Abstract
Background
Persistent fatigue, breathlessness, and reduced exercise tolerance have been reported following acute COVID‐19 infection. Although immuno‐thrombosis has been implicated in acute COVID‐19 pathogenesis, the biological mechanisms underpinning long COVID remain unknown. We hypothesized that pulmonary microvascular immuno‐thrombosis may be important in this context.
Methods
One hundred fifty COVID‐19 patients were reviewed at St James's Hospital Dublin between May and September 2020 at a median of 80.5 (range 44–155) days after initial diagnosis. These included patients hospitalized during initial illness (n = 69) and others managed entirely as out‐patients (n = 81). Clinical examination, chest x‐ray, and 6‐min walk tests were performed. In addition, a range of coagulation and inflammatory markers were assessed.
Results
Increased D‐dimer levels (>500 ng/ml) were observed in 25.3% patients up to 4 months post‐SARS‐CoV‐2 infection. On univariate analysis, elevated convalescent D‐dimers were more common in COVID‐19 patients who had required hospital admission and in patients aged more than 50 years (p < .001). Interestingly, we observed that 29% (n = 11) of patients with elevated convalescent D‐dimers had been managed exclusively as out‐patients during their illness. In contrast, other coagulation (prothrombin time, activated partial thromboplastin time, fibrinogen, platelet count) and inflammation (C‐reactive protein, interleukin‐6, and sCD25) markers had returned to normal in >90% of convalescent patients.
Conclusions
Elucidating the biological mechanisms responsible for sustained D‐dimer increases may be of relevance in long COVID pathogenesis and has implications for clinical management of these patients.
Keywords: coagulation parameter, COVID‐19, D‐dimer, out‐patient follow‐up
ESSENTIALS
-
•
Persistent fatigue, breathlessness, and reduced exercise tolerance following acute COVID‐19 infection has been termed long COVID syndrome.
-
•
We observed sustained elevated D‐dimer levels in 25% of patients reviewed a minimum of 6 weeks following initial diagnosis. Although D‐dimers remained elevated, other coagulation and inflammation markers had returned to normal in the majority of patients.
-
•
Despite ongoing symptoms and elevated D‐dimers, computed tomography pulmonary angiogram screening in eight patients failed to demonstrate pulmonary embolism.
-
•
Further adequately powered studies will be required to elucidate the mechanisms underlying elevated D‐dimer levels during COVID‐19 convalescence.
Alt-text: Unlabelled Box
1. INTRODUCTION
The coronavirus SARS‐CoV‐2 is responsible for the current global pandemic that has resulted in significant morbidity and mortality.1 Features of the acute illness are well described, ranging from mild disturbance in smell and coryzal symptoms to acute respiratory failure and the need for mechanical ventilation.2., 3. Changes in coagulation parameters are common in patients presenting with acute COVID‐19. In particular, elevated plasma levels of fibrin degradation D‐dimers represent a common finding in hospitalized patients. Furthermore, progressive increases in D‐dimers in COVID‐19 are associated with significantly worse clinical outcomes.4 In keeping with coagulation activation in severe COVID‐19, high rates of thrombotic complications, particularly pulmonary emboli, have also been described.5 These data led to initial suggestions that D‐dimer levels may have a role to play in decisions regarding clinical management for patients with SARS‐CoV‐2 infection.6
Importantly, the biological mechanisms underpinning the elevated plasma D‐dimer levels associated with COVID‐19 remain poorly understood. Initial studies from Wuhan, China suggested that the increase in D‐dimers was associated with disseminated intravascular coagulopathy (DIC).7 In contrast, however, more recent studies from Europe and North America suggest that overt DIC is actually rare in COVID‐19 patients receiving prophylactic dose low molecular weight heparin (LMWH). Nevertheless, high D‐dimer levels remain a consistent finding in this latter cohort despite their anticoagulant therapy. In addition, elevated D‐dimers have been shown to be an independent biomarker for poor prognosis even in COVID‐19 patients being treated with LMWH.8 In this context, ongoing clinical trials are comparing the efficacy and safety of different doses of LMWH in COVID‐19. Moreover, extended duration anticoagulant therapy has been proposed for COVID‐19 patients following discharge, particularly after prolonged intensive care unit (ICU) admission.
