Abstract
BACKGROUND AND PURPOSE:
The extensive application of advanced MR imaging techniques to the study of ALS has undoubtedly improved our knowledge of disease pathophysiology, even if the actual spread of the neurodegenerative process throughout the central nervous system is not fully understood. The present study aimed to detect WM patterns of microstructural abnormalities to better investigate the pathologic process in ALS, within but also beyond CSTs, in a whole-brain analysis.
MATERIALS AND METHODS:
DTI was performed in 19 patients with ALS and 20 matched healthy controls, by using whole-brain TBSS and VOI analyses.
RESULTS:
We observed a significant decrease of FA in the body of CC of the ALS group (P < .05). At the VOI level, both FA decrease and RD increase in the body of CC significantly correlated with the UMN score (P = .003 and P = .02). Additionally, significant voxelwise positive correlations between FA and the ALSFRS-R were detected in the WM tracts underneath the left premotor cortex (P < .05).
CONCLUSIONS:
The correlations between reduction of FA and increase of RD in the body of CC with the UMN score indicate that the WM degeneration in the CC is strictly related to the ALS pyramidal impairment, while the correlation between FA and ALSFRS-R in the associative tracts underneath the left premotor cortex might reflect the progressive spread of the disease from the motor toward the extramotor areas.
The diagnosis of ALS, a progressive neurodegenerative disorder affecting both upper and lower motor neurons, is mainly based on clinical criteria. Lower motor neuron injury can be detected even at a subclinical level by suitable techniques, such as needle electromyography. UMN pathology often starts in the primary motor and premotor cortices, with secondary degeneration of motor fibers and gliosis along the CSTs.1 Nevertheless, it may often be difficult to detect the involvement of the UMN. Transcranial magnetic stimulation measurements may contribute to the diagnosis of ALS by revealing a clinically undetectable UMN dysfunction, and particularly the triple stimulation technique can increase the sensitivity to UMN lesions. However, transcranial magnetic stimulation is not yet being used routinely, and its role in monitoring disease progression is unclear.2
DTI is a relatively new method for structural neuroimaging, which allows visualizing the orientation of the fiber tracts and assessing their integrity in the WM by measuring anisotropic water diffusion properties of the brain with MR imaging.3 DTI has already produced promising results in assessing UMN pathology in patients with ALS,2,4–9 suggesting that FA, the most sensitive DTI measure, is reduced along the CSTs due to axonal degeneration and loss of fiber integrity,2,4–6 though AD, MD, and RD changes along the CSTs and other WM structures may also be indicative of degenerative injury.8,9 Significant correlations of diffusion parameters with duration, progression, and severity of the disease also have been reported in some previous studies.2,9–12
Even if the clinical signs of ALS consist of motor impairments, recent evidence suggests that ALS is not an isolated motor neuron disorder13 and that the variability in the location and extension of FA reduction in patients with ALS is such that relying on a priori ROIs to assess DTI changes may not give a consistent and complete picture of ALS neurodegeneration. Therefore, a novel approach based on whole-brain DTI analysis may result in improved accuracy in detecting subclinical microstructural disease-related WM changes. Previous whole-brain DTI analyses reported regions of WM damage in ALS via voxel-based6,11,14,15 and TBSS8,16–19 techniques. Most of these studies reported changes of FA and MD mainly in the CSTs, but also in the CC6,8,15,17–19 and the frontal and temporal lobes.8,9,15,17,20
Here we used TBSS and VOI DTI analyses to evaluate FA, MD, RD, and AD in whole-brain WM of a heterogeneous group of patients with ALS. These 2 quantitative methods are quite different; TBSS performs statistical analysis on the so-called “FA skeleton” derived from mean FA maps, whereas VOI analysis is based on the Johns Hopkins University WM tractography atlas maps21,22 obtained from the direct fiber tracking. So, the results by 2 different approaches could be inconsistent. Particularly, the VOI method, which averages the values within the VOI, is prone to errors due to partial-volume effects and would, therefore, be considered inferior to the voxel-based TBSS method. However, there are also cases in which a VOI-based method can take advantage of the local averaging and produce more robust results. Therefore, in the present study, we performed both methods of analysis to correlate the regional DTI parameters with the degree of clinical UMN impairment, motor disability, and disease progression.
