of contrast enhancement (arrows) are noted at infarcted area on immediate postprocedural nonenhanced CT. F, 2-day postprocedure CT shows no intracranial hemorrhage (NIHSS score is 3 points at days postprocedure).
Discussion
In South Korea, IV tPA is the standard treatment for acute ischemic stroke, when given to patients within 3 hours of stroke symptom onset. The most important step for improved clinical outcome in acute ischemic stroke patients is the time to recanalization, limited to 3 hours. However, only a small number of patients are admitted within this time window. In addition, the efficacy of intravenous tPA for recanalization of large-vessel arterial occlusion is limited. The recanalization rate with intravenous tPA is only 25% for M1 MCA occlusion and 10% for ICA occlusion.1 The Diffusion Weighted Imaging Evaluation for Understanding Stroke Evolution study demonstrated that patients with MCA or ICA occlusion have low rates of complete (22%) or partial (22%) early recanalization after intravenous tPA.8 As the European Cooperative Acute Stroke Study III trial showed a positive result, the window for thrombolysis now extends to 4.5 hours from the time of stroke symptom onset.9 This extended time window may slightly increase the number of patients who receive intravenous rtPA, but it is unlikely to increase the rate of recanalization. Intra-arterial thrombolysis extends the time window for patients with MCA occlusion up to 6 hours. Direct intra-arterial thrombolysis appears to improve the likelihood of successful recanalization. In the Prolyse in Acute Cerebral Thromboembolism II trial, the recanalization rate for M1 occlusions was 66% for intra-arterial pro-urokinase.10 The Interventional Management of Stroke II trial demonstrated better recanalization rate (53%) in the group treated with combined intravenous plus intra-arterial recanalization.11 Mechanical thromboembolectomy is proposed for treatment in cases of failed recanalization after thrombolysis or in patients with contraindications for thrombolytic therapy. The FDA-approved Merci retriever system (Concentric Medical, Mountain View, California) failed to achieve recanalization in a large proportion of patients (43–54%) treated up to 8 hours after symptom onset.12 Mechanical recanalization may have lower rates of intracranial hemorrhage and higher rates of recanalization compared with intra-arterial thrombolytics,13 but a recent clinical registry of 1000 patients treated with mechanical clot retrieval suggested the opposite.14 The failure of thrombolysis to achieve sufficient recanalization led to the advent of mechanical recanalization using intracranial stent placement.15 The main advantage of intracranial stents over other mechanical thrombolysis is rapid flow restoration. There are 2 types of stent: a self-expandable stent and a balloon-mounted stent. The Wingspan is the only FDA-approved self-expandable stent for the treatment of symptomatic intracranial stenosis. A self-expandable Wingspan stent has several advantages over a balloon-mounted stent. Wingspan stents can be delivered to the target vessel with reduced barotrauma, theoretically decreasing the risk of parent vessel dissection or rupture. They adapt to the shape and diameter of the affected artery much better than balloon-mounted stents. Modifications to delivery microcatheters have made Wingspan stents much easier to navigate to target vessels, particularly in patients with tortuous arterial anatomy.16 Intracranial stent placement for recanalization of cerebral arteries has been performed in a limited number of acute stroke patients.2–4 Zaidat et al5 reported on 9 patients with acute stroke who underwent acute self-expanding intracranial stent placement. They had partial and complete recanalization (TICI 2 and 3) in 89% of patients who were treated at a mean time of 5.1 hours from the time of stroke onset, and 6 of the patients had a good clinical outcome (mRS ≤2) at 90-day follow-up. Brekenfeld et al6 retrospectively reported the feasibility and the safety of a self-expanding Wingspan stent for acute cerebral artery occlusion. Although the number of patients (n = 12) was small, partial or complete recanalization (TIMI 2 and 3) was successful in 92%. However, because the patient selection was not proper (vertebrobasilar occlusion in 6 patients), clinical outcome was poor. In the SARIS trial,7 the first FDA-approved prospective trial investigating stent placement using Neuroform or Wingspan for the treatment of acute stroke was completed. Twenty adult patients with poor NIHSS scores (mean 14 ± 3.8) were treated, on average, within 5.5 hours of symptom onset. Partial or complete recanalization was successful in all treated patients (TIMI 2 = 40%, TIMI 3 = 60%), and moderate to good clinical outcomes (mRS ≤3) were achieved in 60% of patients at 30-day follow-up. Compared with the SARIS trial, we only used a Wingspan stent for recanalization. Our patients improved on the NIHSS at 7 days after stent placement (mean improvement of 8.3 points), and their NIHSS score was 4.4 ± 4.7 (median 4; range 0–13) at 7 days after stent placement. At discharge, an mRS of ≤3 was achieved in our all patients and an mRS of ≤2 was achieved in 7 of 10 patients (70%). In the MERCI trial, the functional outcome of an mRS ≤2 was achieved in only 20% of the patients.12 The Penumbra trial showed an outcome of mRS ≤2 in only 29%.17 In the study by Brekenfeld et al, they achieved the functional outcome of a mRS ≤2 in only 25% of the patients.6 Our mean time interval from stroke symptom onset to stent placement (344.8 versus 393 minutes) was shorter than the Brekenfeld et al study. This might explain the better functional outcome of our cases. Even though the number of patients in our study was small, our results concerning neurologic and functional outcomes showed that a Wingspan-assisted recanalization was a good rescue technique for acute MCA occlusion, if the patient was properly selected. But there are some limitations of the Wingspan-assisted recanalization in our case series. The small number of patients limits the good clinical outcome and recanalization rate in our case series. When standard treatment was ineffective or not available for recanalization in case of acute MCA occlusion, we attempted stent placement as a salvageable method, regardless of whether the occlusion had an embolic or atherosclerotic origin. It is difficult to completely distinguish whether it was embolic or atherosclerotic in origin by clinical symptoms and images. In addition, we need to follow-up on the long-term stent patency and the recurrence of in-stent thrombosis of Wingspan stents in our patients.
