Abstract
BACKGROUND AND PURPOSE:
No study has previously examined the feasibility of using EA to remove any peripherally located, solid components remaining after treatment of benign solid thyroid nodules by RFA. The aim of this study was to assess the efficacy of EA in removing remnant solid components following the incomplete ablation of benign solid thyroid nodules by RFA.
MATERIALS AND METHODS:
During a 1-year period, RFA was performed in 18 benign solid thyroid nodules in 17 patients. EA was subsequently performed on 8 of these nodules. The success rate of EA, size and vascularity of the remaining solid components, amount of injected ethanol, degree of intranodular echo staining just after ethanol injection, and number of EA sessions were assessed.
RESULTS:
Of 18 post-RFA nodules, 8 nodules were subsequently treated with EA because of incomplete ablation, as defined by the presence of peripherally located vascularized solid components. On follow-up US, 2 nodules showed marked hypoechogenicity and no vascularity of the remaining solid components, while 3 nodules showed considerably decreased echogenicity and vascularity of the remaining solid components. Three nodules showed no significant decrease or mild decrease in the echogenicity and vascularity of the remaining solid components. No serious complications were observed during or after RFA or EA, with the exception of 1 patient who experienced diffuse glandular hemorrhage during these procedures.
CONCLUSIONS:
EA was effectively used to remove incompletely ablated components of benign solid thyroid nodules remaining after RFA.
Percutaneous RFA has recently been accepted as an effective treatment method for benign solid thyroid nodules1–5 and is considered an appropriate alternative to thyroid surgery in South Korea.1–3,5 Although RFA is a low-risk easy-to-perform procedure that can be conducted in an outpatient setting, it has several limitations. These include cost, dependence on operator ability, and insufficient data on long-term results.1–5 In the author's experience, procedures performed by less experienced operators may result in the incomplete ablation of the peripheral regions of nodules due to technical difficulty or pain experienced by patients.
A potential alternative to RFA for the treatment of benign thyroid nodules is US-guided percutaneous EA. The mechanisms of ablation following treatment with ethanol include coagulative necrosis and small-vessel thrombosis associated with hemorrhagic infarction.6 The ablated portion of the nodule is then replaced by fibrogranulation tissue, followed by progressive shrinkage. Although EA has been established as the first-line treatment for benign cystic thyroid nodules,7–9 its use in the treatment of solid thyroid nodules has been limited due to controversy over its efficacy. Studies that have examined the use of EA to treat benign solid thyroid nodules have shown varying levels of success, depending on nodule size, the volume of ethanol instilled, and the presence of nodule toxicity.10–14 However, the author of this study has recently found that EA may be a viable alternative to RFA or surgery for the treatment of these nodules.15 In addition, to the best of my knowledge, no study has previously examined the feasibility of using EA to remove any peripherally located solid components remaining after treatment of benign solid thyroid nodules with RFA.
The aim of this study was, therefore, to evaluate the use of EA to remove incompletely ablated nodule components remaining after RFA.
Materials and Methods
Patients
This study was approved by our institutional review board. Seventeen patients who had a palpable anterior neck mass between January 2009 and December 2009 and who chose to undergo RFA for treatment were included in the present study. A single radiologist performed RFA of 18 benign solid thyroid nodules, defined as a nodule with a cystic component composing <10% of the total volume in 17 patients (16 women, 1 man; age range, 12–56 years; mean age, 34 years); 2 nodules were treated simultaneously in 1 patient. None of these patients had a history of thyroid function abnormality or medication related to thyroid abnormality. Furthermore, none of the thyroid nodules showed malignant US characteristics before RFA, and all were confirmed to be benign following 1 or 2 sessions of US-guided fine-needle aspiration. Serum thyroid hormone (total triiodothyronine; normal range, 80–200 ng/dL), free thyroxine (normal range, 0.93–1.71 ng/dL), thyrotropin (normal range, 0.27–4.20 mIU/L), and thyroid peroxidase antibody (normal range, 0–35 IU/mL) levels were also determined by chemiluminescent immunoassay (Modular E170; Roche Diagnostics, Mannheim, Germany), both before RFA and EA treatment and during follow-up. Routine scintiscans were not included in this study. The ellipsoid formula (width × length × height × 0.52) was used to calculate both the initial nodule volumes and the volumes of vascularized solid components remaining following RFA. In the case of multifocal remnant solid components, the sum of the volumes of all calculated solid components was used.
