Abstract
Background
This critical appraisal aims to clarify which systematic reviews on COVID‐19 treatment are based on high‐value evidence. Hereby, the most profitable medicines can be suggested.
Methods
The mesh terms of "COVID‐19 drug treatment" (Supplementary Concept) and "COVID‐19 drug treatment" were sequentially utilized as search strategies in Medline and Science direct on October 18, 2020. Searches were confined to systematic reviews/meta‐analyses. The Cochrane database was searched on November 1, 2020 with "COVID." With adding up four articles from other resources, 84 systematic reviews were considered for initial screening. Finally, 22 articles fulfilled the criteria and were assessed using PRISMA guidelines.
Results
Increasing number of clinical trials from the onset of the COVID‐19 pandemic has revealed that hydroxychloroquine and chloroquine are not only profitable but also deleterious. Lopinavir/ritonavir failed to maintain their initial efficacy in improving clinical symptoms and mortality rate. Steroids and tocilizumab were suggested in patients with intensely severe symptoms. Steroids reduced mechanical ventilation and death in severely ill patients. Plasma or immunoglobulins effects are absolutely controversial. Favorable impressions of remdesivir have been relied on for the early onset of this drug. Hypotension and abnormal liver function tests were realized as its side effects. Favipiravir has resulted in a higher viral clearance than remdesivir. However, this claim needs to be proved with subsequent clinical trials.
Conclusions
Currently, remdesivir and favipiravir are advantageous drugs that should be administered in the early phases. Their side effects are not well known and need to be found in the following research projects. Steroids and tocilizumab have been considered beneficial in the cytokine storm phase.
Keywords: Coronavirus, COVID‐19, pandemic, treatment
1. INTRODUCTION
According to the World Health Organization, within the past two decades, the world has experienced three coronaviruses outbreaks with consequential health concerns. The first one was a severe acute respiratory syndrome due to the coronavirus (SARS‐CoV) reported in 2002 and 2003. Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) was the second one, which was identified in 2012. Unfortunately, the mortality rate of MERS was reported as approximately 34% in infected patients. 1 , 2 The outbreak of pneumonia cases, which initially occurred in Hubei, China, evolved into the 2019 Coronavirus Disease pandemic (COVID‐19). 3 The disease is caused by the Coronavirus‐2 of the severe acute respiratory syndrome (SARS‐CoV‐2). 4
SARS‐CoV‐2 (formerly known as COVID‐19) caused a pandemic, which first appeared in December 2019 in Wuhan, China, with no known available treatment or vaccine making it a disastrous disease and rapidly provoked a global concern. 5 , 6 , 7 Genetic assessments showed that SARS‐CoV‐2 has a similar genome structure to the group‐2 coronaviruses and belongs to β‐coronaviruses subfamily including severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV). There is lack of knowledge of the long‐term effects of COVID‐19 infections and the role of possible reinfection and relapses that are recently reported. 8 , 9 Coronavirus is an enveloped, nonsegmented, positive‐sense single‐stranded RNA virus with genome size ranging from 26 to 32 kilobases (the largest known viral RNA genome). 10
It has a diverse clinical presentation ranging from asymptomatic infection to mild flu‐like symptoms up to more severe complications of pneumonia and life‐threatening situations including acute respiratory distress syndrome (ARDS) or myocarditis. 6 The most common documented symptoms are fever, nonproductive cough, muscle aches and/or fatigue, dyspnea, headache, sore throat, and gastrointestinal symptoms. Some of the other less common presentations are rhinorrhea, hemoptysis, chest pain, loss of smell, and loss of taste that intensify suspicion of COVID‐19 disease. 11 According to previous studies, symptoms that were significantly associated with hospitalization included fever, dyspnea, nausea, diarrhea, abdominal pain, and fatigue. Symptoms that were significantly associated with mechanical ventilation included fever, dyspnea, nausea, and diarrhea. Only fever and dyspnea were significantly associated with mortality. 12 This novel virus mortality rate is approximately 3.7% based on reports till March 12, 2020, and caused many more deaths than its predecessors. 13
A large number of studies have reported various treatments for MERS, SARS, and COVID‐19, whereas the major problem in this period of time is the lack of definite specific therapeutic drugs or vaccines for prevention. In spite of identifying various drug choices, scientific evidence is still incipient and of low methodological quality, and some previous systematic reviews and meta‐analysis claimed the poor quality of the included studies. 4 , 14 , 15
Previous treatment strategies were explained and compared to find the most efficient drug regimen. The therapeutic protocols include supportive standard care, antivirals, antibiotics, vitamins, immunomodulators, antimalarial drugs, corticosteroids, antiretroviral drugs, convalescent plasma hemoperfusion, and extracorporeal membrane oxygenation (ECMO). 4 , 15 , 16 Owing to the lack of definite therapeutic protocol, this complementary critical appraisal on previous systematic reviews was carried out upon multiple databases to assess the current evidence regarding COVID‐19 treatment strategies.
As a surprisingly large number of systematic reviews were published within a short time period, we decided to precisely assess the usefulness and quality of them. Considering the day‐to‐day increase in the population of infected people and its mortality rate, we came to this decision to compare the varieties of currently available treatments to determine the high‐quality documented ones as well as knowledge gaps. This study assists to reveal whether the treatment strategies offered in previously published systematic reviews are based on strong evidence such as randomized clinical trials (RCTs) with large enough samples or weak ones such as case series and case reports. The most evidential systematic reviews are done including mostly RCTs, not case series and reports. In the second place, it can give a guide to the other researchers and inform them which treatments need to be proved with more clinical trials and which ones not properly assessed so far. Through this study and comparing the current evidence, it could offer treatments that are more evidently useful.
2. METHOD
This is a critical appraisal of previous systematic reviews performed on present chemical treatments of COVID‐19 disease. The electronic literature searches were conducted to identify all systematic reviews in the field of treatment of COVID‐19 diseases. Hence, Medline (accessed from PubMed), Science Direct, and Cochrane library were three databases taken into account for searching. The Mesh term of "COVID‐19 drug treatment" (Supplementary Concept) was applied to the search in Medline on October 18, 2020. The Science direct database was searched with "COVID‐19 drug treatment" as a search strategy on October 18, 2020. Then, the Cochrane Database of Systematic Reviews electronic databases was searched on November 1, 2020 with "COVID" as the search strategy.