Evidence is emerging of persistent symptoms following acute SARS‐CoV‐2 illness in a high proportion of patients. This “long COVID” phenomenon has been defined as “not recovering for several weeks or months following the start of symptoms that were suggestive of COVID.”9 Although fatigue, breathlessness, and reduced exercise tolerance have been reported as common features,10 the pathological mechanisms underlying these persistent symptoms remain unknown. Given the role of coagulation activation, fibrinolysis, and pulmonary micro‐vascular immuno‐thrombosis in acute COVID‐19 pathogenesis, we hypothesized that similar mechanisms may also be important in long COVID. To address this hypothesis, we investigated clinical parameters together with coagulation and inflammation biomarkers in COVID‐19 patients during convalescence.
2. METHODS
Patients were enrolled from the post‐COVID‐19 review clinic in St James's Hospital (SJH), Dublin, Ireland between May and September 2020. Appointments for this clinic were offered to all individuals diagnosed with a positive SARS‐CoV‐2 nasopharyngeal swab polymerase chain reaction (PCR) at SJH. Patients were reviewed at a minimum of 6 weeks following (i) resolution of symptoms or (ii) hospital discharge. Informed written consent was obtained from all participants in accordance with the Declaration of Helsinki.11 Ethical approval for the current study was obtained from the Tallaght University Hospital/SJH Joint Research Ethics Committee. Demographic, treatment, and outcome data were derived from the hospital electronic patient record. Coagulation assays were performed in the National Coagulation Laboratory (SJH) including prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen as previously described.12 D‐dimer levels were measured using the HemosIL D‐Dimer HS 500 latex enhanced immunoassay, and a manufacturer derived cut‐off upper limit of normal of 500 ng/ml. Serum C‐reactive protein (CRP), soluble CD25 (sCD25), and interleukin‐6 (IL‐6) were measured in a single laboratory in SJH. A 6‐min walk test (6 MWT) and chest x‐ray were performed at out‐patient department review, with distance covered, perception of maximal exertion (measured by Modified Borg Scale), and lowest arterial oxygen saturation recorded during 6 MWT. Fatigue was also assessed using the Chalder fatigue scale.13., 14. Statistical analysis was carried out using STATA v15.0. Univariate analysis was performed on important demographic and acute illness variables to examine differences between those with elevated convalescent D‐dimers and those with normal D‐dimers, using t‐test, Wilcoxon rank‐sum, and Chi‐squared test as appropriate. Multivariate analysis was then performed (see Appendix S1 in supporting information for details) to explore potential predictors of elevated D‐dimer.
3. RESULTS AND DISCUSSION
A total of 150 patients (85 female, 56.7%) were consecutively enrolled with a mean age of 47.3 (SD 15.4) years (Table 1 ). One hundred and seven patients (71.3%) were White, 30 (20%) were Asian, 10 (6.7%) were African, and 3 patients (2%) were of Latino/Hispanic ethnicity. Underlying co‐morbidities were present in 81 (54%) with a median comorbidity count of 1 (interquartile range [IQR] 0–2). Common comorbidities in our cohort were: hypertension (27/150, 18%), type 2 diabetes mellitus (14/150, 9.3%), asthma (14/150, 9.3%), hyperlipidemia (12/150, 8%), ischemic heart disease (10/150, 6.7%), and malignancy (10/150, 6.7%). Obesity, (body mass index [BMI] ≥30 kg/m2) was observed in 23/150 patients (15.3%), and 28/150 patients (18.7%) were overweight (BMI 25–30 kg/m2). One patient (0.7%) was underweight (BMI <18.5 kg/m2) and the remaining patients (65.3%) had BMIs within normal range (BMI 18.5–25 kg/m2). Four patients had a pre‐existing diagnosis of atrial fibrillation and were on long‐term direct oral anticoagulants (DOACs). In all cases, DOAC therapy was continued during acute COVID‐19 infection through to time of convalescent review. Participants with mild illness (n = 81, 64%) were not hospitalized and did not receive thromboprophylaxis. Sixty‐nine patients (46%) with moderate‐‐severe disease were hospitalized with 16 (10.7%) requiring ICU admission (Table 1). All hospitalized patients received standard doses of LMWH prophylaxis throughout admission. In‐patient thromboprophylaxis was not adjusted according to D‐dimer levels or level of care (e.g., ward‐based or ICU) but doses were adjusted according to weight and renal function.12., 15. Weight based dosing was performed as follows: weight <50 kg: enoxaparin 20 mg once daily, 50 kg‐100 kg: enoxaparin 40 mg once daily, 101–150 kg: enoxaparin 40 mg twice daily and >150 kg: enoxaparin 60 mg twice daily. Two hospitalized patients developed objectively confirmed pulmonary embolism (PE) during their admissions. These individuals were initially treated with therapeutic LMWH and switched to apixaban on discharge. No patients with mild illness have had a diagnosis of venous thromboembolism (VTE) to date.