Thyroid US
Each nodule was evaluated with real-time thyroid US, including a color Doppler study both before and after RFA and EA treatments. Thyroid US was performed by a single radiologist (D.W.K.) with a high-resolution sonography instrument (iU22; Phillips Medical Systems, Andover, Maine) equipped with a 5- to 12-MHz linear probe.
During color Doppler US examination, a low value of pulse repetition frequency, 700 Hz, was used for evaluation of the vascularity of thyroid nodules. The vascularity of thyroid nodules was compared with adjacent normal parenchymal vascularity on color Doppler US and classified as follows: scant vascularity (no vascular signal intensity or only a few vascular spots), low vascularity (the vascular signal intensity is lower than that of adjacent normal parenchyma), iso-vascularity (the vascular signal intensity is the same as that of adjacent normal parenchyma), mildly increased vascularity (the vascular signal intensity is greater than that of adjacent normal parenchyma and covers ≤30% of the solid component), and markedly increased vascularity (the vascular signal intensity covers at least 30% of the solid component).
RFA Protocol
Written informed consent was obtained from all patients before each RFA. All RFAs were performed by the same operator (D.W.K.) with an RF generator (Cool-tip RF System, Covidien, Boulder, Colorado; SSP-2000, Taewoong Medical, Gimpo, Korea; M-1004, RF Medical, Seoul, Korea), an internally cooled electrode (Cool-tip,Taewoong Medical), and an active needle tip 1-, 1.5-, or 2-cm length, depending on the nodule volume. RF power varied from 30 to 100 W, depending on the size of the active needle tip, nodule vascularity, and nodule volume. The position of a patient was different according to the location of a nodule: A general position where an operator faced the patient's face was used in RFA of the right thyroid nodule or right isthmic nodule, but a reverse position where an operator faced the patient's feet was applied in RFA of the left thyroid nodule or left isthmic nodule. After local anesthesia, all procedures were performed under real-time US guidance. An electrode was placed in the thyroid nodule by using a transisthmic approach, followed by an attempt to achieve complete ablation while simultaneously minimizing thermal injury to surrounding critical structures. Patients were not treated with antibiotics before or after ablation.
EA Protocol
Written informed consent was obtained from all patients before each EA. Eight patients required EA, which was performed on an outpatient basis by the same operator (D.W.K.) at least 6 months after RFA to remove the solid nodule components that remained following treatment. During the entire procedure, the operator maneuvered the US probe with his left hand and maneuvered a 10-mL plastic syringe filled with 99.9% absolute ethanol attached to either a conventional 21- or 23-ga needle with his right hand. The US probe was adjusted to center the target nodule on the US monitor, and a single-puncture technique was used with no local anesthesia. The operator rapidly inserted the needle almost perpendicular to the neck and applied positive pressure to the syringe piston with the thumb of his right hand to prevent an influx of blood into the needle lumen during insertion. Adequate coverage of the target nodule, as indicated by its echogenicity (called “intranodular echo staining”), was achieved by adjusting the injection of ethanol under US guidance. In all cases, the amount of injected ethanol did not exceed 10 mL in 1 session.
Intranodular echo staining was roughly estimated on the basis of real-time US and classified as follows: no staining (nearly complete washout of injected ethanol), poor staining (≤10% of the injected area), mild staining (10%–50% of the injected area), and moderate staining (≥50% of the injected area).
