Abstract
Aims and objectives
Our objective was to rapidly adapt and scale a registered nurse‐driven Coordinated Transitional Care (C‐TraC) programme to provide intensive home monitoring and optimise care for outpatient Veterans with COVID‐19 in a large urban Unites States healthcare system.
Background
Our diffuse primary care network had no existing model of care by which to provide coordinated result tracking and monitoring of outpatients with COVID‐19.
Design
Quality improvement implementation project.
Methods
We used the Replicating Effective Programs model to guide implementation, iterative Plan‐Do‐Study‐Act cycles and SQUIRE reporting guidelines. Two transitional care registered nurses, and a geriatrician medical director developed a protocol that included detailed initial assessment, overnight delivery of monitoring equipment and phone‐based follow‐up tailored to risk level and symptom severity. We tripled programme capacity in time for the surge of cases by training Primary Care registered nurses.
Results
Between 23 March and 15 May 2020, 120 Veterans with COVID‐19 were enrolled for outpatient monitoring; over one‐third were aged 65 years or older, and 70% had medical conditions associated with poor COVID‐19 outcomes. All Veterans received an initial call within a few hours of the laboratory reporting positive results. The mean length of follow‐up was 8.1 days, with an average of 4.2 nurse and 1.3 physician or advanced practice clinician contacts per patient. The majority (85%) were managed entirely in the outpatient setting. After the surge, the model was disseminated to individual primary care teams through educational sessions.
Conclusion
A model based on experienced registered nurses can provide comprehensive, effective and sustainable outpatient monitoring to high‐risk populations with COVID‐19.
Keywords: coordinated transitional care, COVID‐19, outpatient monitoring, transitional care
WHAT DOES THIS PAPER CONTRIBUTE TO THE WIDER GLOBAL CLINICAL COMMUNITY?
Unexpected and sudden events such as the COVID‐19 pandemic present a tremendous challenge to healthcare systems with a diffuse network of primary care teams.
RNs are uniquely qualified to provide the assessment, care coordination, education, and evaluation of home safety and care needs that COVID‐19‐positive patients require.
The results of this clinical innovation project demonstrate that a monitoring programme based primarily on RN effort was feasible and effective in optimising COVID‐19 care.
1. INTRODUCTION
As of November 2020, 9.7 million United States (US) citizens have been diagnosed with COVID‐19 and over 230,000 have died, accounting for 19% of deaths from COVID‐19 worldwide (Coronavirus Resource Center, 2020). The pandemic has exposed multiple weaknesses of the US healthcare system, including its lack of universal healthcare coverage and under‐resourced public health services (Huston et al., 2020). Existing pandemic plans were outdated and gave no guidance for management of outpatient populations to primary care practices (Pandemic Influenza Plan, 2017 Update, 2017). In addition, the United States had not kept pace with other nations in the development of telehealth – models that deliver healthcare at a distance through phone, video or other virtual means – due to a lack of reimbursement, concerns about confidentiality and insufficient investment in telehealth technology (Peabody et al.2019). As a result, US healthcare systems were forced to rapidly create models of virtual care for outpatients with COVID‐19.
In March 2020, Boston was one of the first major US cities to be affected by the pandemic, and our Veterans Affairs (VA) Healthcare System turned to international experience for guidance. Evidence from the response in Asia and Italy suggested that that the majority of patients could be safely managed at home and that doing so would limit unnecessary exposures, decrease the burden on acute care resources and save personal protective equipment (PPE; Report of the WHO‐China Joint Mission on Coronavirus Disease (COVID‐19), 2020). Primary care settings in Asia and Africa had a history of effectively leveraging telehealth to respond to SARS and Ebolavirus (Keshvardoost et al., 2020). In addition to medical monitoring, existing guidance emphasised the importance of appropriate triage of systems, assessment of home safety and resources, education of patients and families and care coordination, suggesting that experienced registered nurses (RNs) were well suited to play a key role in the model (Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19), 2020). We therefore sought to develop a nurse‐driven system of outpatient monitoring and support for Veterans with known or suspected COVID‐19.
2. BACKGROUND
The VA Boston Healthcare System provides primary care for over 30,000 Veterans within a 40‐mile radius of the greater Boston area. The VA is unique in the United States as a federally funded, single‐payer healthcare system mandated to provide healthcare access to Veterans across the nation. As such, it has been an early implementer and leader of telehealth services. Compared to general populations, our Veterans are substantially older, predominantly male and have a higher prevalence of the comorbidities associated with worse COVID‐19 outcomes, such as chronic lung disease, heart disease and diabetes (Kazis et al., 2004). The first Veteran with COVID‐19 presented to VA Boston for testing on 17 March 2020. At that time, ordering of COVID‐19 testing for outpatients was performed by the Emergency Department (ED) and Urgent Care (UC) staff due to the limited supply of tests and swabs available. This created a disconnect in communication with the patient's primary care team, who lacked the necessary systems to respond immediately with close monitoring that would include weekends. There was an urgent need for a mechanism to track and report test results and a provide a coordinated approach to clinical follow‐up of outpatients with COVID‐19. Our institution's Incident Command Team asked our Coordinated Transitions of Care (C‐TraC) programme to fill this need.