All the following searches were conducted and repeated by three authors (Hamidreza Dehghan, Dorna Kheirabadi, and Fatemeh Haddad). The three authors achieved the same findings in their searching. To ensure literature saturation, the reference lists of the included studies or relevant reviews identified through the search were scanned. No time and language restriction was considered for the searching. To be included, systematic reviews had to be concerned specifically with the chemical treatment strategies of COVID‐19. Systematic reviews not relevant to the study's aim, on herbal treatments, written in non‐English language, or systematic reviews which depended upon in vitro studies, or performed on other coronaviruses except COVID‐19 were excluded, through reading the title and the abstract (Mohammad Rezaeisadrabadi [MR] and Aylar Fazlzadeh).
Then, the full texts of the studies were evaluated by two authors (MR and Razieh S. Mousavi‐Roknabadi [RSM]); they decided whether these met the inclusion criteria, independently. Disagreements were resolved by discussion between all authors, and finally, the articles were selected based on consensus. Neither of the authors was blind to the journal titles or to the study authors or institutions. The following data were extracted from the included studies: Study authors, study designs, methods, treatment, main findings, complications, and conclusion. The report of this systematic review was made according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 17 (Figure 1).
Figure 1.
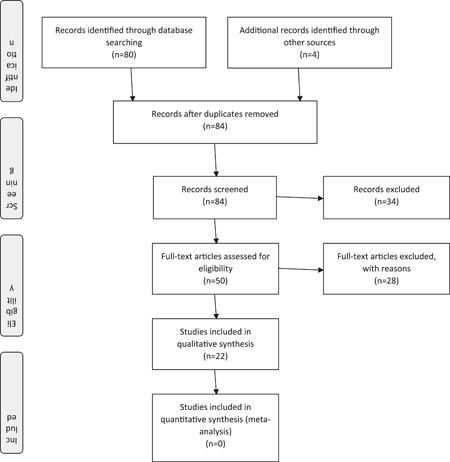
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow diagram of the study
The methodological quality of the included studies was assessed by the authors (RSM and MR). The quality of the systematic reviews was assessed using PRISMA guidelines through an evidence‐based set of items to report the quality of the systematic review. For each item in PRISMA guideline, "Yes" was given if any of the items were fully complied within the study, "Not Suitable" if any of the items were not suitable, and "No" if any of the items have not complied. If a study gets more than 80% of the items as "Yes," it was considered as high‐quality, if it gets 50%–80%, it was considered as medium, and if it gets less than 50%, it was considered as low. The review protocol was not previously registered.
3. RESULTS
Totally, 84 (46 articles in Medline, 33 articles in Cochrane Database of Systematic Reviews, 1 article from Science Direct, and 4 articles from other resources) were achieved at the first step by searching. After an initial assessment, no duplication was found. After the identification and the screening, 50 systematic reviews were selected as potential studies. No article from Science Direct fulfilled the inclusion criteria. After reading the full text of these articles, 22 articles formed the final sample (19 articles from Medline, 1 from Cochrane library, and 2 from other resources). 4 , 14 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 Inter‐rater agreement following the first round of screening between the two investigators was 93.90% (Cohen's k = 0.842). Within the second round of screening, the inter‐rater agreement was raised to 100%. The results are shown in Table 1. All studied were published in 2020. Our investigation for previous studies illustrated that most of the available reported evidence (59.1%) did not have high quality (Table 2).
Table 1.
An overview of the systematic reviews provided for COVID‐19 treatment
| Study authors (year) | Country | Type | Title | Aim | Sample Size (no. of studies) | Method | Drug | Treatment Complication | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| AminJafari and Ghasemi (2020) 32 | Iran | Systematic review | The possibility of immunotherapy for COVID‐19: A systematic review | To evaluate the existing evidence about immunotherapy for COVID‐19. | 7 | Not reported | Immunotherapy: Polyclonal antibody by plasma therapy, polypeptide hormone for maturation of T cells, immunoglubolins, ACE2 immunoadhesin and monoclonal antibody against the interleukin‐6 (IL‐6) | Not reported | No serious research has been done on this subject, but similar studies on the related viruses showed remarkable effects. It was suggested that immunotherapy (immunoglobulin and plasma therapy) can be used to improve the clinical outcomes in patients with COVID‐19. |
| Andrade et al. (2020) 4 | Brazil | Systematic review | Pharmacological therapies for patients with human coronavirus infections: a rapid systematic review | To evaluate the effects of drug therapies for human coronavirus infections. | 36 | 6 RCTs, 2 clinical trials, 16 retrospective cohorts, 2 prospective cohorts, 1 case‐reports, 6 case series, 3 systematic review | Antimalarial drugs, antivirals, and antiretroviral drugs, immunomodulators, anticoagulant, corticoid, combined therapies | Not reported | The available scientific evidence is preliminary and of low methodological quality. |
| Piechotta et al. (2020) 33 | Europe | Systematic review | Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review | To evaluate the safety and effectiveness of convalescent plasma or hyperimmune immunoglobulin transfusion in the treatment of patients with COVID‐19. | 19 | 2 RCTs, 17 clinical trials | Convalescent plasma or hyperimmune immunoglobulin transfusion | Allergic or respiratory, thrombotic or thromboembolic, and cardiac events | It was not clear whether convalescent plasma decreases all‐cause mortality at hospital discharge. It may lead to little to no difference in the improvement of clinical symptoms at 7 days. It may increase improvement of clinical symptoms at up to 15 days, and at up to 30 days |
| Chowdhury et al. (2020) 27 | USA | Systematic review | A rapid systematic review of clinical trials utilizing CQ and HCQ as a treatment for COVID‐19 | To review the literature regarding the clinical use of CQ and HCQ as treatment of COVID‐19. | 7 | 7 clinical trials | HCQ and CQ | Potential risk of QTc prolongation in combination of HCQ plus azithromycin | HCQ or CQ is efficacious compared to supportive care and to LPV/r in the treatment of COVID‐19. |
| Cortegiani et al. (2020) 34 | Italy | Systematic review | A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19 | To summarize the evidence regarding CQ for the treatment of COVID‐19 | 6 | 1 narrative letter, 1 in vitro, 1 editorial, 1 expert consensus paper, 2 national guidelines, ongoing clinical trial | CQ | Anemia, thrombocytopenia or leukopenia, hepatic, renal dysfunction, development of QT interval prolongation or bradycardia, visual and/or mental disturbance/deterioration | CQ seems to be effective in limiting the replication of SARS‐CoV‐2 in vitro. There is sufficient preclinical rationale and evidence regarding the effectiveness of CQ for treatment of COVID‐19 and safety from long‐time use in clinical practice for other indications. Although the use of CQ may be supported by expert opinion, clinical use of this drug should be approved. |
| Ford et al. (2020) 16 | Switzerland | Systematic review | Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS, or COVID‐19: Initial assessment | To evaluate the clinical outcomes of using antiretroviral drugs for the prevention and treatment of coronaviruses and planned clinical trials. | 26 | 23 antiviral drugs for treatment (2 RCTs, 21 observational studies), 3 antiviral drugs for prevention | LPV/r, emtricitabine, tenofovir, atazanavir, ritonavir, darunavir, nelfinavir, indinavir, saquinavir, lamivudine, and zidovudine | LPV/r: Mortality, gastrointestinal complaints (nausea, vomiting, and diarrhea) in | It is ambiguous whether LPV/r and other antiretrovirals improve clinical outcomes or prevent infection among patients at high risk of COVID‐19. |