One or 2 further EAs were performed at least 1 month following the initial treatment if the outcome of the preliminary EA was determined to be unsuccessful on follow-up US. The amount of infused ethanol, degree of intranodular echo staining just after ethanol injection, and the presence of pain or other complications during or after the procedure were recorded for each patient. Any patients experiencing a sensation of inebriation following EA were not allowed to drive themselves home.
Assessment of EA Outcomes
All 8 nodules were sonographically followed up at 1 and 6 months after EA. The decreased echogenicity and vascularity of solid components were used as criteria to assess the ability of EA to remove any components remaining after RFA. The nodule echogenicity was classified as follows according to the comparison with adjacent parenchyma and strap muscle: isoechogenicity (defined as the same echogenicity compared with the adjacent normal thyroid parenchyma), hypoechogenicity (defined as decreased echogenicity compared with the adjacent normal thyroid parenchyma and the increased echogenicity compared with the strap muscle), and marked hypoechogenicity (defined as the same or decreased echogenicity compared with the strap muscle). The vascularity of solid components was assessed by using real-time color Doppler US just before EA and following the final EA. Cases in which the solid components showed marked hypoechogenicity without vascularity on follow-up US were considered successful. Nodule volumes and the volume of the remaining solid components were calculated before RFA and EA, respectively, and at the time of the final follow-up thyroid US following EA treatment; and the change in volume was assessed.
Results
In this study, RFA of 18 benign solid thyroid nodules (mean of the largest diameter, 3.3 cm; range of the largest diameter, 1.2–7.0 cm) was performed in 17 patients (Table 1). All patients had normal serum thyroid hormone levels before RFA and EA and during the follow-up period (3–6 months after the final session). One patient had a low serum level of thyrotropin before RFA and EA; however, this low level was also observed following EA. One patient had severe pain and anterior neck swelling during the RFA because of severely diffuse glandular hemorrhage. The procedure was therefore stopped, and the patient was given oral analgesics for 3 days. However, diffuse glandular hemorrhage did not remain on follow-up US at 1 month after RFA. Three patients who experienced mild pain during and after RFA also took oral analgesics for 1 day following the procedure. On follow-up US, 10 nodules showed marked hypoechogenicity and scant or no vascularity, while 8 nodules had peripherally located solid components remaining following RFA.
Table 1:
Results of RFA for 18 benign solid thyroid nodules
| Sex/Age (yr) | Sizea (cm) | Volume (cm3) | Location | PRF (W) | TRF (min) | TPT (min) | NoS | Cx | Resultb (%) |
|---|---|---|---|---|---|---|---|---|---|
| F/34 | 2.4 | 4.3 | Rt | 30–60 | 15 | 25 | 1 | Mild pain | 30.2 |
| F/20 | 3.7 | 9.1 | Lt | 30–60 | 20 | 30 | 1 | – | 1.1 |
| F/56 | 5.5 | 49 | Lt | 30–60 | 40 | 60 | 2 | Mild pain | 51.6 |
| F/45 | 2.0 | 1.9 | Lt | 30–40 | 5 | 10 | 1 | – | 0 |
| F/36 | 2.9 | 5.2 | Lt | 30–60 | 20 | 30 | 1 | – | 61.5 |
| M/30 | 7.0 | 87.4 | Lt | 50–100 | 10 | 20 | 1 | Diffuse glandular hemorrhage | 81.2 |
| F/37 | 3.0 | 7.8 | Rt | 30–60 | 20 | 30 | 1 | – | 0 |
| F/21 | 1.7 | 1.5 | Isth | 20–30 | 6 | 15 | 1 | – | 0 |
| F/26 | 4.0 | 10 | Lt | 30–40 | 10 | 20 | 1 | – | 13 |
| F/34 | 5.0 | 31.2 | Lt | 30–50 | 26 | 45 | 1 | – | 0 |
| F/45 | 4.3 | 13.9 | Lt | 50–70 | 20 | 40 | 1 | – | 10.8 |
| F/40 | 2.0 | 1.8 | Lt | 20–30 | 10 | 20 | 1 | – | 0 |
| F/40 | 1.2 | 0.5 | Rt | 20–30 | 2 | 5 | 1 | - | 0 |
| F/30 | 3.7 | 5.3 | Lt | 30–60 | 19 | 30 | 1 | Mild pain | 20.8 |
| F/12 | 2.7 | 4.8 | Rt | 50–70 | 13 | 25 | 1 | – | 0 |
| F/50 | 1.6 | 0.8 | Isth | 30–35 | 5 | 10 | 1 | – | 0 |
| F/20 | 4.5 | 17.2 | Lt | 70–100 | 16 | 30 | 1 | – | 0 |
| F/37 | 1.3 | 0.5 | Isth | 30–40 | 3 | 5 | 1 | – | 0 |
| Mean | 3.3 | 14 | 14.4 | 25 |
Note:—TRF indicates total ablation time; TPT, total procedure time; NoS, number of RFA sessions; Cx, complications; PRF, the power of RFA; Rt, right; Lt, left; isth, isthmus.