TABLE 1.
Demographic and clinical parameters for patients reviewed in COVID‐19 convalescent clinic
| Parameters | Normal range | Total cohort (n = 150) | D‐dimer within normal range (n = 112) | D‐dimer elevated (n = 38) |
|---|---|---|---|---|
| Demographic & clinical parameters | ||||
| Age – years (SD) |
47.3 (15.4) | 44.6 (14.2) | 55.6 (16.2) | |
| Sex – female (%) | 85 (56.7) | 68 (60.7) | 17 (44.7) | |
| BMI – kg/m2 (SD) | 27.8 (4.8) | 28.3 (5.2) | 26.9 (4.5) | |
| Co‐morbidities median (IQR) |
1 (0–2) | 0 (0–2) | 2 (1–4) | |
| Time to follow‐up median (IQR) |
80.5 (67–112) | 82 (68–114) | 72.5 (62–86) | |
| Hospitalization – n (%) | 69 (46) | 42 (37.5) | 27 (71) | |
| Parameters at convalescent review | ||||
| D‐dimer (ng/ml) median (IQR) |
0–500 | 327 (224–502) | 262.5 (215–355) | 744 (607–1038) |
| CRP (mg/ml) median (IQR) |
0–5 | 1.23 (1–2.65) | 1 (1–2.5) | 1.9 (1.2–3.6) |
| IL−6 (pg/ml) median (IQR) |
0–7.26 | 0 (0–3.41) | 0 (0–0) | 0 (0–5.1) |
| PT (sec) median (IQR) |
9.9–13.1 | 11 (10.5–11.7) | 10.9 (10.5–11.7) | 11.1 (10.5–11.7) |
| APTT (sec) median (IQR) |
24–36 | 30.7 (29–32.5) | 30.9 (29.2–32.9) | 30 (28.6–31.4) |
| Fibrinogen (g/L) median (IQR) |
1.9–3.5 | 3 (2.7–3.4) | 2.9 (2.6–3.3) | 3.35 (2.9–3.6) |
| Platelets (×109/L) median (IQR) |
140–450 | 263 (222–301) | 266 (227–304) | 242 (218–282) |
| 6 MWT distance (m) median (IQR) |
400–700 | 450 (380–520) | 465 (415–528) | 380 (310–500) |
| Maximal Borg Score median (IQR) |
3 (2–5) | 3 (2–5) | 3 (2–6) | |
| Lowest desaturation (%) median (IQR) |
95 (94–96) | 95 (94–96) | 95 (94–96) | |
| Abnormal chest X‐ray n (%) |
14 (9) | 7 (6) | 7 (18) | |
Notes:
The demographic data and laboratory results are shown for the entire cohort (n = 150 patients). In addition, the cohort have been subdivided into patients with either normal or elevated D‐dimer levels at time of out‐patient assessment.
Abbreviations: 6 MWT, 6 min walk test; APTT, activated partial thromboplastin time; BMI, body mass index; CRP, C‐reactive protein; IL‐6, interleukin‐6; IQR, interquartile range; PT, prothrombin time.