Materials and Methods
Patients and Controls
We examined 19 patients with ALS (9 men, 10 women; mean age, 61.1 ± 11.2 years; ranging from 34 to 80 years), with definite (n = 9) or probable (n = 10) sporadic ALS, according to the revised El Escorial criteria of the World Federation of Neurology.23 The mean disease duration, estimated from the time of symptom onset to scanning, was 4.1 ± 3.6 years, ranging from 1 to 14 years. In all patients, we assessed the ALSFRS-R for evaluation and monitoring of functional status24; the rate of disease progression (calculated according to Ellis et al2 by the following formula: 40 − ALSFRS-R / disease duration); and the UMN score, a measure of pyramidal dysfunction25 (for more details, see the Table). Two of the 19 patients had disease durations of >10 years and were receiving artificial respiratory support (home mechanical noninvasive ventilation; mean duration, 68 months). We administered the Frontal Systems Behavior Scale to our patients and their caregivers,26 a questionnaire that measures executive dysfunction; in patients with ALS, we found a frontal behavioral impairment (Table).
Detailed patient characteristics
| Patient | Sex | Age (yr) | Disease Duration (yr) | UMN Score | ALSFRS-R | Disease Progression Rate | FrSBe Scale (t-score)a |
|---|---|---|---|---|---|---|---|
| 1 | M | 70 | 4 | 14 | 45 | −0.104 | 142 |
| 2 | F | 65 | 2 | 10 | 22 | 0.75 | 89 |
| 3 | M | 42 | 4 | 1 | 44 | −0.083 | 107 |
| 4 | F | 80 | 5 | 3 | 25 | 0.25 | 106 |
| 5 | F | 72 | 1 | 12 | 18 | 1.83 | 143 |
| 6 | F | 60 | 3 | 4 | 42 | −0.05 | 90 |
| 7 | F | 62 | 13 | 2 | 35 | 0.03 | 74 |
| 8 | M | 34 | 4 | 9 | 39 | 0.02 | 106 |
| 9 | F | 70 | 2 | 5 | 22 | 0.75 | 125 |
| 10 | M | 66 | 2 | 3 | 42 | −0.083 | 106 |
| 11 | M | 59 | 14 | 16 | 29 | 0.083 | 143 |
| 12 | F | 68 | 1 | 8 | 31 | 0.75 | 60 |
| 13 | M | 59 | 4 | 6 | 44 | −0.083 | 102 |
| 14 | M | 59 | 1 | 7 | 39 | 0.083 | 112 |
| 15 | F | 48 | 3 | 2 | 47 | −0.194 | 82 |
| 16 | F | 66 | 2 | 11 | 38 | 0.083 | 119 |
| 17 | M | 65 | 1 | 12 | 21 | 1.583 | 92 |
| 18 | M | 69 | 2 | 3 | 32 | 0.333 | 106 |
| 19 | F | 48 | 4 | 7 | 36 | 0.083 | 123 |
| 61.1 ± 11.2 | 4.1 ± 3.6 | 7.1 ± 4.4 | 34.2 ± 9.1 | 0.31 ± 0.57 | 106.6 ± 22.7 |
Note:—FreSBe indicates Frontal Systems Behavior.
Values represent mean ± SD. T-score (assessed by FrSBe scale) was derived from the caregiver form, referring to the present time.
The control group comprised 20 healthy controls (10 men, 10 women) from 46 to 78 years of age (mean age, 62.1 ± 8.5 years) with no history of neurologic or psychiatric diseases and without any abnormalities detected on conventional T1- and T2-weighted images.
All 39 subjects included in the study were right-handed. Additionally, before enrollment in the present study, FLAIR images of 40 patients and 50 caregivers (not consanguineous of patients) were reviewed, and 39 subjects without periventricular WM disease were recruited.
Ethics approval for all procedures was obtained before the study. Written informed consent was obtained from all participants.
MR Imaging
MR imaging was acquired on a 3T scanner equipped with an 8-channel parallel head coil (GE Healthcare, Milwaukee, Wisconsin). Whole-brain DTI was performed by using a gradient-recalled echo EPI sequence (TR = 10,000 ms, TE = 88 ms, FOV = 320 mm, isotropic resolution = 2.5 mm, b = 1000 s/mm2, 32 isotropically distributed gradients, frequency encoding right-left). DTI datasets were processed with the FSL software package (www.fmrib.ox.ac.uk/fsl). Preprocessing included eddy currents and motion correction and brain-tissue extraction. After preprocessing, we averaged and concatenated DTI images into 33 (1 b = 0 + 32 b = 1000) volumes and a diffusion tensor model was fitted at each voxel, generating FA, MD, and eigenvalue (λ1, λ2, λ3) maps. The average of the second and third eigenvalues of the diffusion tensor was used for the definition of the RD, whereas AD corresponded to the first eigenvalue.