The largest diameter of the nodule before RFA.
The percentage of remaining vascularized solid components of the nodule after RFA (in comparison with the original nodule before RFA).
Of the 18 nodules that were initially treated with RFA, 8 nodules subsequently underwent EA for complete ablation of peripherally located remaining solid components after RFA (Table 2 and Figs 1 and 2). The patient who experienced severely diffuse glandular hemorrhage at RFA also presented with the same symptom during EA, leading to discontinuation of the procedure. This patient again took oral analgesics for 3 days. Three of the 8 patients (37.5%) experienced mild pain either during or several minutes after the procedure; however, oral analgesics were not used for management of local pain. On follow-up US, 2 nodules showed marked hypoechogenicity and no vascularity of the previous remnant solid components, while 3 nodules showed considerably decreased echogenicity and vascularity of the previous remnant solid components after 1 EA session. The remaining 3 nodules showed no significant decrease (n = 1) or mild decrease (n = 2) in the echogenicity or vascularity of the remnant solid components following the first EA session; 1 of these was stopped with a single EA session and 2 were subsequently treated with 1 or 2 further procedures. Therefore, EA for 5 nodules (62.5%) was considered successful.
Table 2:
Results of EA for 8 incompletely RF-ablated nodules
| Sex/Age (yr) | Volumea (mL) | Vasb | MTAE (cm3) | IESc | NoS | VasEA | Cx | VRR (%) | LFUUS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| F/34 | 1.3 | 3 | 1 | 3 | 1 | 1 | 94.7 | 6 | |
| F/20 | 0.1 | 3 | 1 | 2 | 1 | 0 | 91.2 | 12 | |
| F/56 | 25.3 | 3 | 10 | 2 | 3 | 2 | Mild pain | 45.9 | 6 |
| F/36 | 3.2 | 3 | 6 | 2 | 2 | 1 | Mild pain | 36.5 | 6 |
| M/30 | 71 | 4 | 5 | 1 | 1 | 4 | Diffuse glandular hemorrhage | 8.4 | 6 |
| F/26 | 1.3 | 2 | 3 | 3 | 1 | 1 | 72.5 | 6 | |
| F/45 | 1.5 | 3 | 1 | 2 | 1 | 0 | Mild pain | 59.0 | 6 |
| F/30 | 1.1 | 3 | 0.8 | 2 | 1 | 3 | 73.6 | 6 | |
| Mean | 13.1 | 2.3 | 1.4 | 6.8 |
Note:—MTAE indicates the total amount of injected ethanol in the entire series of EA sessions; IES, intranodular echo staining; NoS, the number of EA sessions; VasEA, the vascularity of solid components after the final EA on follow-up US; Cx, complications; VRR, the percentage of decreased nodule volume between the original nodule before RFA and the postablated nodule on the last follow-up US after EA; LFUUS, the last follow-up US.