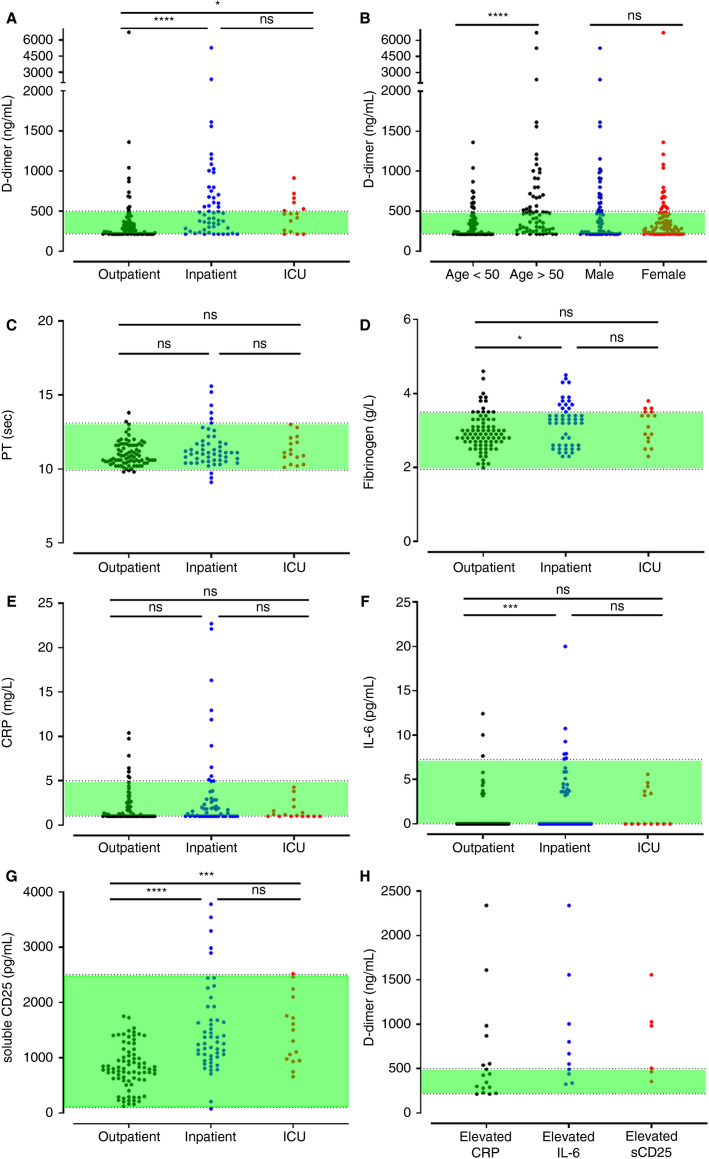
Participants were assessed at a median of 80.5 (range 44–155) days after initial diagnosis. Median D‐dimer level in the entire cohort of COVID‐19 convalescent patients was 327 ng/ml, which was within our local normal range. However, we observed marked inter‐individual variation, with D‐dimer levels ranging from 215 to 6726 ng/ml. Importantly, elevated D‐dimer levels were observed in 38 (25.3%) patients at time of follow‐up (Figure 1A ). Median D‐dimer levels in this subgroup was 744 ng/ml (range 504–6726 ng/ml). Furthermore, 12/150 (8%) of the COVID‐19 patients studied during recovery phase had markedly increased D‐dimer levels more than twice the upper limit of normal (ULN). On univariate analysis, elevated convalescent D‐dimers were significantly more common among COVID‐19 patients who had required hospital admission compared to those with mild disease (X2 12.86; p < .001; Table 1, Figure 1A). Nevertheless, significant inter‐individual variability in D‐dimer levels during convalescence was observed even among hospitalised COVID‐19 patients (Figure 1A). For example, D‐dimer levels had normalized by time of review in 10/16 (62.5%) patients who had required ICU support. Interestingly, we observed that 29% (n = 11) of patients with abnormally elevated D‐dimer at time of follow‐up had been managed exclusively as out‐patients during their illness (Figure 1A). On univariate analysis, elevated convalescent D‐dimers were significantly more frequent in patients aged >50 years (t −3.98 p < .001) and in those with comorbidities (comorbidity count ‐ z 2.03 p < .001; Table 1, Figure 1B). Collectively, these novel data demonstrate that sustained elevations in D‐dimers are a common finding in patients following SARS‐CoV‐2 infection and occur more frequently in those with severe acute disease and in older patients.
FIGURE 1.