DTI group analyses included voxel-based TBSS and atlas-based VOI analyses. For these group analyses, DTI images were warped to the MNI 152 template, available as standard T1 dataset in the FSL software package.
TBSS was run with FA maps to create the “skeleton,” which represented the center of all fiber bundles in common to all subjects, and which was used for all other maps. To this purpose, FA images of all subjects (N = 39) were aligned to a common target (FMRIB 58_FA standard space) by using nonlinear registration; thereby, FA, MD, AD and RD maps were calculated by using the FSL Diffusion Toolbox tool and aligned to a 1 × 1 × 1 mm MNI 152 standard space.16 A mean FA skeleton was then created with a threshold of FA > 0.2.
Individual skeleton images were smoothed before being submitted to a GLM analysis with appropriate design matrices and linear contrasts defined for group comparisons and correlation with the clinical scores. Voxelwise correlations were performed to compare between all diffusivity parameters (FA, RD, MD, AD) on one side and all clinical parameters (the UMN, ALSFRS-R, and rate of disease progression scores) on the other side. The results were shown on the skeleton map after correction for multiple comparisons with the threshold-free cluster enhancement technique.
A VOI analysis was also performed to correlate the TBSS results with standard anatomic VOI data. VOIs were defined by anatomic marks obtained from the International Consortium of Brain Mapping DTI-81 WM labels atlas (Johns Hopkins University, Baltimore, Maryland).21,22 Individual diffusivity parameters were extracted by first aligning the specific VOI to the DTI maps of all subjects with the Nonlinear Image Registration Tool in FMRIB. Then, all subject maps were masked with this VOI, and the mean values of all diffusivity parameters were obtained. DTI values were evaluated in the CC, divided into splenium, body, and genu.
Pearson correlation coefficients were calculated to evaluate the relationship between some clinical variables (ALSFRS-R, UMN score, and disease duration) and the mean values of all diffusivity parameters within the VOIs, and P values < .05 were considered statistically significant, after correction for multiple comparisons with the Bonferroni method.
Results
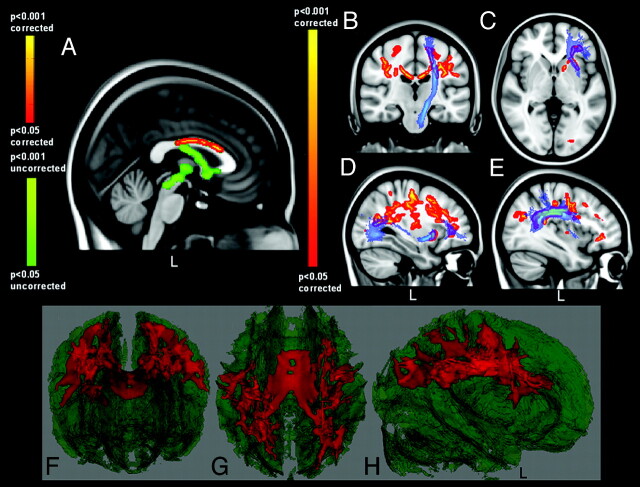
Comparing the FA skeletons of patients with ALS and controls in the TBSS analysis, we observed reduced FA values in the anterior and middle body of the CC and bilaterally in WM tracts from the central CC to the primary motor and premotor cortices, also including, with slight prevalence in the left hemisphere, the corona radiata; anterior cingulate; and superior longitudinal, inferior longitudinal, inferior occipitofrontal, and uncinate fasciculi (P < .05, corrected) (Fig 1). At an uncorrected level of significance, regional differences were also observed caudally in the CST at the left pontomesencephalic junction (P < .001, uncorrected) (Fig 1).
Fig 1.