Volume of vascularized solid components of an incompletely ablated nodule after RFA and before EA.
Nodule vascularity on color and power Doppler US: no vascularity, 0; low vascularity, 1; iso-vascularity, 2; mildly increased vascularity, 3; markedly increased vascularity, 4.
Intranodular echo staining (depending on the degree of nodule echogenicity due to infused ethanol): no staining (nearly complete washout), 0; poor staining (≤10%), 1; mild staining (10%–50%), 2; moderate staining (≥50%), 3.
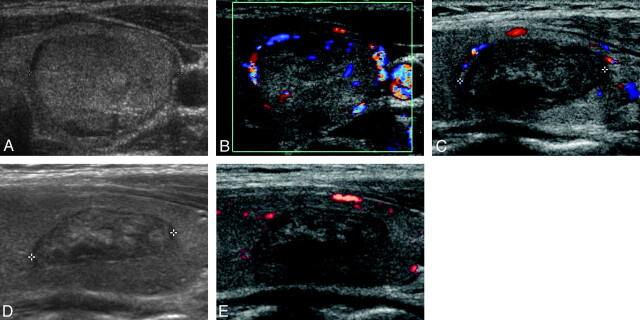
Fig 1.
An example of successful US-guided percutaneous EA for an eccentric peripherally located solid component remaining in a 20-year-old woman following RFA. A and B, Transverse gray-scale and color Doppler US images of a thyroid nodule (1.7 × 2.5 × 3.7 cm) in the left lobe show isoechogenicity and mildly increased vascularity, respectively. C, Longitudinal color Doppler follow-up US image obtained 6 months after the RFA session shows moderate shrinkage (1.0 × 1.4 × 2.2 cm, decrease in volume of 82.5%), while the presence of a vascularized solid component remaining in the upper portion of the nodule was also detected. D and E, Longitudinal gray-scale and power Doppler follow-up US images obtained 6 months after a single session of EA (total amount of injected ethanol, 1 mL) show no visualization of the solid component previously detected in the upper portion of the nodule, as well as a further decrease in nodule volume (0.7 × 1.1 × 2.0 cm, decrease in volume of 91.2%).
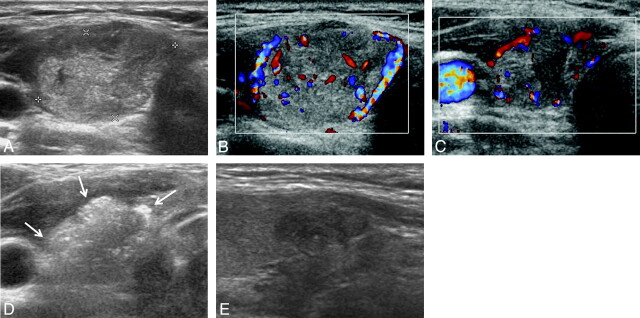
Fig 2.
An example of successful US-guided percutaneous EA for a circumferential peripherally located solid component remaining in a 34-year-old woman following RFA. A and B, Transverse gray-scale and color Doppler US images of a thyroid nodule (1.5 × 2.3 × 2.4 cm) in the right lobe show isoechogenicity and mildly increased vascularity, respectively. C, Transverse color Doppler follow-up US image obtained 6 months after the RFA session shows mild shrinkage (1.1 × 1.5 × 1.5 cm, decrease in volume of 70.1%) and the presence of vascularized solid components in the peripheral regions of the nodule. D, Transverse gray-scale US images just after EA show complete replacement of the nodule with intranodular echo staining (arrows) due to injected ethanol (total amount of injected ethanol, 1 mL). E, Longitudinal gray-scale follow-up US image obtained 6 months after a single session of EA shows marked hypoechogenicity and no vascularity (0.5 × 0.8 × 1.1 cm, decrease in volume of 94.7%).