Coagulation and inflammatory parameters in COVID‐19 patients at convalescent follow‐up. Out‐patient results are grouped according to whether acute infection was managed as an out‐patient, in‐patient, or in‐patient requiring intensive care unit (ICU) admission showing: (A) D‐dimers according to initial illness severity; (B) D‐dimers stratified by age and gender; (C) prothrombin time; (D) fibrinogen; (E) C‐reactive protein (CRP); (F) interleukin‐6 (IL‐6); (G) soluble CD25 (SCD25); (H) D‐dimers in patients with elevated CRP, IL‐6, and sCD25 at convalescence. Dotted lines represent the lower limit of detection and the upper limit of normal for D‐dimer and IL‐6. Dotted lines represent the upper and lower limit of the normal reference ranges for all other parameters with results in the green‐shaded areas falling within the normal reference range. Differences assessed by Kruskal‐Wallis testing with Dunn's post hoc test. *p < .05; **p < .01; ***p < .001; ****p < .0001; ns, not significant
To investigate the biological mechanisms responsible for the sustained increase in D‐dimers, we investigated other coagulation and inflammatory markers in convalescent COVID‐19 patients. In contrast to the D‐dimer results, PT and APTT had returned to normal range in a much larger proportion of patients (Figure 1C, Table 1). Moreover, convalescent patients had no evidence of hypofibrinogenemia (Figure 1D) or thrombocytopenia (Table 1) during recovery. These results suggest that neither low‐grade DIC nor systemic coagulation activation can explain the elevation in D‐dimer levels in convalescent patients. Similarly, the elevated plasma D‐dimer levels were not associated with evidence of ongoing acute phase responses. Mild increases in CRP were only present in 17/150 (11%) patients (Figure 1E), increased IL‐6 levels in 10/150 (6.7%; Figure 1F), and increased sCD25 in only 6/150 (4%; Figure 1G) of convalescent COVID‐19 patients. Some patients with ongoing elevated inflammatory markers also had elevated D‐dimer levels (Figure 1H). Importantly, however, all three inflammatory markers (CRP, IL‐6, and sCD25) were within the normal range in the majority of convalescent COVID‐19 patients with elevated D‐dimers (26/38; 68.5%).
Multivariate analysis was used to explore potential predictors of elevated convalescent D‐dimers. For COVID‐19 patients who had required hospitalization, the relationship between elevated D‐dimer levels and previous coagulation‐inflammation assays performed during acute SARS‐CoV‐2 illness was investigated. Elevated D‐dimers at follow‐up had no relationship with peak D‐dimers, fibrinogen, platelets, or CRP (Table S1 in supporting information). Similarly, no significant relationships were observed between elevated convalescent D‐dimers and other markers of coagulation or inflammation at convalescence, or time to follow‐up Table S1). The relationship between acute illness severity and age with elevated convalescent D‐dimers was not seen on multivariate analysis. Finally, we examined the relationship between elevated convalescent D‐dimers and clinical features in convalescent COVID‐19 patients. Ongoing symptoms consistent with long COVID were common in our cohort. Seventy‐seven patients (51%) met the case definition for fatigue, 43 of whom (43/77, 56%) had experienced mild COVID‐19 and were managed entirely as out‐patients. On univariate analysis, abnormal chest x‐rays during convalescence were more common in patients with elevated D‐dimers compared to patients in whom D‐dimer levels were within normal range (χ2 6.01, p = .01). However, on multivariate analysis, there was no relationship between any of the clinical parameters assessed and elevated convalescent D‐dimers (Table S2 in supporting information).
Notwithstanding the limited size of our cohort, the lack of a complete dataset on all patients, and the range in time for patient review in follow‐up convalescent clinic, the observation of prolonged increased D‐dimer levels in COVID‐19 has potential implications with respect to the management of these patients. Based upon clinical findings (including chest pain and shortness of breath), eight symptomatic patients in our cohort proceeded to undergo computed tomography pulmonary angiogram (CTPA). All these patients also had elevated D‐dimer levels. Thus, CTPA was performed in 8/38 (21%) of our total cohort with increased D‐dimer levels at convalescent clinic review. Importantly, none of these scans identified any evidence of PE. These findings suggest that the utility of standard VTE diagnostic algorithms may need to be reviewed in this context. Further clinical studies will be necessary to consider whether D‐dimer cut‐off levels should be amended to reduce the risk of excessive CTPA testing in convalescent COVID‐19 patients.
Interestingly, two patients in our elevated D‐dimer group subsequently developed vascular complications. A 51‐year‐old female was admitted with bilateral PE. In addition, a 65‐year‐old female presented with non‐ST‐elevation myocardial infarction, which required percutaneous coronary intervention and subsequent coronary artery bypass grafting. No patients in our normal D‐dimer group have suffered thrombotic complications to date.