| Hernandez, et al. (2020) 26 | Peru, USA | Systematic review | HCQ or CQ for Treatment or Prophylaxis of COVID‐19 | To evaluate the benefits and harms of HCQ or CQ for the treatment or prophylaxis of COVID‐19. | 23 | 4 RCTs, 10 cohort studies, 9 case‐series | HCQ or CQ | QTc interval ≥ 500 ms | Evidence on the benefits and harms is very weak and conflicting. There were no assessments of these drugs for prophylaxis against COVID‐19. |
| Li et al. (2020) 19 | China | Systematic review and meta‐analysis | Impact of corticosteroid therapy on outcomes of persons with SARS‐CoV‐2, SARS‐CoV, or MERS‐CoV infection: a systematic review and meta‐analysis | To determine the safety and efficacy of corticosteroids in SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV infections | 11 | 1 RCT, 10 cohort studies | Corticosteroids | Not reported | Corticosteroid was associated with delayed virus clearing, but no significant reduction in death and ICU admission were observed. Hospital length of stay was prolonged, and use of mechanical ventilation increased. |
| Lima et al. (2020) 31 | Brazil, Canada | Systematic review | The potential of drug repositioning as a short‑term strategy for the control and treatment of COVID‑19 (SARS‑CoV‑2): a systematic review | To evaluate the drug repositioning strategy against SARS‐CoV‐2. | 12 | 1 RCT, 2 retrospective studies, 2 case reports, 2 in vitro, 5 in silico | Antivirals, antiretroviral, antibiotics, antitumoral, antipsychotic, antifungal, antiemetic, antiplatelet agent, sedative‐hypnotic, hipolipemiant, | Not reported | LPV/r had low effectiveness on COVID‐19, but arbidol, remdesivir, and CQ/HCQ showed promising effects. |
| Liu et al. (2020) 35 | China | Systematic review and meta‐analysis | Efficacy and safety of antiviral treatment for COVID‐19 from evidence in studies of SAR‐SCoV‐2 and other acute viral infections: a systematic review and meta‐analysis | To evaluate the benefits and harms of 7 antiviral treatments for COVID‐19. | 19 | 7 RCTs, 11 cohorts, 1 case‐control | 7 antivirals: Ribavirin, CQ, HCQ, umifenovir (arbidol), favipiravir, interferon, LPV/r | Ribavirin: Anemia and bradycardia HCQ: Diarrhea, vomiting, headache, rash, and blurred vision Arbidol: Diarrhea and decreased appetite Favipiravir: diarrhea Interferon‐a: Need for granulocyte colony‐stimulating factor in patients with leukopenia LPV/r: Diarrhea, nausea and vomiting, and stomach ache | Very low‐quality evidence with little or no suggestion of benefit for most treatments and outcomes in both non‐severe and severe COVID‐19 were found. LPV/r with low‐quality evidence was shown to be effective in decreasing in length of stay in ICU and hospital stay. With moderate‐quality evidence, it may increase diarrhea, nausea, and vomiting |
| Musa et al. (2020) 28 | USA | Systematic review | Remdesivir for the treatment of COVID‐19: a systematic review of the literature | To determine the outcomes and adverse events associated with this investigational, antiviral medication | 8 | 8 clinical trials | Remdesivir: 5 trials: 200‐mg intravenous loading dose following by maintenance dose of 100 mg for 9 days. 2 trials: A single, 100‐mg IV infusion | The side‐effects profile of remdesivir remains not well defined | The clinical effectiveness of IV remdesivir for treatment of COVID‐19 and potential side effects remain incompletely defined in the human population. |
| Nasir et al. (2020) 29 | Bangladesh | Systematic review | Systematic review on repurposing use of favipiravir against SARS‐CoV‐2 | To evaluate evidence regarding the safety of the repurposing clinical use of favipiravir for the treatment of COVID‐19. | 19 | 2 RCTs, 17 ongoing trials | Favipiravir in combination with other treatments | Increased serum uric acid level | Favipiravir has significantly better treatment effects on disease progression, viral clearance, improved the latency to relief for pyrexia and cough in patients with COVID‐19. |
| Nasir et al. (2020) 30 | Bangladesh | Systematic review | Use of remdesivir in the management of COVID‐19: a systematic review on current evidence | To evaluate the efficacy and safety to identify any promising role for compassionate use of remdesivir in patient who suffered from severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). | 7 | 2 RCTs, 1 clinical trial, 4 case reports | Remdesivir | Hypotension, skin rashes, abnormal liver function, and diarrhea | Although remdesivir did not have a significant effect on the time to clinical improvement, its benefit may significantly depend on the time of administration (2 h after infection). |
| Patel et al. (2020) 24 | India, USA | Systematic review and meta‐analysis | Does adding of HCQ to the standard care provide any benefit in reducing the mortality among COVID‐19 patients?: a systematic review | To evaluate the early trends of mortality in patients with COVID‐19 treated with HCQ. | 6 | 1 nonrandomized controlled trial, 5 retrospective observational studies | HCQ and supportive Care HCQ plus azithromycin HCQ plus azithromycin and supportive care | QTc interval prolongation | HCQ had no additional benefit for reducing mortality in patients with COVID‐19 when it was added to the standard treatment. |
| Shah et al. (2020) 21 | India | Systematic review | A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease‐19 (COVID‐19) | The evaluate the role of CQ or hydroxychloroquine (HCQ) in preventing the spread of COVID‐19 | 5 | 3 in vitro, 2 clinical opinion | CQ or HCQ | Not reported | Clinical opinions advocated the prophylactic use of CQ and HCQ against COVID‐19. The prophylactic use of CQ or HCQ against COVID‐19 needs to be further reviewed as more data pour in. |
| Siemieniuk et al. (2020) 20 | Canada | Systematic review and meta‐analysis | Drug treatments for covid‐19: living systematic review and network meta‐analysis | To compare the effects of treatments for COVID‐19 | 27 | 27 RCTs | Glucocorticoids, remdesivir, HCQ, favipiravir, HCQ plus azithromycin, LPVr, umifenovir | Not reported | Glucocorticoids probably reduce mortality and mechanical ventilation in severe COVID ‐19. Remdesivir probably reduces time to symptom resolution, but whether it has an impact on other patient‐important outcomes such as mortality remains uncertain. HCQ may not reduce mortality or mechanical ventilation, and it seems unlikely to have any other benefits. The certainty effects of other drugs are low. |
| Singh et al. (2020) 23 | India | Systematic review and meta‐analysis | HCQ in patients with COVID‐19: A systematic review and meta‐analysis | To evaluate the effect of HCQ on viral clearance by RT‐PCR negativity and death due to all causes in patients with COVID‐19, as well as the efficacy and safety of HCQ. | 7 | 3 RCTs, 2 clinical trials, 5 retrospective, and prospective cohorts | HCQ | QTc prolongation, nausea, vomiting, and blurred vision | The rate of PCR negativity found no benefit with HCQ. The death due to all causes showed a two‐times increase in HCQ treatment. |
| Singh et al. (2020) 25 | India | Systematic search and narrative review | CQ and HCQ in the treatment of COVID‐19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. | To evaluate the efficacy of CQ and HCQ in the treatment of patients with COVID‐19 with or without diabetes. | 11 | 9 in vitro, 2 human trial | CQ and HCQ | Azithromycin plus HCQ may increase the risk of QTc prolongation. | Although evidence of CQ and HCQ is limited considering the potentially favorable benefit‐risk balance of any other valid treatment option, this treatment could be useful in the current context of pandemic COVID‐19 outbreak. |