Regional FA reductions in patients with ALS compared with controls. Blue shows the CST (B) and the uncinate (C), the inferior occipitofrontal (D) and the superior longitudinal (E) fasciculi (derived from the Johns Hopkins University WM tractography atlas21,22). F−H, 3D renderings of the FA skeleton (green), in which red shows regional FA reductions in patients. Prominent involvement of the CC (A) and rostral CST (B) is revealed in patients with ALS (red-yellow scale), with caudal CST changes seen in uncorrected FA results (A, green-only scale). Reduced FA values in the ALS group are also detected in frontal (associative) tracts (C−H).
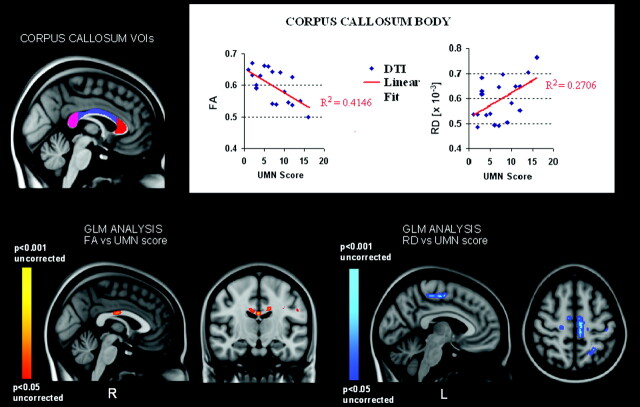
Within the patient group, significant correlations between FA and clinical measures were observed. Specifically, individual FA data, extracted via the VOI analysis selectively from the TBSS results, showed a strong inverse correlation with UMN scores in the body of the CC (P = .003) (Fig 2). Even at the voxel level, although at an uncorrected level, GLM analyses on the group of patients showed an inverse correlation between FA and UMN scores inside the midbody of CC (P < .001, uncorrected) (Fig 2).
Fig 2.
VOI (top panel) and GLM (lower panel) correlation analyses of FA and RD measures with UMN score values. VOI analysis (top panel) shows significant inverse and positive correlations, respectively, of FA and RD values (expressed in mm2 × s−1) with the UMN score in the body of CC (P = .003, P = .02). GLM analysis (lower panel) also shows an inverse correlation between FA and the UMN score in the midbody of CC (red-yellow), while RD values correlate with the UMN score mainly within the left paracentral lobule WM (blue) but not in the CC (uncorrected level of significance).
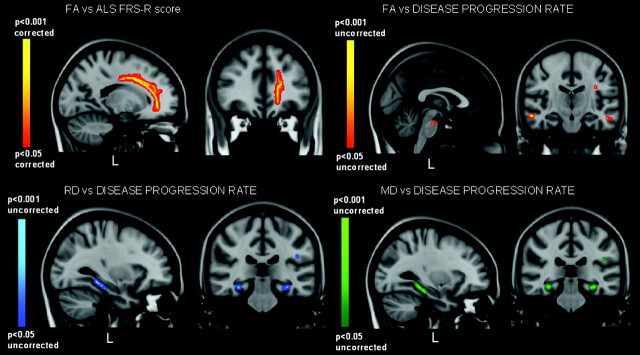
Voxel-level GLM analyses also showed that FA was positively correlated with ALSFRS-R in the WM underneath the left premotor cortex. This pattern included the left paracentral lobule, the anterior cingulate and superior longitudinal fasciculus (P < .05, corrected) (Fig 3); and at an uncorrected level, FA was negatively correlated with the disease progression rate in the CSTs, the pons, and the WM underneath the middle temporal gyrus (bilaterally) and the left inferior frontal gyrus (P < .001, uncorrected) (Fig 3).
Fig 3.
GLM correlation analyses of FA, RD, and MD measures with ALSFRS-R and disease progression-rate values. A positive correlation between FA and ALSFRS-R is revealed in the WM underneath the left premotor cortex (especially the paracentral lobule and cingulate gyrus) (P < .05, corrected) (top left), while an inverse correlation is identified between FA and the disease-progression rate within the CSTs (pons) and frontotemporal WM (middle temporal and left inferior frontal gyri) (P < .01, uncorrected) (top right). Additionally, positive correlations are identified with both RD (low left) and MD (low right) measures in the hippocampus and within the inferior longitudinal fasciculus (P < .01, uncorrected).
Trends of group differences between patients with ALS and controls were also observed in both the genu and splenium of the CC when comparing the MD and RD values (P < .001, uncorrected). Within the CC, this pattern of MD-RD increase in patients with ALS was strikingly complementary to the pattern of FA decrease.