In conclusion, our findings clearly demonstrate that elevation in D‐dimers is a common finding in COVID‐19 patients during convalescence. Importantly, this increase in D‐dimer was seen at a median of greater than 2 months following resolution of acute COVID‐19 infection and was observed in a cohort comprising predominantly young patients (median age 47 years) who mostly (64%) recovered without hospitalization. Interestingly, the increase in convalescent D‐dimers remained despite normalization of inflammatory markers and other coagulation parameters in most patients. Further adequately powered studies will be required to elucidate the mechanisms underpinning elevated D‐dimer levels during recovery phase. However, previous studies have highlighted disturbances in the balance between pulmonary coagulation and fibrinolytic pathways in models of acute respiratory distress syndrome, pneumonia, and ventilator‐associated lung injury.16., 17., 18. Furthermore, it seems likely that extravascular pulmonary fibrinolysis may be important in the etiology of elevated D‐dimers during COVID‐19 recovery. Given emerging data regarding post‐infection long COVID syndrome, as well as ongoing discussions regarding optimal duration of thromboprophylaxis in COVID‐19 patients following discharge, defining these mechanisms may be of direct clinical relevance.
CONFLICTS OF INTEREST
J.S.O. has served on the speaker's bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma. He has also served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer. J.S.O’D, has also received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk.
AUTHOR CONTRIBUTIONS
LT, HF, CNC, JSO: conception, patient enrolment, data collection, and interpretation. All authors contributed to literature review, final draft writing, and critical revision. All the authors have participated sufficiently in this work, take public responsibility for the content, and have made substantial contributions to this research.
ACKNOWLEDGMENTS
This work was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning and the Health and Social Care, Research and Development Division, Northern Ireland. The Irish COVID‐19 Vasculopathy Study (ICVS) is supported by a Health Research Board COVID‐19 Rapid Response award (COV19‐2020‐086). The research was also supported by a philanthropic grant from 3 M to the RCSI University of Medicine and Health Sciences in support of COVID‐19 research. J.O.D. was supported by the National Children’s Research Centre Project Award (C/18/1).
Wellcome/HRB Irish Clinical Academic Training (ICAT) Programme203930/B/16/Z
Health Research Board COVID‐19 Rapid Response awardCOV19‐2020‐086
Philanthropic grant from 3M to the RCSI University of Medicine and Health Sciences in support of COVID‐19 researchNA
National Children’s Research Centre Project AwardC/18/1
Footnotes
Liam Townsend and Helen Fogarty contributed equally to this article.
Manuscript handled by: Saskia Middeldorp
Final decision: Saskia Middeldorp, 08 February 2021
Supporting Information
App S1
REFERENCES
- 1.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr C., Hughes G., Mckenna L., Bergin C. Prevalence of smell and taste dysfunction in a cohort of CoVID19 outpatients managed through remote consultation from a large urban teaching hospital in Dublin, Ireland. Infect Preven Pract. 2020;2(3):100076. doi: 10.1016/j.infpip.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogarty H., Townsend L., Ni Cheallaigh C., et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakr Y., Giovini M., Leone M., et al. Pulmonary embolism in patients with coronavirus disease‐2019 (COVID‐19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:1–13. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu A., Liu Y., Zayac A.S., Olszewski A.J., Reagan J.L. Intensity of anticoagulation and survival in patients hospitalized with COVID‐19 pneumonia. Thromb Res. 2020;196:375–378. doi: 10.1016/j.thromres.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidali S., Morosetti D., Cossu E., et al. D‐dimer as an indicator of prognosis in SARS‐CoV‐2 infection: a systematic review. ERJ Open Res. 2020;6(2):00260‐2020. doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of post‐acute covid‐19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 10.Townsend L., Dyer A.H., Jones K., et al. Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Association W.M. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373. [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty H., Townsend L., Ni Cheallaigh C., et al. COVID‐19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalder T., Berelowitz G., Pawlikowska T., et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 14.Jackson C. The Chalder fatigue scale (CFQ 11) Occup Med. 2015;65:86. doi: 10.1093/occmed/kqu168. [DOI] [PubMed] [Google Scholar]
- 15.Fogarty H., Townsend L., Ni Cheallaigh C., et al. More on COVID‐19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1060–1061. doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glas G.J., Van Der Sluijs K.F., Schultz M.J., Hofstra J.J., Van Der Poll T., Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost. 2013;11:17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 17.O'Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J., O'Donnell J.S. Endothelial cells orchestrate COVID‐19 coagulopathy. Lancet Haematol. 2020;7:e553–e555. doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1