C‐TraC is a protocolised, telephone‐based intervention originally developed at VA Madison to provide high‐risk patients an extra layer of care during the transition from hospital to home and the immediate post‐discharge period (Kind et al., 2012). The model relies on experienced RNs who provide intensive case management after discharge, and the protocols and training manuals are publicly available (Kind, 2012). In their pre‐COVID‐19 role, C‐TraC RNs meet inpatients prior to discharge, participate in the discharge plan and then call patients soon after discharge and once a week for four weeks. C‐TraC goals are to educate and empower patients and caregivers, identify ‘red flag’ symptoms that indicate the need to seek medical attention, provide their direct contact information for issues that arise and ensure that the planned medical follow‐up is obtained. The RNs work in close collaboration with patient's primary and specialty care teams. This intervention was shown to decrease the risk of readmission among high‐risk patients in our institution by more than half (Reese et al., 2019). As such, C‐TraC was perfectly positioned to rapidly leverage its existing infrastructure, protocols and expertise to provide COVID‐19‐positive Veterans a high quality, in‐depth outpatient assessment and monitoring system. Using an implementation science framework, our goal was to adapt our programme into a primarily RN‐driven model of care for outpatients with COVID‐19 that would achieve rapid reporting of test results and close and coordinated monitoring of all positive patients at home.
3. METHODS
3.1. Project goals and design
The goals of this clinical innovation project were to: (1) report negative COVID‐19 tests to patients within 24 h, (2) report COVID‐19 positive tests and assess patient disease severity and care needs within 4 h (for results received during working hours, 7 days a week), (3) provide protocolised follow‐up of COVID‐19 positive patients by phone or video visit and facilitate timely referral for acute care evaluation when needed and (4) rapidly scale and disseminate the model within our facility and to regional VA hospitals.
We used a modified version of the US Center for Disease Control's (CDC) Replicating Effective Programs (REP) framework, a conceptual model to ensure evidence‐based adaptation of programmes across variations in settings and contexts (Kilbourne et al., 2007). The four phases of the REP model include: (1) establishing pre‐conditions, (2) pre‐implementation, (3) implementation and (4) maintenance and evolution. REP has been used to successfully adapt C‐TraC to other sites and purposes (Kind et al., 2016). In this case, we adapted our protocol from a post‐discharge intervention delivered over 30 days to an intensive phone‐based home monitoring programme delivered over 2 weeks. We used iterative Plan‐Do‐Study‐Act cycles (Langley et al., 2009) to refine and adapt the initial protocol to meet changing needs during the first wave of the pandemic. This project was reviewed by the VABHS Research and Development Committee and determined to be non‐research.
3.2. Data collection and reporting
In order to determine whether tests were reported in a timely fashion, a spreadsheet was updated daily to record when test results were reported by the lab and when the patient was contacted by a member of our team. We performed chart review to extract the key variables needed to describe the cohort and assess programme efficacy. We used percentages to describe categorical variables and means and standard deviations to describe continuous variables. We based our manuscript on the SQUIRE 2.0 (Appendix S1) Revised Standards for Quality Improvement Reporting Excellence (Ogrinc et al., 2016).
4. RESULTS
4.1. Establishing Pre‐conditions
Pre‐conditions in the REP model are pre‐existing characteristics of the health system and local context that are critical to consider in building a new intervention. Our resources included a large primary care network with an established practice of video visits (Veteran's Video Connect) and a Home Telehealth programme (nurse‐driven home monitoring programme which sends vital sign and blood sugar monitoring technology to the home within 24 h), a Hospital in Home programme (acute care in the home to Veterans within a 20‐mile radius of the hospital), and robust local and regional health informatics groups. System barriers included: (1) absence of expertise in outpatient management of COVID‐19, (2) lack of equipment such as thermometers and fingertip pulse oximeters that would allow for adequate home monitoring and (3) no existing clinical entity that was staffed to provide frequent follow‐up by phone/video for the first two weeks of symptomatic disease with coverage on weekends.
4.2. Pre‐Implementation
4.2.1. Critical stakeholders
This REP phase involves assembling key stakeholders to help customise delivery of the protocol, putting logistics in place and training team members. Our stakeholders included the Deputy Chief of Staff, two Infectious Disease (ID) specialists and the Chief of Primary Care Service. The group determined that initially, it would be best to have the C‐TraC team manage all COVID‐19 test results and monitor‐positive patients. This would allow rapid development of experience quickly define the needed tools and clinical pathways, as well as training materials. It was felt that experienced RN case managers were best qualified to play the leading roles in the team as most of the work entailed result reporting, care coordination, symptom assessment and patient education.