No significant group differences were found in AD bilaterally in the CSTs and the CC.
RD, MD, and AD did not correlate with the UMN score in the anterior and midbody of the CC according to the voxel-based GLM analysis, though in the body of the CC, mean RD data, extracted by VOI analysis of the TBSS results, showed a significant correlation with UMN score (P = .02) (Fig 2). At an uncorrected level of significance, these GLM analyses allowed identifying positive correlations between the RD and UMN score within the left paracentral lobule WM (P < .001, uncorrected) (Fig 2) and between the MD and UMN score in the CSTs, mainly in the cerebral peduncles, principally at the left pontomesencephalic junction and within the genu of left internal capsule and thalamus (left lateral posterior nucleus) (P < .001, uncorrected). Finally, the disease-progression rate showed significant positive correlation with both RD and MD bilaterally in the hippocampus and with slight left lateralization in the insular gyri and inferior longitudinal and uncinate fasciculi (P < .001, uncorrected) (Fig 3).
No significant correlation was observed between all diffusivity parameters and disease duration in both the CSTs and the CC.
Discussion
Even if currently ALS is not considered basically a motor disease, the actual spread of the neurodegenerative process throughout the central nervous system is not fully understood. The present study depicted the microstructural pattern of WM impairment in ALS within but also beyond the motor system and allowed us to hypothesize the underlying mechanisms of cortical spreading of the pathologic process.
We showed a reduced anisotropy in the body of the CC, which correlated significantly with clinical UMN involvement in patients with ALS. These results are consistent with previous studies of CC impairment in ALS.6,8,15,17,18 Conversely, in a recent study,19 the callosal involvement in ALS resulted independent of clinical UMN involvement, and changes of FA were significantly correlated with UMN bilaterally in the CSTs but not in the CC.
With respect to data reported by Filippini et al,19 we observed a similar anisotropic pattern in the body of CC, though it was associated with a significant inverse correlation between the FA and UMN score. We argue that these diverging results depend on a possible more advanced pathologic stage in the process of axonal loss in our patients with ALS, which showed a modest clinical UMN involvement but longer disease course. Given that the staging of ALS depends on the clinical characterization and the grade of disability, most DTI studies were performed in patients with mildly disabling ALS (ALSFRS-R ≥ 20) and a mean disease duration of ≤40 months.8,9,15 Therefore, despite making less evident the effects of reactive gliosis (typical of extremely disabled patients), the selection of mildly disabled patients could not allow depicting structural changes that characterize more advanced stages of disease. Thus, we speculated that in advanced stages of ALS, the CC diffusivity pattern and its relation with UMN involvement might more strikingly reflect a progressive cortical spread of the disease across the 2 hemispheres.
The more rostral impairment of the CSTs has already been described before17 and might be interpreted as an advanced stage of more cranial and widespread distribution of the pathologic degenerative process, similar to what has been seen and reported in postmortem examinations of patients with sporadic ALS who had survived for long periods.27,28
The reduction of FA in the midpart of the CC and mainly in the cranial parts of the CSTs is probably a result of the prevalent axonal loss within the motor fiber pathway, as was also demonstrated by neuropathologic evidence.29 Moreover, because the preferential loss of water diffusion in the fiber direction (decreased FA) was not accompanied by higher diffusion perpendicular to the CC fibers (measured by RD) in the body of CC, these effects seem to reflect a wallerian degeneration (atrophy) of the axons rather than a loss of myelin integrity. We would also have expected to detect corresponding changes of AD, because an increase of AD along fiber tract bundles may be an index of axonal degeneration in ALS.8 Additionally, we did not find an increase of MD within the CC or along the CSTs. We hypothesized that such a diffusivity pattern probably was due to a conspicuous process of reactive gliosis, secondary to tissue loss, which might have lead to a “pseudonormalization” of MD, RD, and AD values, while still reducing FA (glial cells do not have the same anisotropic morphology as the tissue they replace).15
Previous evidence showed that both neuronal and non-neuronal elements may be involved in neurodegeration during the different phases of the disease process,30 though the specific neuropathologic substrate underlying the DTI abnormalities reported in ALS has not yet been clarified. However, in extremely disabled patients, a prominent effect of reactive gliosis as a counterbalance of tissue loss has been detected.31 Indeed, in the postmortem examinations of patients with ALS, there was loss of pyramidal motor neurons in the primary motor cortex and axonal degeneration of the CSTs, with proliferation of glial cells, extracellular matrix expansion, and whole-brain intracellular abnormalities.29 In terms of age and global atrophy, our ALS population did not significantly differ from the control population. Atrophy was assessed by computing the average brain parenchymal fraction score (ratio of brain parenchymal volume to the intracranial volume) obtained by a fully automated method (structural imaging evaluation of normalized atrophy,32data not shown).