| Subramanian et al. (2020) 14 | India | Systematic review | Perspectives on the early quality of evidence guiding the therapeutic management of Sars‐CoV‐2: a systematic literature review | To evaluate the quality of early clinical evidence currently guiding the treatment strategies for COVID‐19 and the therapeutic recommendations of different treatment guidelines | 22 | 5 RCTs, 9 prospective cohorts, 3 retrospective cohorts, 2 case‐series, 3 case reports | CQ and HCQ, remdesivir, corticosteroids, immunotherapy with convalescent plasma/sera, tocilizumab, other antivirals | Not reported | The current evidence provides ambiguous results because of the study designs and the endpoints assessed and different national treatment guidelines. |
| Verdugo‐Paiva et al. (2020) 22 | Chile, Argentina | Systematic review and meta‐analysis | LPV/r for COVID‐19: A living systematic review | To provide a summary of the evidence on the role of LPV/r in the treatment of patients with COVID‐19. | 2 | 2 RCTs | LPV/r | Not reported | LPV/r could reduce the mortality and the risk of requiring invasive mechanical ventilation, developing respiratory failure, or acute respiratory distress syndrome. But, it did not have an effect on the duration of hospitalization and may lead to an increase in the number of total adverse effects. |
| Yang et al. (2020) 18 | China | Systematic review and meta‐analysis | The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta‐analysis | To evaluate the influence of corticosteroids on patients with coronavirus infection. | 15 | Retrospective studies | Corticosteroid | Bacterial infection, hyperglycemia, hypocalcaemia | Patients with severe conditions are more likely to require corticosteroids. Using corticosteroid is associated with increased mortality in patients with coronavirus pneumonia. |
| Zhao et al. (2020) 15 | China | Systematic review and meta‐analysis | Efficacy of tocilizumab treatment in severely ill COVID‐19 patients | To evaluate the effects of tocilizumab treatment in severely ill COVID‐19 patients. | 10 | 1 RCT, 9 retrospective cohort studies | Tocilizumab | Not reported | Tocilizumab could be useful in the treatment of severely ill COVID‐19 patients. |
Abbreviations: CQ, chloroquine; HCQ, hydroxychloroquine; ICU, intensive care unit; RCT, randomized controlled trial.
Table 2.
Quality assessment of the systematic reviews using Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines
| Authors (Year of publication) | PRISMA items | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | #Yes | Overall quality | |
| AminJafari and Ghasemi (2020) 32 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | NS | Y | Y | Y | 15/27 | Medium |
| Andrade et al. (2020) 4 | Y | NS | NS | NS | N | Y | Y | Y | Y | Y | Y | N | Y | N | N | N | Y | Y | N | N | N | N | N | Y | Y | Y | Y | 14/27 | Medium |
| Piechotta et al. (2020) 33 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 27/27 | High |
| Chowdhury et al. (2020) 27 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | N | N | N | N | N | N | Y | Y | N | 16/27 | Medium |
| Cortegiani A, et al. (2020) 34 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | NS | N | N | N | N | N | Y | Y | N | N | N | N | N | Y | N | Y | Y | 13/27 | Medium |
| Ford et al. 16 (2020) | Y | NS | Y | NS | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | Y | Y | Y | Y | 14/27 | Medium |
| Hernandez et al. (2020) 26 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y | Y | N | N | N | N | N | N | NS | Y | Y | 14/27 | Medium |
| Li et al. (2020) 19 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 26/27 | High |
| Lima et al. (2020) 31 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | Y | Y | N | N | N | N | N | N | N | Y | Y | 15/27 | Medium |
| Liu et al. (2020) 35 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 27/27 | High |
| Musa et al. (2020) 28 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | NS | Y | N | N | N | N | N | N | Y | Y | Y | 14/27 | Medium |
| Nasir M, et al. (2020) 29 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | N | Y | Y | N | 14/27 | Medium |
| Nasir M, et al. (2020) 30 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | N | Y | Y | N | 14/27 | Medium |
| Patel et al. (2020) 24 | NS | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 24/27 | High |
| Shah et al. (2020) 21 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | Y | Y | Y | Y | 16/27 | Medium |
| Siemieniuk et al. (2020) 20 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 27/27 | High |
| Singh et al. (2020) 23 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 26/27 | High |
| Singh et al. (2020) 25 | Y | Y | Y | Y | N | Y | NS | Y | N | Y | NS | N | N | N | N | N | N | Y | N | N | N | N | N | Y | Y | Y | Y | 12/27 | Low |
| Subramanian et al. (2020) 14 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N | Y | Y | N | N | N | N | N | Y | NS | Y | Y | 16/27 | Medium |
| Verdugo‐Paiva et al. (2020) 22 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 27/27 | High |
| Yang et al. (2020) 18 | Y | NS | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NS | Y | Y | Y | Y | Y | Y | Y | 24/27 | High |
| Zhao et al. (2020) 15 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NS | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 25/27 | High |
Note: PRISMA items: Title: (1) Identify the report as a systematic review, meta‐analysis, or both. Abstract (2) Structured summary: Provide a structured summary including, as applicable: Background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. Introduction: (3) Describe the rationale for the review in the context of what is already known. (4) Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). Methods: (5) Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. (6) Specify study characteristics (e.g., PICOS, length of follow‐up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. (7) Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. (8) Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. (9) State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta‐analysis). (10) Describe the method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. (11) List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. (12) Describe methods used for assessing the risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. (13) State the principal summary measures (e.g., risk ratio, difference in means). (14) Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I 2) for each meta‐analysis. (15) Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). (16) Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta‐regression), if done, indicating which were prespecified. (17) Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. (18) For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow‐up period) and provide the citations. (19) Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). (20) For all outcomes considered (benefits or harms), present, for each study: (a) Simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot. (21) Present results of each meta‐analysis done, including confidence intervals and measures of consistency. (22) Present results of any assessment of risk of bias across studies. (23) Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta‐regression). (24) Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policymakers). (25) Discuss limitations at study and outcome level (e.g., risk of bias), and at review‐level (e.g., incomplete retrieval of identified research, reporting bias). (26) Provide a general interpretation of the results in the context of other evidence, and implications for future research. (27) Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review.