4.3. Customising delivery, logistics and training
We adapted the C‐TraC protocol to the typical clinical course of COVID‐19, as shown in Figure 1. While we expected most patients to have mild or moderate symptoms, it was critical to recognise worrisome signs of dehydration and hypoxia, which most often present at the end of the first week and beginning of the second. Initial triage and follow‐up were tailored based on CDC risk factors, severity of symptoms and day since symptom onset, as shown in Figure 2 and Table S1 (Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19), 2020). Symptoms were considered mild if they did not limit a patient's activities of daily living (ADL) and moderate if they impacted ADL but did not reach the threshold for referral to the emergency department (ED). Severe symptoms, such as decreasing oxygen saturation, difficulty speaking in full sentences, falls, confusion, angina and hypotension, almost always required ED referral.
FIGURE 1.
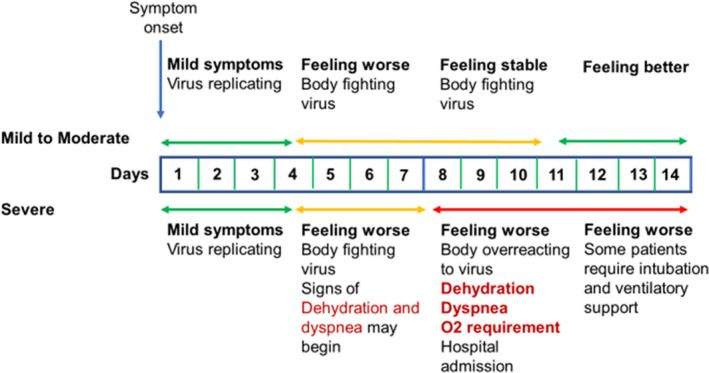
Typical clinical progression of COVID‐19 infection by symptom severity. While most symptomatic patients have mild to moderate symptoms, up to 20% can develop severe disease and require inpatient care
FIGURE 2.
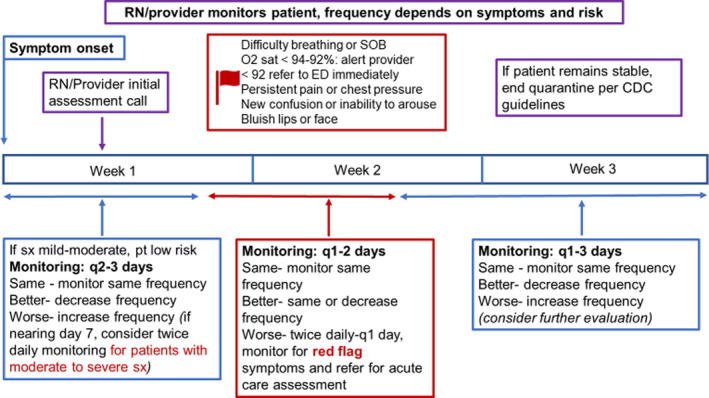
Protocol for outpatient follow‐up of patients with confirmed or presumed positive COVID‐19. The protocol is primarily nurse‐driven with MD or advanced practice clinician support for patients with moderate to severe symptoms
We created detailed note templates designed to help RNs determine if a call from a MD or advanced practice clinician or ED referral was indicated (Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19), 2020). Key elements are shown in Table S2. An electronic referral to our team could be used to request follow‐up of outpatients who had known or suspected COVID‐19. Based on events unfolding in New York City, we worked with our Palliative Care service to obtain training on conversations about advanced care planning for COVID‐19 and identified helpful resources for these discussions (COVID Ready Communication Playbook, 2020). We created a weekend coverage system to report results and provide ongoing home monitoring to patients with worrisome symptoms. The C‐TraC RNs and supervising MD were briefed by the Infectious Diseases team and read guidance from the CDC and World Health Organization on COVID‐19 care (Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19), 2020; Report of the WHO‐China Joint Mission on Coronavirus Disease (COVID‐19), 2020).
4.4. Implementation
On 23 March 2020, the C‐TraC team began working directly with an ID specialist to contact ambulatory COVID‐19‐positive patients who had been tested in the ED, UC or outpatient testing tents. The RNs called patients with negative results. The initial call to positive patients was made by an RN, MD or advanced practice clinician (nurse practitioner or physician's assistant) depending on patient acuity, and RNs made most follow‐up calls. We requested the purchase of thermometers and fingertip pulse oximeters through our COVID‐19 Incident Management Team. Because patients could decompensate very quickly once they began showing signs of worsening oxygenation, we adapted the protocol to allow for calls twice or three times daily when needed at the peak of symptoms. We also noted that patients over 70 were more likely to present with functional decline rather than typical symptoms, so updated the triage template accordingly.