In the 3 failed cases, 1 showed a very large (volume, 71 cm3) highly vascularized remnant solid component remaining before EA and poor intranodal echo staining during and immediately after 1 session of EA, while another was found to have a large (volume, 25.3 cm3) peripherally located remnant solid component with mildly increased vascularity and poor intranodal echo staining during and immediately following 3 sessions of EA. In the final failed case, a small (volume, 3.2 cm3) highly vascularized remnant solid component was observed before EA, which maintained poor intranodal echo staining during and after 2 sessions of EA.
Discussion
The treatment of benign thyroid nodules by RFA has recently been accepted as a viable alternative to radioiodine therapy, surgery, and EA.1–5 In particular, RFA has proved to be a feasible and effective tool for treatment of benign solid thyroid nodules. Baek et al5 previously reported that the treatment of benign solid thyroid nodules by RFA had a high success rate (100%) and was effective in both reducing the volumes of benign solid thyroid nodules, with a mean volume reduction rate of 79.7%, and relieving nodule-related clinical problems during a 6-month period of US follow-up. In the present study, the incidence of RFA success, defined as the absence of vascularized solid components remaining on follow-up US, was 55.6% (10/18). In the author's opinion, an important factor that determines the success of RFA in the treatment of benign solid thyroid nodules is operator experience, in addition to nodule characteristics, such as vascularity, location, and volume.
In this study, 3 failed cases were found to have remnant solid components with high vascularization before EA and poor intranodular echo staining during and immediately following EA. This result corresponds with a previous study by this author that demonstrated that high vascularity and poor intranodular echo staining are significantly correlated with the success of EA in the treatment of benign solid thyroid nodules.15 Moreover, remnant solid components in 2 failed cases showed a volume of >5 cm3 before EA, while remnant solid components in 6 successful cases showed a volume of <5 cm3. Therefore, the author recommends the limited use of EA following incomplete RFA in cases in which the remaining component has a volume of <5 cm3 and is not highly vascularized.
The operator who performed all the procedures in this study had a low level of experience in using RFA to treat thyroid nodules (≤20 RFA cases/year); this may have been a factor in the considerably high incidence of peripherally located nonablated components remaining after RFA treatment. In cases in which there is incomplete nodule ablation by RFA, resulting in peripherally located solid components remaining, additional RFA may not be recommended due to technical difficulty and cost. Indeed, RFA is significantly more expensive than EA and has been reported to cost approximately 3–6 times more than EA in South Korea.
The possible serious complication of EA is the direct damage of adjacent nerves or critical structures by leakage of ethanol following injection.16–18 However, substantial operator experience and a precise US-guided injection may help decrease the incidence of or avoid complications. With the exception of transient neck pain and diffuse glandular hemorrhage, no serious complications of EA occurred in this study. The author therefore recommends that EA be used to remove any nonablated components of benign solid thyroid nodules remaining after RFA treatment.
There were several limitations to this study, one of which is the small sample size. To address these issues, large-scale studies examining the treatment of solid thyroid nodules by RFA followed by EA are recommended. In addition, a single operator performed all of the RFA and EA procedures included in this report. Therefore, the author plans a multicenter study for further investigation into the efficacy of EA in the treatment of benign symptomatic thyroid nodule components remaining following RFA. Finally, thyroid scans were not performed before RFA or EA treatments, and long-term US follow-up of >12 months was not undertaken.
Conclusions
Despite the limitations of this study, the findings described in this report suggest that following incomplete RFA for benign symptomatic solid thyroid nodules, EA can be considered as a treatment, instead of a second round of RFA, when the solid components show peripheral localization, have a volume of <5 cm3, and are not highly vascularized.
ABBREVIATIONS:
- EA
sonography-guided percutaneous ethanol ablation
- RF
radio-frequency
- RFA
sonography-guided percutaneous radio-frequency ablation
- US
sonography
Footnotes
This work was supported by the Busan Paik Hospital Imaging Research Institute.