Abbreviations: N, no; NS, not suitable; Y, yes; #Yes, number of "Yes".
In a systematic review and meta‐analysis, Yang et al. 18 analyzed 3176 patients who were treated with corticosteroids and 1780 were treated with noncorticosteroids. Seven studies described the use of corticosteroids in critical and noncritical patients. The effects of corticosteroids may be influenced by other therapeutic options, such as antiviral drugs. Critical patients were more likely to require corticosteroid therapy. Corticosteroid treatment was associated with higher mortality, longer length of stay, higher rate of bacterial infection, and hypokalemia, but not hyperglycemia or hypocalcemia. The study limitations were that most of the studies were retrospective cohort and historical control studies, with a low level of evidence with a lack of RCTs with optimized design. There was no uniform standard for the time and dosage used in different studies. In addition, publication bias due to the rapid evolution of the SARS‐CoV‐2 situation and some unpublished studies may influence the results. 18
In another meta‐analysis, Li et al. 19 obtained that corticosteroid use in patients with SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV infection was associated with delayed virus clearing, but no significant reduction in death and intensive care unit (ICU) admission were observed. Hospital length of stay was prolonged, and use of mechanical ventilation increased. Several adverse effects were reported. The main limitations of this study were that the majority of studies were observational with selection and publication biases. 19
On the contrary, Siemieniuk et al. 20 in their high‐quality meta‐analysis on 22 randomized controlled trials (RCTs) stated that glucocorticoids probably reduce death, mechanical ventilation, and hospital length of stay. The impact of remdesivir on mortality, mechanical ventilation, and duration of hospitalization is unclear, but it probably reduces the duration of symptoms and probably does not increase adverse effects leading to drug discontinuation. Hydroxychloroquine (HCQ) may not reduce the risk of death or mechanical ventilation. The certainty in effects for all other drugs was low or very low. 20 Shah et al.'s 21 study, suggested that it seems premature to recommend CQ and HCQ drugs as a universal cure for prophylaxis of COVID‐19 due to lack of high‐quality evidence and clinical studies to support their clinical efficacy. 21
Verdugo‐Paiva et al. 22 in their meta‐analysis on two RCTs with a total of 250 adult inpatients evaluated the effects of lopinavir/ritonavir (LPV/r) plus standard care in comparison with standard care alone. They concluded that LPV/r could reduce the mortality and the risk of requiring invasive mechanical ventilation, developing respiratory failure, or ARDS. But, it did not have effects on the duration of hospitalization and may lead to an increase in the number of total adverse effects. The certainty of the evidence was reported low or very low. 22
Ford et al. 16 evaluated the clinical outcomes of using antiretroviral drugs for the prevention and treatment of coronaviruses. They demonstrated that most studies reported outcomes of using LPV/r as treatment and two studies reported outcomes among HIV‐positive patients, who were on a combination of antiretroviral drugs for management of HIV. Their obtained results were not pooled in meta‐analysis due to the limited number of studies. In total, 0.83% of patients who received LPV/r died; but the certainty of the evidence was very low. Three studies reported a possible protective effect of LPV/r as postexposure prophylaxis with very low certainty of the evidence due to limited sample size. In the first founded trial (n = 199), LPV/r (400/100 mg twice/day) for 14 days was not associated with a statistically significant difference in time to clinical improvement. LPV/r given within 12 days of symptoms was associated with a shorter time to clinical improvement. Twenty‐eight‐day mortality was not statistically different. Gastrointestinal adverse effects (nausea, vomiting, and diarrhea) were higher in the LPV/r group. The second trial (n = 42) was conducted on patients with mild/moderate COVID‐19 admitted to hospital with the treatment of LPV/r (200 mg/50 mg twice/day). Both studies showed that LPV/r had no clinical benefit and superiority. Due to the risk of bias, the certainty of the evidence in both studies was low and very low. Both RCTs demonstrated. 16
The results of a meta‐analysis by Patel et al. 24 showed that HCQ did not improve the mortality in patients with COVID‐19. In treatment with HCQ plus azithromycin, the risk of mortality was higher than those who received neither of these drugs. Also, they recommended that the combination of HCQ and azithromycin should be avoided in the treatment of COVID‐19 patients. It is worth saying that their analysis only included one non‐RCT and five retrospective observational studies. Singh et al. 23 in another meta‐analysis of three studies (n = 210) showed that the rate of PCR negativity found no benefit with HCQ. Their meta‐analysis of three trials (n = 474) reported that death due to all causes showed a two‐times increase in HCQ treatment.