By week 2 after launch, the C‐TraC team was making calls independently and consulted the ID specialist as needed. Oximeters became available for overnight delivery and placed in the ED and UC clinics for distribution. Oximeters were generally supplied to patients with mild to moderate symptoms of COVID‐19 who had risk factors for poor outcome but could be ordered for any COVID‐19‐positive patient with worrisome symptoms.
By week three, we had created an initial educational presentation on the outpatient management of COVID‐19 that was updated with each successive week of experience and used to train new team members. We presented two webinars on our programme to VA Primary Care groups across the New England region. By this time, the volume of tests had grown substantially due to the growing incidence of COVID‐19 and more rapid test turnaround.
4.5. Maintenance and evolution
4.5.1. Local dissemination
During this, REP phase decisions are made about sustainment, expansion and dissemination. The stakeholders agreed that the model was successful but needed rapid expansion as the number of cases was beginning to overwhelm the capacity of the C‐TraC RNs. Primary Care had created a virtual clinic into which the Patient Call Center could schedule any patient with influenza‐like‐illness. This ‘TeleFlu’ team could evaluate patients by phone or video, and schedule them for COVID‐19 testing. The Teleflu clinic was both a first‐line resource for triaging worried well from sick patients and a control point for appropriate utilisation of COVID‐19 testing supplies. Experience derived from establishing the TeleFlu clinic and managing demand for COVID‐19 testing positioned Primary Care to take on patient notification of negative COVID‐19 results. This allowed the C‐TraC team to focus exclusively on patients with known or suspected COVID‐19.
By week four after launch, the Primary Care Service had identified staff who could be temporarily re‐assigned to replicate the C‐TraC model. The RNs had both acute care and telehealth experience. Based on estimates that patient volume might triple, the Primary Care COVID‐19 Outpatient Intensive Management Team (OIMT) consisted of two full‐time RNs and two supervising physicians. Patients who needed more comprehensive home monitoring could be followed by Home Telehealth RNs, thus expanding the pool of nurses with COVID‐19 experience. Due to the prevalence of social isolation, limited support and anxiety in our population, a part‐time social worker and a psychologist was added. This multidisciplinary team quickly adapted the C‐TraC education materials to the Primary Care setting and created their own referral mechanism and virtual clinics. In week four, OIMT team members were oriented by the C‐TraC team and began calling patients alongside C‐TraC staff. The OIMT programme fully launched in week five and received continuing mentoring from C‐TraC during a daily morning huddle. Coordination between OIMT and TeleFlu was facilitated by having both led by the same medical director. The evolution of the clinical implementation and average weekly census is displayed in Figure 3.
FIGURE 3.
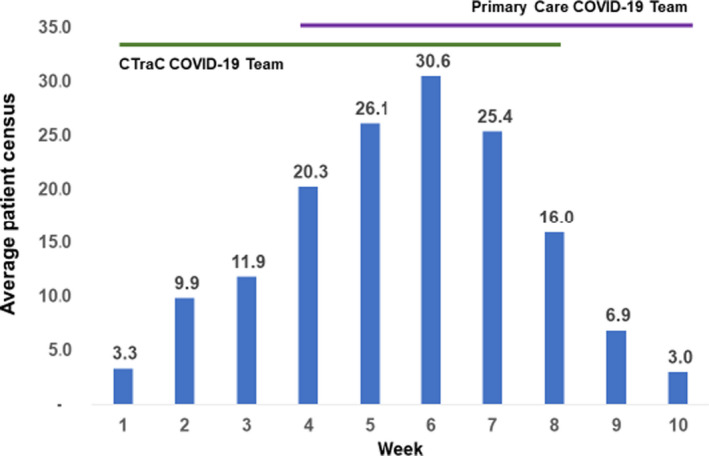
Average weekly patient census of outpatient COVID teams for the first 10 weeks of the outbreak at VA Boston. The Coordinated‐Transitions of Care (C‐TRAC) COVID‐19 Team trained and overlapped with the primary care COVID‐19 Outpatient Intensive Management Team (OIMT) for 5 weeks
4.6. Evolution
By week seven, there were signs that the incidence of COVID‐19 in the Boston area was declining, but there was a growing awareness that additional surges of COVID‐19 were possible. This required a long‐term plan based on existing resources. In week 9, C‐TraC returned to transitional care and he OIMT team helped to prepare individual primary care teams to manage the cases in their own patient panels. The training consisted of lectures on outpatient management of COVID‐19 that incorporated case presentations and time for questions. Members of the OIMT team made themselves available for consultation, with a plan to re‐constitute the team if needed in the face of a second surge.