The significant correlations shown within the patient group between the UMN score and RD in the body of the CC at the VOI level and between the UMN score and RD and MD along the CSTs, via voxel-based GLM analysis (uncorrected level of significance), might reflect the frequently observed CST histopathologic association of axonal loss with secondary pallor and injury of the myelin sheaths throughout the extent of axonal projections.29,33
If we take these results together, our data support the hypothesis of an active and at least partially independent bilateral cortical process of degeneration with secondary damage to the CC, particularly in its midbody, known to be significantly involved in the process of axonal degeneration in ALS34 and substantially formed by interhemispheric fibers connecting the motor cortices, as also demonstrated by diffusion tensor tractography.35
According to previous neuropathologic findings,27,34,36 ALS degenerative changes in the cerebral cortex mainly involve motor areas, though recent whole-brain voxel-based morphometry analyses have largely reported GM abnormalities even in extramotor regions.12,14,15,20,31,37 Thereby, the trends of MD and RD changes, which largely overlapped in our patients in both the genu and splenium of the CC, may represent an increase in the extracellular volume secondary to axonal loss associated with injury to the myelin sheaths, as previously reported in human and animal models of demyelination and axonal degeneration.2,38,39 Thus, our results also suggest that in advanced stages of ALS, FA changes in the middle body of the CC reflect the degenerative process of axonal damage, inducing loss of motor function, whereas the increase in RD and MD in both the genu and splenium of the CC reflect more subtle axonal damage that could affect fine motor or extramotor skills. Moreover, the mild microstructural changes that we observed in both the genu and splenium of the CC in our patients, affected by a frontal cognitive dysfunction, are highly reminiscent of what has been previously found in callosal areas of the healthy population in relation to aging40 and, to a greater extent, in the case of the compounded effect of age and alcohol abuse.41
The left lateralization of the impairment of the CSTs observed in our patients was probably due to the fact that all subjects were right-handed. This finding, with the recent observation of concordance between handedness and laterality of the upper limb onset in ALS,42 allowed us to further support the hypothesis of an independent cortical vulnerability to the disease of the 2 hemispheres (ie, our results confirm the hypothesis that, despite the systemic clinical symmetry, lateralized motor degeneration is possible). Moreover, this is not the first study in which ALS effects were manifest as lateralized in the motor system.4,10,15,20,37,43,44 Our findings are also in agreement with the descriptions of asymmetry in ALS pathology of the CSTs,45 though a correspondence between the side of greater MR imaging differences and the side of greater muscle strength deficit of the limbs was not found,10 probably because the muscle-strength score is a result of the upper and lower motor neuron involvement, whereas MR imaging detects only the UMN damage.
Using TBSS and voxel-based GLM analyses of the FA maps, we were able to detect significant positive correlations between FA and ALSFRS-R in the WM underneath the whole left premotor frontal cortex, especially the paracentral lobule (corrected level of significance), and between FA and the disease-progression rate along the CSTs in the pons (though at an uncorrected level of significance). These results are consistent with previous DTI correlation analyses between FA and clinical measure of disease severity and progression within the pyramidal system.2,10,16,46 However, not all previous MR imaging studies reported significant correlations between diffusivity parameters and clinical scores in ALS,4,7,11,15 probably due to the following: 1) the intrinsic heterogeneity of the pathologic process (in ALS, degeneration of fiber tracts may not be a classic wallerian degeneration in terms of the contribution from intra- and extracellular changes, astrocytosis, disorders of axonal transport, and accumulation of axonal spheroids29); 2) the different clinical characteristics of the patients, with predominantly upper or lower motor neuron signs and different disease duration and disability; 3) the lack of clinical scores highly specific for the detection of UMN impairment (associations between clinical symptoms and pathologic findings are weak because it has been shown that prominent clinical disability may occur even in the absence of impairment of the CSTs, and vice versa)29; and 4) the different methodologies used.