References
- 1. Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16:361–67 [DOI] [PubMed] [Google Scholar]
- 2. Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244–50 [DOI] [PubMed] [Google Scholar]
- 3. Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009;33:1971–77 [DOI] [PubMed] [Google Scholar]
- 4. Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 2009;19:219–25 [DOI] [PubMed] [Google Scholar]
- 5. Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 2010;194:1137–42 [DOI] [PubMed] [Google Scholar]
- 6. Crescenzi A, Papini E, Pacella CM, et al. Morphological changes in a hyperfunctioning thyroid adenoma after percutaneous ethanol injection: histological, enzymatic and sub-microscopical alterations. J Endocrinol Invest 1996;19:371–76 [DOI] [PubMed] [Google Scholar]
- 7. Monzani F, Lippi F, Goletti O, et al. Percutaneous aspiration and ethanol sclerotherapy for thyroid cysts. J Clin Endocrinol Metab 1994;78:800–02 [DOI] [PubMed] [Google Scholar]
- 8. Kim DW, Rho MH, Kim HJ, et al. Percutaneous ethanol injection for benign cystic thyroid nodules: is aspiration of ethanol-mixed fluid advantageous? AJNR Am J Neuroradiol 2005;26:2122–27 [PMC free article] [PubMed] [Google Scholar]
- 9. Bennedbak FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab 2003;88:5773–77 [DOI] [PubMed] [Google Scholar]
- 10. Livraghi T, Paracchi A, Ferrari C, et al. The treatment of autonomous thyroid nodules with percutaneous ethanol injection: 4-year experience. Radiology 1994;190:529–33 [DOI] [PubMed] [Google Scholar]
- 11. Mazzeo S, Toni MG, De Gaudio C, et al. Percutaneous injection of ethanol to treat autonomous thyroid nodules. AJR Am J Roentgenol 1993;161:871–76 [DOI] [PubMed] [Google Scholar]
- 12. Papini E, Panunzi C, Pacella CM, et al. Percutaneous ultrasound-guided ethanol injection: a new treatment of toxic autonomously functioning thyroid nodules? J Clin Endocrinol Metab 1993;76:411–16 [DOI] [PubMed] [Google Scholar]
- 13. Monzani F, Caraccio N, Goletti O. Five-year follow-up of percutaneous ethanol injection for the treatment of hyperfunctioning thyroid nodules: a study of 117 patients. Clin Endocrinol 1997;46:9–15 [DOI] [PubMed] [Google Scholar]
- 14. Tarantino L, Giorgio A, Mariniello N, et al. Percutaneous ethanol injection of large autonomous hyperfunctioning thyroid nodules. Radiology 2000;214:143–48 [DOI] [PubMed] [Google Scholar]
- 15. Kim DW, Rho MH, Park HJ, et al. Ultrasonography-guided ethanol ablation of predominantly solid thyroid nodules: factors predicting the outcome. Br J Radiol 13 December 2011; doi: 10.1259/bjr/81849588 [DOI] [PMC free article] [PubMed]
- 16. Zingrillo M, Collura D, Ghiggi MR, et al. Treatment of large cold benign thyroid nodules not eligible for surgery with percutaneous ethanol injection. J Clin Endocrinol Metab 1998;83:3905–07 [DOI] [PubMed] [Google Scholar]
- 17. Papini E, Pacella CM, Verde G. Percutaneous ethanol injection (PEI): what is its role in the treatment of benign thyroid nodules? Thyroid 1995;5:147–50 [DOI] [PubMed] [Google Scholar]
- 18. Mauz PS, Maassen MM, Braun B, et al. How safe is percutaneous ethanol injection for treatment of thyroid nodule? Report of a case of severe toxic necrosis of the larynx and adjacent skin. Acta Otolaryngol 2004;124:1226–30 [DOI] [PubMed] [Google Scholar]