4.7. Patient Population and Outcomes
From the beginning of the COVID‐19 C‐TraC programme on 23 March 2020 to its final day on 18 May 2020, 120 Veterans received outpatient monitoring. Patient characteristics are displayed in Table 1. The census peaked at thirty patients during the height of the surge, week 6 of the implementation. Eighty‐three Veterans were diagnosed with COVID‐19 at our medical centre and 37 at community hospitals; seven were initially tested as inpatients and then followed by our team.
TABLE 1.
Characteristics of COVID‐19‐positive Veterans followed in outpatient programme
| Variable | All patients (n = 120) | Tested in VA (n = 83) | Tested outside VA (n = 37) |
|---|---|---|---|
| Age categories, n (%) | |||
| <55 | 53 (44.2) | 40 (48.2) | 13 (35.1) |
| 55–65 | 25 (20.8) | 18 (21.7) | 7 (18.9) |
| 65–75 | 24 (20.0) | 17 (20.5) | 7 (18.9) |
| >75 | 18 (15.0) | 8 (9.6) | 10 (27.0) |
| Sex | |||
| Male, n (%) | 101 (84.2) | 69 (83.1) | 32 (86.5) |
| Female, n (%) | 19 (15.8) | 14 (16.9) | 5 (13.5) |
| Race | |||
| White (including Hispanic) | 88 (73.3) | 64 (77.1) | 24 (64.9) |
| Black/African American | 25 (20.8) | 13 (15.7) | 12 (32.4) |
| Unknown | 7 (5.9) | 6 (7.2) | 0 (0.0) |
| Lives Alone, n (%) | 30 (25.0) | 19 (24.4) | 11 (29.7) |
| Existing Medical Condition, n (%) | 84 (70.0) | 56 (67.5) | 28 (75.7) |
| Pulmonary disease | 26 (21.7) | 15 (18.1) | 11 (29.7) |
| Diabetes | 33 (27.5) | 22 (26.5) | 11 (29.7) |
| Cardiovascular disease | 64 (53.3) | 41 (49.4) | 23 (62.2) |
| Renal disease | 10 (8.3) | 3 (3.6) | 7 (18.9) |
| Liver disease | 10 (8.3) | 7 (8.4) | 3 (8.1) |
| Immunocompromised | 6 (5.0) | 4 (4.8) | 2 (5.4) |
| Neurologic disease | 8 (6.7) | 6 (7.2) | 2 (5.4) |
| Obesity | 32 (26.7) | 19 (22.9) | 13 (35.1) |
| Sleep apnoea | 17 (14.2) | 13 (15.7) | 4 (10.8) |
| Smoker | |||
| Current | 19 (15.8) | 12 (14.5) | 7 (18.9) |
| Former | 53 (44.2) | 38 (45.8) | 15 (40.5) |
| Severity of symptoms at initial call | |||
| None or Mild | 72 (60.0) | 47 (56.6) | 25 (67.6) |
| Moderate | 38 (31.7) | 28 (33.7) | 10 (27.0) |
| Severe | 10 (8.3) | 8 (9.6) | 2 (5.4) |
| Presenting symptoms | |||
| Cough | 76 (63.3) | 53 (63.9) | 23 (62.2) |
| Fever (overall) | 73 (60.8) | 55 (66.3) | 18 (48.6) |
| Documented | 39 (32.5) | 30 (36.1) | 9 (24.3) |
| Subjective | 34 (28.3) | 25 (30.1) | 9 (24.3) |
| Shortness of breath | 58 (48.3) | 44 (53.0) | 14 (37.8) |
| Myalgia | 58 (48.3) | 37 (44.6) | 21 (56.8) |
| Nasal congestion | 57 (47.5) | 37 (44.6) | 20 (54.1) |
| Headache | 49 (40.8) | 36 (43.4) | 13 (35.1) |
| Diarrhoea | 40 (33.3) | 27 (32.5) | 13 (35.14) |
| Chills and sweats | 37 (30.8) | 27 (32.5) | 10 (27.0) |
| Loss of taste/smell | 35 (29.2) | 23 (27.7) | 12 (32.4) |
| Chest pain | 21 (17.5) | 10 (12.1) | 10 (12.1) |
| Nausea/vomiting | 19 (15.8) | 14 (16.9) | 5 (13.5) |
| Abdominal Pain | 10 (8.3) | 5 (6.0) | 7 (18.9) |
The mean age of the cohort was only 54.7 years (SD 18.1), reflecting the predominance of work‐related exposures early in the epidemic. Patients were mostly male (n = 101; 84.2%) and white (n = 88; 73.3%), which is typical of the Veteran population in the Boston area. As expected, the cohort was at high risk of poor COVID‐related outcomes, with 84 (70%) having at least one of the coexisting medical conditions defined by the CDC. Cardiovascular disease, diabetes, obesity and chronic lung disease were the most common. Symptoms at initial presentation were mild in 72 (60%), moderate in 38 (31.7%) and severe in 10 (8.3%). Overall, 15 (12.5%) of Veterans developed severe symptoms during follow‐up. The most common symptoms on initial presentation were cough, followed by fever, dyspnoea and myalgia. Home Telehealth was engaged for thirteen patients, Hospital in Home for six, Social Work for four and Hospice or Palliative Care for two. About half of patients (n = 69) met criteria and received a fingertip pulse oximeter.