Singh et al. 25 reported that chloroquine (CQ) and antimalaria agent has antiviral effects against multiple viruses. They enrolled 11 studies which only 2 of them were human trials conducted with CQ and HCQ in patients with COVID‐19 and significant improvements in some parameters were observed. The first study (n = 100) found that CQ (500 mg, twice daily in mild to severe COVID‐19 pneumonia) was superior to the control group in reducing symptom duration, exacerbation of pneumonia including radiological improvement and promoting virus‐negative seroconversion without any severe side effects. The second study (n = 36) with a nonrandomized trial design found that HCQ alone, as well as HCQ plus azithromycin, was significantly effective in clearing viral nasopharyngeal carriage in 3–6 days in patients with COVID‐19 due to a synergistic effect of azithromycin with HCQ. Guidelines varied in the recommended dosage and the duration of treatment, as well as drug combinations. The authors stated that reporting only two small human trials was their study's limitation. 25
Cortegiani et al.'s 34 systematic review claimed that there is no contraindication for CQ phosphate tablets (500 mg twice/day for 10 days) for patients with mild, moderate, and severe SARS‐CoV‐2 pneumonia. Blood testing to rule out the improvement of anemia, thrombocytopenia or leukopenia, serum electrolyte disturbances and/or hepatic and renal dysfunction was recommended. Also, electrocardiography was suggested to rule out the development of QT‐interval prolongation or bradycardia. Visual and/or mental disturbance/deterioration should be noticed as well. The Dutch Center for Disease Control recommended that treat severe infections require admission to the hospital and oxygen therapy or admitted to the ICU with CQ (in adults consists of 600 mg of CQ base (six tablets A‐CQ 100 mg) followed by 300 mg after 12 h on Day 1, then 300 mg × 2/die per os on Days 2–5), ceasing the treatment at Day 5 to reduce the risk of side effects. CQ phosphate is recommended at 500 mg in the first dose, and then 300 mg of the second. The Italian Society of Infectious and Tropical Disease mentioned the use of CQ (500 mg × 2/die) or HCQ (200 mg die) for 10 days, although according to clinical severity the treatment can be varied 5–20 days. Finally, they stated that although the use of CQ may be supported by expert opinion, the clinical use of this drug should be approved. 34
In another systematic review by Hernandez et al. 26 about the benefits and harm of HCQ or CQ for the treatment or prophylaxis of COVID‐19, it was found QTc interval ≥ 500 ms that the proportion of patients with this finding varied among the studies. The effects on all‐cause mortality, need for mechanical ventilation, progression to severe disease, symptom resolution, and upper respiratory viral clearance with HCQ were found conflicting, but mostly no different from conventional therapy. A nonsignificant increase in all‐cause mortality, ICU admission, mechanical ventilation, and ventricular arrhythmias with a higher‐dose therapy was found. There were no assessments of these drugs for prophylaxis against COVID‐19. 26
Chowdhury et al. 27 stated that HCQ or CQ is efficacious compared to supportive care and to LPV/r in the treatment of patients with COVID‐19 in their systematic review. But the potential risk of QTc prolongation in combination with HCQ plus azithromycin was observed. The small sample size in assessed trials was the main limitation. Finally, it was reported that no sufficient data was available to support the route use of these drugs as therapies for these patients. 27
Andrade et al. 4 enrolled 36 studies in their systematic review. Most of the studies were retrospective cohort. They reported there is no significant difference in the probability of negative viral load by RT‐PCR between the HCQ and conventional treatment. But, the results of other cohort studies were inconsistent with each other. HCQ plus azithromycin had no significant effect. Patients on HCQ had a higher risk of mortality. Also, this rate was worse in patients on HCQ plus azithromycin. They also reported that the rate of viral load after 7 days was 35% in treatment with LPV/r, 37.1% for arbidol, and 41.2% for not receiving antiviral therapy. LPV/r could decrease the duration of hospitalization. Moreover, viral clearance took about 8 days in patients receiving interferon‐α2b, 6.5 days in patients with interferon and arbidol, and 10 days in patients with arbidol. On the contrary, heparin can be used as a treatment in COVID‐19 infection due to its anti‐inflammatory effects. Methylprednisolone can decrease the time of symptom improvement in COVID‐19, but another study showed that corticosteroids increased the need for invasive mechanical ventilation, ICU admission, and the mortality rate in MERS‐CoV patients. Corticosteroids with antivirals did not affect the mortality after 28‐days hospitalization. But ribavirin associated with corticosteroids could improve the chest images' infiltrations. A combination of Meplazumab with other drugs led to 94% patients' discharge, 3 days for negative viral load, and an increase in C‐reactive protein in 82.4% of the patients. But the authors stated that lack of allocation secrecy, blinding, and small sample size in the RCTs; unclear information about controlling the confounding variables, length of follow‐up, and patient eligibility criteria were the limitations of the enrolled studies. 4
Zhao et al. in their meta‐analysis showed that severe COVID‐19 patients received intravenous or subcutaneous tocilizumab had a significant difference in mortality rate compared to ones received standard care (i.e. HCQ, LPV/r, remdesivir, azithromycin, low molecular weight heparin, and/or methylprednisolone). So, they suggested tocilizumab treatment for severe COVID‐19 due to its efficacy. However, high heterogeneity was observed. They reported several limitations, for example, only one RCT was included and the rest of the studies were retrospective cohort studies, the uniformity of the diagnostic criteria for severe COVID‐19, and incompleted extraction of the original data, and lack of relevant data. 15
Musa et al. 28 systematically reviewed eight eligible clinical trials about remdesivir. They finally stated that the clinical effectiveness of remdesivir for patients with COVID‐19 and potential side effects remain incompletely defined in the human population.