Comparing our patients with ALS with controls, we detected reduced FA values in the WM underneath the left medial frontal lobe, including the anterior cingulate and superior longitudinal fasciculus, whereas we also found a positive correlation between FA changes and the ALSFRS-R. The DTI pattern of predominantly frontal WM injury clearly reflects the frontal executive dysfunction that characterizes our patients with ALS and has been also extensively described in other cohorts of patients with ALS.47,48 However, we cannot directly validate this hypothesis in our population because our patients did not show overt signs of cognitive impairment, and we did not perform an extensive neuropsychological assessment. Nonetheless, the location of the diffusivity changes in the associative tracts of the frontal lobes that we report in the present study is in line with similar FA and RD changes described in patients with the behavioral variant of frontotemporal dementia.49
The correlations between the disease-progression rate and FA in WM bilaterally underneath the middle temporal gyrus and the left inferior frontal gyrus and between the disease-progression rate and RD and MD bilaterally within the hippocampus, the left insula, and the left inferior longitudinal and uncinate fasciculi might provide further structural evidence of the progressive development in ALS of a multisystem disorder. Furthermore, the highly distributed and widespread pattern of our results is in agreement with the recent findings that in ALS, the cerebral expression of a new neuropathologic marker of disease, the transactivating responsive sequence DNA–binding protein inclusions, involves the nigrostriatal system, the neocortical and allocortical areas, and the cerebellum, more often in case of ALS dementia than in ALS without cognitive dysfunction (eg, Geser et al13).
Conclusions
Even if longitudinal follow-up studies with larger patient groups and extensive clinical and neuropsychological testing are needed to validate the use of DTI parameters as possible biomarkers for neurodegeneration and/or disease progression, our findings underline the crucial feature of CC impairment as neuroradiologic marker of UMN and widespread cortical involvement in ALS, especially to better define the different clinical phenotypes and to more accurately assess and eventually follow, in vivo, the pathologic spread of the disease within and beyond motor areas.
ABBREVIATIONS:
- AD
axial diffusivity
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
revised ALS functional rating scale
- CC
corpus callosum
- CST
corticospinal tract
- FA
fractional anisotropy
- GLM
general linear model
- GM
gray matter
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- RD
radial diffusivity
- TBSS
tract-based spatial statistics
- UMN
upper motor neuron
References
- 1. Davidoff RA. The pyramidal tract. Neurology 1990;40:332–39 [DOI] [PubMed] [Google Scholar]
- 2. Ellis CM, Simmons A, Jones DK, et al. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999;53:1051–58 [DOI] [PubMed] [Google Scholar]
- 3. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion tensor MRI. J Magn Reson 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 4. Toosy AT, Werring DJ, Orrell RW, et al. Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003;74:1250–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham JM, Papadakis N, Evans J, et al. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004;63:2111–19 [DOI] [PubMed] [Google Scholar]
- 6. Sach M, Winkler G, Glauche V, et al. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004;127:340–50 [DOI] [PubMed] [Google Scholar]
- 7. Iwata NK, Aoki S, Okabe S, et al. Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 2008;70:528–32 [DOI] [PubMed] [Google Scholar]
- 8. Metwalli NS, Benatar M, Nair G, et al. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 2010;1348:156–64 [DOI] [PubMed] [Google Scholar]
- 9. Agosta F, Pagani E, Petrolini M, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 2010;31:1457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciccarelli O, Behrens TE, Altmann DR, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain 2006;129:1859–71 [DOI] [PubMed] [Google Scholar]
- 11. Sage CA, Peeters RR, Gorner A, et al. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 2007;34:486–99 [DOI] [PubMed] [Google Scholar]
- 12. Thivard L, Pradat PF, Lehéricy S, et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry 2007;78:889–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 2009;66:180–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abe O, Yamada H, Masutani Y, et al. Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR Biomed 2004;17:411–16 [DOI] [PubMed] [Google Scholar]
- 15. Agosta F, Pagani E, Rocca MA, et al. Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 2007;28:1430–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505 [DOI] [PubMed] [Google Scholar]