The programme successfully achieved all its goals. Nearly, all outpatients tested at VA Boston were informed of negative results in less than 24 hours and positive results within 4 hours after lab reporting. The programme was primarily driven by nurses, with 509 RN patient contacts compared to 162 MD (or advanced practice clinician) contacts. The mean length of follow‐up was 8.1 days (SD 5.6). Almost all contacts (n = 671; 97%) were by phone rather than video; video visits were strategically used for patients with moderate to severe symptoms. Nine (7.5%) patients were referred to the ED and discharged home, and 18 (15%) had an acute care admission. The mean age of hospitalised patients was 64.7 years; however, the five patients who received ICU care (of whom three were intubated) had a mean age of only 52.0 years. Two of the 120 patients died (1.7%), both of COVID‐19. Considering the high‐risk nature of our population, clinical outcomes were better than might be expected. The programme was easily and rapidly disseminated using existing resources to meet the needs of COVID‐19 outpatients throughout the healthcare system.
5. DISCUSSION
By quickly adapting an existing RN‐driven transitional care model, we were able to provide a coordinated response, call outpatient Veterans with COVID‐19 within a few hours of their test results, rapidly provide home monitoring equipment and deliver proactive evaluation and care. Using this model, 85% of our high‐risk population was safely managed in the outpatient setting with virtual care, primarily by nurses and almost entirely by phone. The approach was quickly scalable to serve the needs of our large and geographically dispersed outpatient population during the surge and was then adapted again to a more sustainable model disseminated across individual primary care teams.
Ours is one of a number of virtual care models that arose during the first months of the pandemic in the United States (Annis et al., 2020; Blazey‐Martin et al., 2020; Sinha et al., 2020; Wosik et al., 2020). Our outcomes were very similar to that of a virtual COVID programme in non‐VA academic hospital in the Boston area, in which 54 of 305 (18.0%) outpatients receiving intensive monitoring required acute care admission, 6 of whom died (2.0%) (Blazey‐Martin et al., 2020). Compared to these other models, which were driven by physicians, residents or advanced practice clinicians, our approach relied more heavily on protocolised triage and follow‐up by RNs. Another distinction is that our programme rarely used video telehealth despite the wide availability of a video telehealth infrastructure in VA. This was often due to patients inability to access or navigate video technology at home, a common occurrence among populations of lower socioeconomic status or older age in the US (Jaklevic, 2020). In addition, early in the pandemic the process of creating and accessing the video visits was time‐consuming and the system was unable to handle the large volume of users. VA has since responded by streamlining the technology and making tablets with Wi‐Fi readily available to Veterans. However, our findings suggest that video telehealth is not necessary to provide effective and safe outpatient monitoring; an RN and phone‐based model may be a low‐cost solution that would benefit low resource settings.
Several limitations should also be considered in the interpretation of our results. All our patients were US Veterans and predominantly male, potentially limiting the generalisability of our findings. As all outpatients with COVID‐19 received the intervention, we had no comparison group with which to establish efficacy. While no Veterans refused follow‐up by the programme, some were overwhelmed by the intensity of contact, especially those with psychiatric illness. In these cases, we had to balance our clinical concern with respect for the patient's privacy and use other means of communication such as text messaging or secure email.
Nurses have been described as the linchpin of healthcare systems and have played a central role in the COVID‐19 (Watkins & Neubrander, 2020). The value of telehealth nursing practice has been increasingly recognised in the United States, and a clear evidence base suggests that it may be as effective as face‐to‐face visits (Speyer et al., 2018). Currently, virtual transitional care and chronic disease management visits by RNs are reimbursable by the Centers for Medicare and Medicaid Services. However, the recent legislation that has expanded healthcare reimbursement for telehealth during the pandemic neglected to include RNs as eligible providers (Watkins & Neubrander, 2020). Our programme provides further evidence that experienced RNs can work assume more responsibility than is currently promoted by reimbursement practices while remaining within their scope. RNs expertise in triage, patient and family education, assessment of home safety, care coordination and self‐management support positioned them extremely well to serve on the front lines of our population‐based monitoring programme.