Subramanian et al. 14 evaluated the quality of early clinical evidence currently guiding the treatment strategies for COVID‐19 and the therapeutic recommendations of different treatment guidelines. They indicated that various national guidelines suggested remdesivir, convalescent sera, corticosteroids, and HCQ in different groups of patients. Remdesivir may improve the oxygen‐support class, but it had no difference with placebo in regard to time to clinical improvement. Using corticosteroids was associated with virus clearance time, hospital length of stay, or duration of symptoms in a cohort study. They finally stated that these pieces of evidence provided ambiguous results due to their designs and the endpoints assessed. 14
Nasir et al. 16 concluded that favipiravir (7–28 days) is one of the proposed antiviral drugs. But, no study evaluated the efficacy of favipiravir alone for the treatment. Although there was insufficient evidence, the studies showed significantly better treatment effects on disease progression, viral clearance, improved the latency to relief for pyrexia and cough in patients with COVID‐19. The adverse effects were reported to be mild and manageable. 29
In another systematic review, although they stated that remdesivir did not have a significant effect on the time to clinical improvement, they found that the benefit of remdesivir may significantly depend on the time of administration (2 h after infection). However, the available evidence was not high‐quality and sufficient about the safety and efficacy of this drug. 30
In another systematic review on different types of drugs in the treatment of COVID‐19, Lima et al. 31 found that antivirals, especially antiretrovirals, were the most frequently studied class of therapeutic agents (30%). After that, antitumor (16%), antimalarial (7%), antibacterial (5%), anticoagulant (3.5%), anti‐inflammatory (3.5%), phosphodiesterase (PDE)‐inhibiting (3.5%), anti‐rheumatic (3.5%), sedative‐hypnotic (3.5%), and antivenous insufficiency agents (3.5%) were placed. They concluded that LPV/r had low effectiveness on COVID‐19, but arbidol, remdesivir, and CQ/HCQ showed promising effects. 31
In a systematic review done by AminJafari and Ghasemi, 32 it was reported that no serious research has been done on the role of immunotherapy for the treatment of COVID‐19, but similar studies on the related viruses showed remarkable effects. It was suggested that immunotherapy (immunoglobulin and plasma therapy) can be used to improve the clinical outcomes in patients with COVID‐19. The authors stated that it is difficult, expensive, and time‐consuming to produce large‐scale monoclonal antibodies for clinical use. 32
On the contrary, Piechotta et al. 33 in their meta‐analysis on two RCTs (n = 198) stated that it was not clear whether convalescent plasma decreases all‐cause mortality at hospital discharge. Also, a convalescent plasma may lead to little or even no difference in improvement of clinical symptoms within 7 days. However, it may increase improvement of clinical symptoms at up to 15 days and even 30 days. No studies were found on quality of life. There was limited information regarding adverse events to determine the effect of convalescent plasma therapy on clinically relevant severe adverse effects. These were predominantly allergic or respiratory, thrombotic or thromboembolic, and cardiac events. 33
Finally, Liu et al. 35 investigated the efficacy and safety of antiviral treatment (ribavirin, CQ, HCQ, arbidol, favipiravir, interferon, LPV/r) in the patients with COVID‐19. They enrolled 7 RCTs, 11 cohorts, and 1 case‐control. It was found that ribavirin had uncertain effects on mortality, but the evidence was very low‐quality. It was reported that this drug increases the incidence of anemia and bradycardia. HCQ was evaluated in three RCTs. Although the quality of evidence was low, it was indicated that it had minimal effects on viral clearance at Day 14, the progression from nonsevere to severe illness or clinical recovery at Day 7. Also, HCQ might result in a shorter duration of fever. Diarrhea, vomiting, headache, rash, and blurred vision were reported as adverse effects (low and very low quality of evidence). Evidence about arbidol was limited, but it was reported that there was a large decrease in mortality. Diarrhea and decreased appetite were its complications (low‐quality of evidence). Favipiravir could increase the clinical recovery and viral clearance at Day 7 with (low‐quality of evidence). Patients who received interferon were found to have a shorter time to viral clearance and duration of hospitalization. Moreover, they stated that Interferon‐α may decrease mortality (low and very low quality of evidence). They also reported that LPV/r has positive effects on mortality, viral clearance at Days 14 or 23, mechanical ventilation, cough alleviation, and length of stay in ICU and hospital in patients with severe COVID‐19. In nonsevere patients, it has little or no reduction in viral clearance at Day 14. Incidence of diarrhea (moderate‐quality of evidence), nausea and vomiting (moderate‐quality of evidence), and stomach ache (low‐quality of evidence). 35
4. DISCUSSION
As corona antiviral drug treatment has not been definitively approved, examining different treatment protocols in different countries can lead to improved treatment methods.
The first line of treatment used was the use of CQ and HCQ. In various studies, the effectiveness of these drugs has been confirmed, whereas in some other studies, this drug has not been more effective than palliative treatments. In addition to the unapproved efficacy of this drug, ocular and cardiac complications of this class of drugs have led to serious harmful effects on patients. For example, prolongation of the QT segment in the EKG can be considered as a life‐threatening complication of these drugs. Over time, studies focusing on the efficacy of HCQ or CQ have shown that these drugs do not have suitable antiviral implications and not decrease the mortality rate. One of the reasons for this alteration in treatment strategies compared to the beginning of the pandemic has been related to the increased number of controlled clinical trial studies. Overall, the authors of this article, by summarizing the existing studies, conclude that the use of antimalarials cannot lead to a reduction in mortality rate, and due to serious cardiac complications, its harm can outweigh the profit. 4 , 12 , 21 , 22 , 23 , 24 , 25 , 26 , 33
Antiretroviral drugs, including LPV/r, have been shown to be less effective in improving clinical symptoms and mortality rates in various studies. Also, the use of these drugs in outpatients did not reduce their hospitalization rate. In general, as mentioned in the studies, the effects of this class of drugs are ambiguous. In some existing studies, the use of remdesivir has been preferred to LPV/r. 14 , 20 , 30 , 33
As one of the approved mechanisms of this disease is the inflammatory cascade, the use of corticosteroids has been considered. In early studies, steroids did not reduce death in the ICU and had no effect on clearing the virus. However, in more recent studies including more clinical trials and high‐quality studies, corticosteroids have reduced the need for mechanical ventilation and death especially in patients with highly severe symptoms. We believe that the use of corticosteroids in severely ill patients can be effective and lead to a reduction in mortality. 16 , 18 On the basis of this pathophysiological justification, the use of tocilizumab has also been considered, and in the present article, has recommended its use in very critically ill patients. 13
The results regarding the use of plasma or immunoglobulin have been quite contradictory. In some studies, it did not improve symptoms or mortality. In others, it has reduced respiratory symptoms and the severity of the disease. Interferon‐α may decrease mortality based on the low and very low quality of evidence. 31 , 32 , 33
Remdesivir and favipiravir have come to the attention of researchers in recent months. In existing clinical trials, favipiravir has led to higher clearance of the virus and cough amelioration. 28 Promising results have been observed in patients using Remdesivir, but its side effects are still less known due to the novelty of the drug. The most side effects which were mentioned were hypotension and abnormal liver function tests. The researchers believed that the clinical improvement following the use of remdesivir depended on the onset of the drug in the early stages of the disease. 27 , 29 It seems that one of the difficulties of clinical research on covid‐19 patients is the multiple treatments or sometimes salvage therapy in critically ill patients. It is recommended that the therapeutic effects of drugs should be assessed in more controlled clinical trials.