Conclusions
Our case series suggests that recanalization with a self-expandable Wingspan stent is safe and feasible in selected patients, and can provide good results in recanalization rates and functional outcomes. If the patient is properly selected, recanalization with a Wingspan stent is a possible option as a rescue strategy for acute ischemic stroke with MCA occlusion when intravenous thrombolysis fails or is contraindicated. Further study of a larger patient population with this stent-assisted recanalization for acute ischemic stroke is needed to verify appropriate indications, safety, durability, and long-term angiographic and clinical follow-up.
ABBREVIATIONS:
- M1
first segment of MCA
- M2
second segment of MCA
- mRS
modified Rankin Score
- SARIS
Stent-Assisted Recanalization in Acute Ischemic Stroke
- TICI
Thrombolysis in Cerebral Infarction
References
- 1. Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator: the rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993; 14: 3–13 [PMC free article] [PubMed] [Google Scholar]
- 2. Levy EI, Ecker RD, Hanel RA, et al. Acute M2 bifurcation stenting for cerebral infarction: lessons learned from the heart: technical case report. Neurosurgery 2006; 58: 458–63 [DOI] [PubMed] [Google Scholar]
- 3. Levy EI, Mehta R, Gupta R, et al. Self expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007; 28: 816–22 [PMC free article] [PubMed] [Google Scholar]
- 4. Sauvageau E, Samuelson RM, Levy EI, et al. Middle cerebral artery stenting for acute ischemic stroke after unsuccessful Merci retrieval. Neurosurgery 2007; 60: 701–06 [DOI] [PubMed] [Google Scholar]
- 5. Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke 2008; 39: 2392–95 [DOI] [PubMed] [Google Scholar]
- 6. Brekenfeld C, Schroth G, Mattle HP, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke 2009; 40: 847–52 [DOI] [PubMed] [Google Scholar]
- 7. Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke). Stroke 2009; 40: 3552–56 [DOI] [PubMed] [Google Scholar]
- 8. Albers GW, Thijs VN, Wechsler L, et al. DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–17 [DOI] [PubMed] [Google Scholar]
- 9. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–29 [DOI] [PubMed] [Google Scholar]
- 10. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA 1999; 282: 2003–11 [DOI] [PubMed] [Google Scholar]
- 11. IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007; 38: 2127–35 [DOI] [PubMed] [Google Scholar]
- 12. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008; 39: 1205–12 [DOI] [PubMed] [Google Scholar]
- 13. Nakano S, Iseda T, Yoneyama T, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke 2002; 33: 2872–76 [DOI] [PubMed] [Google Scholar]
- 14. Rymer MM, Jovin TG, Budzik RF, et al. Interventional acute stroke therapy with the Merci retriever embolectomy device: results from 1000 patients in a open label prospective multicenter registry. International Stroke Conference, Los Angeles, February 8–11, 2011. [Google Scholar]
- 15. Mori T, Kazita K, Seike M, et al. Successful cerebral artery stent placement for total occlusion of the vertebrobasilar artery in a patient suffering from acute stroke. Case report. J Neurosurg 1999; 90: 955–58 [DOI] [PubMed] [Google Scholar]
- 16. Levy EI, Sauvageau E, Hanel RA, et al. Self-expanding versus balloon-mounted stents for vessel recanalization following embolic occlusion in the canine model: technical feasibility study. AJNR Am J Neuroradiol 2006; 27: 2069–72 [PMC free article] [PubMed] [Google Scholar]
- 17. Penumbra Pivotal Stroke Trial Investigators. The Penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009; 40: 2761–68 [DOI] [PubMed] [Google Scholar]