We faced a number of challenges in delivering virtual care to our outpatients with COVID‐19, as outlined in Table 2. Our patients, many of whom have post‐traumatic stress disorder and other psychiatric illnesses, experienced high levels of anxiety; they likely required more frequent calls than other populations. Self‐monitoring with oximeters was very reassuring, provided the patients a sense of control and prevented unnecessary acute care visits and calls to our call centre. Staff unfamiliar with COVID care needed to be trained quickly by staff who were extremely busy. A virtual team huddle every morning allowed us to review patients together and boost team education. We invited a social worker and psychologist to help us address issues such as food insecurity, exacerbation of mental health issues and help with advanced care planning. Occasionally, phone and video‐based support were not enough to adequately assess patients and led us to we reach out to home‐based services. Another RN‐driven team, the Hospital in Home (HIH) Program, partnered with us to provide strategic home‐based acute care to patients who needed blood draws or IV therapies. The HIH team also visited a group home in which seven Veterans with complex mental and medical illness were positive for COVID‐19 to assess the patients, educate the caregivers and help us establish a monitoring plan. The HIH RN manager then visited eight other group homes identified as higher risk for an outbreak to educate the staff on the importance of PPE, cleaning and the monitoring for symptoms. This collaboration allowed our team to transcend the limitations of a virtual programme and optimise Veterans’ outcomes.
TABLE 2.
Obstacles to Implementing the COVID C‐TraC Program
| Obstacle | Response |
|---|---|
| Immediate need for intensive surveillance and management of outpatients with COVID‐19 | Repurpose experienced RNs with phone‐based protocols and case‐management infrastructure |
| Difficulty assessing severity of pulmonary symptoms and dehydration remotely |
Overnight mailing of fingertip oximeters Video visits to visualise patients |
| Rapid clinical decompensation |
Call patients 2–3 times daily at peak of symptoms if clinical concern or if O2 sat <94 Call ED to discuss case if O2 sat <92 Refer to ED immediately if O2 sat 90 or less |
| Lack of typical symptoms in older adults | Update note templates to include assessment of functional decline |
| Increased patient volume |
Offload rapid reporting of negative results to Primary Care Identified primary care RNs with appropriate experience and trained them and supervising PCPs in COVID‐19 protocol |
| Need to address social and mental health issues | Added social worker and psychologist to multidisciplinary team |
| Need to optimise communication of a complex team | Daily morning huddle of COVID‐19 outpatient team |
| Need for flexible, long‐term programme post‐surge | Disseminate training and protocol to individual RN‐PCP teams |
6. CONCLUSION
Our rapid implementation project demonstrates that experienced RNs trained in the C‐TraC model of care can quickly respond to emergencies and play a large role in the triage and outpatient monitoring of COVID‐19 positive patients. Nurses are particularly suited to this role as it requires skills in care coordination, home safety assessment, patient education and caregiver support. Our RN‐driven model did not rely heavily on physicians or technology, and as such is relevant to low resource settings. C‐TraC is an evidence‐based programme that empowers nurses to lead and adapt to meet patient and institutional needs. The C‐TraC training and protocol is a publicly available resource that has potential benefit for healthcare systems worldwide.
7. RELEVANCE TO CLINICAL PRACTICE
An RN‐driven, phone‐based, protocolised intervention is an effective and low‐cost method of providing intensive monitoring and care coordination for high‐risk outpatients with COVID‐19 that is feasible for low resource settings. This approach minimises exposure, saves PPE and results in timely referral to in‐person evaluation when appropriate.
AUTHOR CONTRIBUTIONS
All authors of this manuscript qualify for authorship and have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, been involved in drafting the manuscript or revising it critically for important intellectual content and given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Tables S1‐S2
Supplementary Material
ACKNOWLEDGEMENTS
The Coordinated Transitional Care Program at VA Boston Healthcare System was funded by a Mentored Partnership Grant from VA’s Office of Geriatrics and Extended Care. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System. The contents do not represent the views of the U. S. Department of Veterans Affairs or the United States Government.