- 17. Sage CA, Van Hecke W, Peeters R, et al. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: revisited. Hum Brain Mapp 2009;30:3657–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ciccarelli O, Behrens TE, Johansen-Berg H, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 2009;30:615–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filippini N, Douaud G, Mackay CE, et al. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 2010;75:1645–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Senda J, Kato S, Kaga T, et al. Progressive and widespread brain damage in ALS: MRI voxel-based morphometry and diffusion tensor imaging study. Amyotroph Lateral Scler 2011;12:59–69 [DOI] [PubMed] [Google Scholar]
- 21. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analysis of white matter anatomy and tract-specific quantification. Neuroimage 2008;39:336–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–99 [DOI] [PubMed] [Google Scholar]
- 24. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function: BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21 [DOI] [PubMed] [Google Scholar]
- 25. Kaufmann P, Pullman SL, Shungu DC, et al. Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS). Neurology 2004;62:1753–57 [DOI] [PubMed] [Google Scholar]
- 26. Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment 1999;6:269–84 [DOI] [PubMed] [Google Scholar]
- 27. Nishihira Y, Tan CF, Toyoshima Y, et al. Sporadic amyotrophic lateral sclerosis: widespread multisystem degeneration with TDP-43 pathology in a patient after long-term survival on a respirator. Neuropathology 2009;29:689–96 [DOI] [PubMed] [Google Scholar]
- 28. Nishihira Y, Tan CF, Onodera O, et al. Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 2008;116:169–82 [DOI] [PubMed] [Google Scholar]
- 29. Ince PG, Wharton SB. Cytopathology of the motor neuron. In: Eisen AA, Shaw PJ. eds. Motor Neuron Disorders and Related Diseases: Handbook of Clinical Neurology. 3rd Series. Amsterdam, the Netherlands: Elsevier; 2007:89–119 [DOI] [PubMed] [Google Scholar]
- 30. Clement AM, Nguyen MD, Roberts EA, et al. Wildtype non-neuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 2003;302:113–17 [DOI] [PubMed] [Google Scholar]
- 31. Chang JL, Lomen-Hoerth C, Murphy J, et al. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 2005;65:75–80 [DOI] [PubMed] [Google Scholar]
- 32. De Stefano N, Iannucci G, Sormani MP, et al. MR correlates of cerebral atrophy in patients with multiple sclerosis. J Neurol 2002;249:1072–77 [DOI] [PubMed] [Google Scholar]
- 33. Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neuron disease. J Neurol Neurosurg Psychiatry 1970;33:338–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith MC. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 1960;23:269–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chao YP, Cho KH, Yeh CH, et al. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp 2009;30:3172–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuoka T, Fujii N, Kondo A, et al. An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology 2011;31:71–76 [DOI] [PubMed] [Google Scholar]
- 37. Tedeschi G, Trojsi F, Tessitore A, et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol Aging 2012;33:886–98 [DOI] [PubMed] [Google Scholar]
- 38. Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 39. Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005;26:132–40 [DOI] [PubMed] [Google Scholar]
- 40. Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging 2010;31:464–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging 2006;27:994–1009 [DOI] [PubMed] [Google Scholar]
- 42. Turner MR, Wicks P, Brownstein CA, et al. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2011;82:853–54 [DOI] [PubMed] [Google Scholar]
- 43. Hatazawa J, Brooks RA, Dalakas MC, et al. Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr 1988;12:630–36 [DOI] [PubMed] [Google Scholar]
- 44. Rule RR, Schuff N, Miller RG, et al. Gray matter perfusion correlates with disease severity in ALS. Neurology 2010;74:821–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swash M, Scholtz CL, Vowles G, et al. Selective and asymmetric vulnerability of corticospinal and spinocerebellar tracts in motor neuron disease. J Neurol Neurosurg Psychiatry 1988;51:785–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang S, Poptani H, Woo JH, et al. Amyotrophic lateral sclerosis: diffusion-tensor and chemical shift MR imaging at 3.0 T. Radiology 2006;239:831–38. Epub 2006 Apr 26 [DOI] [PubMed] [Google Scholar]
- 47. Murphy J, Henry R, Lomen-Hoerth C. Establishing subtypes of the continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol 2007;64:330–34 [DOI] [PubMed] [Google Scholar]
- 48. Abrahams S, Goldstein LH, Suckling J, et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005;252:321–31 [DOI] [PubMed] [Google Scholar]
- 49. Whitwell JL, Avula R, Senjem ML, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 2010;74:1279–87 [DOI] [PMC free article] [PubMed] [Google Scholar]