5. CONCLUSION
In conclusion, the use of antimalarial drugs has not been recognized as an effective treatment for covid‐19. There are also serious controversies about the effectiveness of lopinavir and other antiretroviral drugs. Remdesivir and favipiravir have been considered as new effective drugs that should be used in the early phases of this viral disease. However, their side effects are not yet known. The use of steroids and recently developed drugs such as tocilizumab has also been reasonable in the cytokine storm phase. Immunotherapy (immunoglobulin and plasma therapy) needs to be more evaluated by further clinical trials.
Finally, none of the above treatments has been approved as a definitive treatment of the Wuhan coronavirus disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Dorna Kheirabadi was responsible for studies search, data extraction, and the final revision of the manuscript. Fatemeh Haddad, Hamidreza Dehghan, and Aylar Fazlzadeh helped in searching studies and data extraction and were responsible for the manuscript writing. Razieh S. Mousavi‐Roknabadi helped in data searching, data extraction, and manuscript writing. Mohammad Rezaeisadrabadi was responsible for the final revision of the manuscript and the study.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26811ARDP202000455.
Kheirabadi D, Haddad F, Mousavi‐Roknabadi RS, Rezaeisadrabadi M, Dehghan H, Fazlzadeh A. A complementary critical appraisal on systematic reviews regarding the most efficient therapeutic strategies for the current COVID‐19 (SARS‐CoV‐2) pandemic. J Med Virol. 2021;93:2705‐2721. 10.1002/jmv.26811
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gross AE, Bryson ML. Oral ribavirin for the treatment of noninfluenza respiratory viral infections: a systematic review. Ann Pharmacother. 2015;49(10):1125‐1135. [DOI] [PubMed] [Google Scholar]
- 3. Ren L‐L, Wang Y‐M, Wu Z‐Q, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133(9):1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade KRC, Carvalho VKS, Farinasso CM, et al. Pharmacological therapies for patients with human coronavirus infections: a rapid systematic review. Cien Saude Colet. 2020;25:3517‐3554. [DOI] [PubMed] [Google Scholar]
- 5. Momattin H, Mohammed K, Zumla A, Memish ZA, Al‐Tawfiq JA. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS‐CoV)–possible lessons from a systematic review of SARS‐CoV therapy. Int J Infect Dis. 2013;17(10):e792‐e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): a systematic review. J Clin Med. 2020;9(3):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khosrawipour V, Lau H, Khosrawipour T, et al. Failure in initial stage containment of global COVID‐19 epicenters. J Med Virol. 2020;92(7):863‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLOS Med. 2006;3(9):e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arafkas M, Khosrawipour T, Kocbach P, et al. Current meta‐analysis does not support the possibility of COVID‐19 reinfections. J Med Virol. 2020. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4Z):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaur N, Gupta I, Singh H, et al. Epidemiological and clinical characteristics of 6635 COVID‐19 patients: a pooled analysis. SN Compr Clin Med. 2020;2(8):1048‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US Veterans with SARS‐CoV‐2 infection. JAMA Netw Open. 2020;3(9):e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yousefifard M, Zali A, Ali KM, et al. Antiviral therapy in management of COVID‐19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8(1):e45. [PMC free article] [PubMed] [Google Scholar]
- 14. Subramanian K, Nalli A, Senthil V, Jain S, Nayak A, Bhat A. Perspectives on the early quality of evidence guiding the therapeutic management of SARS‐CoV‐2: a systematic literature review. Adv Ther. 2020;37(10):4107‐4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao J, Cui W, Tian B‐p. Efficacy of tocilizumab treatment in severely ill COVID‐19 patients. Crit Care. 2020;24(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID‐19: initial assessment. J Int AIDS Soc. 2020;23(4):e25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta‐analysis. J Infect. 2020;81(1):e13‐e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS‐CoV‐2, SARS‐CoV, or MERS‐CoV infection: a systematic review and meta‐analysis. Leukemia. 2020;34:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siemieniuk R, Bin Sadeghirad B, Sekercioglu N, et al. Br Med J. 2020;370:m2980.32732190 [Google Scholar]
- 21. Shah S, Das S, Jain A, Misra DP, Negi VS. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease‐19 (COVID‐19). Int J Rheum Dis. 2020;23(5):613‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verdugo‐Paiva F, Izcovich A, Ragusa M, Rada G. Lopinavir/ritonavir for COVID‐19: a living systematic review. Medwave. 2020;20(6):e7966. [DOI] [PubMed] [Google Scholar]
- 23. Singh AK, Singh A, Singh S, Misra A. Hydroxychloroquine in patients with COVID‐19: a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14(4):589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel TK, Barvaliya M, Kevadiya BD, Patel PB, Bhalla HL. Does adding of hydroxychloroquine to the standard care provide any benefit in reducing the mortality among COVID‐19 patients?: a systematic review. J Neuroimmune Pharmacol. 2020;15(3):350‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID‐19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID‐19: a living systematic review. Ann Intern Med. 2020;173(4):287‐296. [DOI] [PubMed] [Google Scholar]
- 27. Chowdhury MS, Rathod J, Gernsheimer J. A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID‐19. Acad Emerg Med. 2020;27(6):493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Musa A, Pendi K, Hashemi A, et al. Remdesivir for the treatment of COVID‐19: a systematic review of the literature. West J Emerg Med. 2020;21(4):737‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nasir M, Perveen RA, Saha SK, Talha KA, Selina F, Islam MA. Systematic review on repurposing use of favipiravir against SARS‐CoV‐2. Mymensingh Med J. 2020;29(3):747‐754. [PubMed] [Google Scholar]
- 30. Nasir M, Talha KA, Islam KA, Saha SK, Selina F, Perveen RA. Use of remdesivir in the management of COVID‐19: a systematic review on current evidence. Mymensingh Med. 2020;29(2):481‐487. [PubMed] [Google Scholar]
- 31. Lima WG, Brito JCM, Overhage J, Nizer WSC. The potential of drug repositioning as a short‐term strategy for the control and treatment of COVID‐19 (SARS‐CoV‐2): a systematic review. Arch Virol. 2020;165:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. AminJafari A, Ghasemi S. The possible of immunotherapy for COVID‐19: a systematic review. Int Immunopharmacol. 2020;83:106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piechotta V, Chai KL, Valk SJ, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database Syst Rev. 2020;7(7):CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cortegiani A, Ingoglia I, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care. 2020;57:279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu W, Zhou P, Chen K, et al. Efficacy and safety of antiviral treatment for COVID‐19 from evidence in studies of SARS‐CoV‐2 and other acute viral infections: a systematic review and meta‐analysis. CMAJ. 2020;192(27):E734‐E744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