REFERENCES
- Annis, T. , Pleasants, S. , Hultman, G. , Lindemann, E. , Thompson, J. A. , Billecke, S. , Badlani, S. , & Melton, G. B. (2020). Rapid implementation of a COVID‐19 remote patient monitoring program. Journal of the American Medical Informatics Association, 27(8), 1326–1330. 10.1093/jamia/ocaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey‐Martin, D. , Barnhart, E. , Gillis, J. Jr , & Vazquez, G. A. (2020). Primary care population management for COVID‐19 Patients. Journal of General Internal Medicine, 35(10), 3077–3080. 10.1007/s11606-020-05981-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Resource Center (2020). Retrieved from https://coronavirus.jhu.edu/map.html
- COVID Ready Communication Playbook (2020). https://www.vitaltalk.org/guides/covid‐19‐communication‐skills/
- Huston, P. , Campbell, J. , Russell, G. , Goodyear‐Smith, F. , Phillips, R. L. Jr , van Weel, C. , & Hogg, W. (2020). COVID‐19 and primary care in six countries. BJGP Open, 4(4), 1781–1783. 10.3399/bjgpopen20X101128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19) (2020). https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html
- Jaklevic, M. C. (2020). Telephone visits surge during the pandemic, but will they last? JAMA, 324(16), 1593. 10.1001/jama.2020.17201 [DOI] [PubMed] [Google Scholar]
- Kazis, L. E. , Lee, A. , Spiro, A. III , Rogers, W. , Ren, X. S. , Miller, D. R. , & Haffer, S. C. (2004). Measurement comparisons of the medical outcomes study and veterans SF‐36 health survey. Health Care Financing Review, 25(4), 43–58. [PMC free article] [PubMed] [Google Scholar]
- Keshvardoost, S. , Bahaadinbeigy, K. , & Fatehi, F. (2020). Role of telehealth in the management of COVID‐19: lessons learned from previous SARS, MERS, and Ebola outbreaks. Telemedicine and e‐Health, 26(7), 850–852. 10.1089/tmj.2020.0105 [DOI] [PubMed] [Google Scholar]
- Kilbourne, A. M. , Neumann, M. S. , Pincus, H. A. , Bauer, M. S. , & Stall, R. (2007). Implementing evidence‐based interventions in health care: application of the replicating effective programs framework. Implementation Science, 2, 42. 10.1186/1748-5908-2-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, A. J. H. (2012). The Department of Veterans Affairs Coordinated‐Transitional Care (C‐TraC) Program Toolkit. Madison, WI: Madison VA Geriatric Research and Clinical Centers (GRECC) and the UW Health Innovation Program. Available from: http://www.hipxchange.org/C‐TraC
- Kind, A. J. , Brenny‐Fitzpatrick, M. , Leahy‐Gross, K. , Mirr, J. , Chapman, E. , Frey, B. , & Houlahan, B. (2016). Harnessing protocolized adaptation in dissemination: successful implementation and sustainment of the veterans affairs coordinated‐transitional care program in a non‐veterans affairs hospital. Journal of the American Geriatrics Society, 64(2), 409–416. 10.1111/jgs.13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, A. J. , Jensen, L. , Barczi, S. , Bridges, A. , Kordahl, R. , Smith, M. A. , & Asthana, S. (2012). Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Affairs, 31(12), 2659–2668. 10.1377/hlthaff.2012.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, G. , Moen, R. , Nolan, K. , Nolan, T. , Norman, C. , & Provost, L. (2009). The improvement guide: a practical approach to enhancing organizational performance. John Wiley & Sons. [Google Scholar]
- Ogrinc, G. , Davies, L. , Goodman, D. , Batalden, P. , Davidoff, F. , & Stevens, D. (2016). SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Quality and Safety, 25(12), 986–992. 10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandemic Influenza Plan 2017 Update (2017). https://www.cdc.gov/flu/pandemic‐resources/pdf/pan‐flu‐report‐2017v2.pdf
- Peabody, M. R. , Dai, M. , Turner, K. , Peterson, L. E. , & Mainous, A. G. III (2019). Prevalence and factors associated with family physicians providing E‐visits. The Journal of the American Board of FamilyMedicine, 32(6), 868–875. 10.3122/jabfm.2019.06.190081 [DOI] [PubMed] [Google Scholar]
- Watkins, S. , & Neubrander, J. (2020). Primary‐care registered nurse telehealth policy implications. Journal of Telemedicine and Telecare, 1357633X20940142. 10.1177/1357633X20940142. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19) . (2020). Retrieved from https://www.who.int/publications‐detail/report‐of‐the‐who‐china‐joint‐mission‐on‐coronavirus‐disease‐2019‐(covid‐19)
- Sinha, S. , Kern, L. M. , Gingras, L. F. , Reshetnyak, E. , Tung, J. , Pelzman, F. , McGrath, T. A. , & Sterling, M. R. (2020). Implementation of video visits during COVID‐19: Lessons learned from a primary care practice in New York City. Frontiers in Public Health, 8, 514. 10.3389/fpubh.2020.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer, R. , Denman, D. , Wilkes‐Gillan, S. , Chen, Y. , Bogaardt, H. , Kim, J. , Heckathorn, D. , & Cordier, R. (2018). Effects of telehealth by allied health professionals and nurses in rural and remote areas: A systematic review and meta‐analysis. Journal of Rehabilitation Medicine, 50(3), 225–235. 10.2340/16501977-2297 [DOI] [PubMed] [Google Scholar]
- Wosik, J. , Fudim, M. , Cameron, B. , Gellad, Z. F. , Cho, A. , Phinney, D. , Curtis, S. , Roman, M. , Poon, E. G. , Ferranti, J. , Katz, J. N. , & Tcheng, J. (2020). Telehealth transformation: COVID‐19 and the rise of virtual care. Journal of the American Medical Informatics Association, 27(6), 957–962. 10.1093/jamia/ocaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Supplementary Material


