Abstract
High‐throughput droplet‐based digital PCR (ddPCR) is a refinement of the conventional polymerase chain reaction (PCR) methods. In ddPCR, DNA/RNA is encapsulated stochastically inside the microdroplets as reaction chambers. A small percentage of the reaction chamber contains one or fewer copies of the DNA or RNA. After PCR amplification, concentrations are determined based on the proportion of nonfluorescent partitions through the Poisson distribution. Some of the main features of ddPCR include high sensitivity and specificity, absolute quantification without a standard curve, high reproducibility, good tolerance to PCR inhibitor, and high efficacy compared to conventional molecular methods. These advantages make ddPCR a valuable addition to the virologist's toolbox. The following review outlines the recent technological advances in ddPCR methods and their applications in viral identification.
Keywords: Droplet digital PCR, microfluidic, virus
1. INTRODUCTION
Our knowledge about virus biology has improved over time. However, the mortality rate of well‐characterized viruses is still high. 1 , 2 , 3 Viral epidemics spread quickly across the world, causing high mortality rates in a short time. There is always an urgent need to develop a rapid, sensitive, cost‐effective, and high‐performance test to detect viral antigens. Early sensitive detection of the virus is an essential parameter in patients management, 4 , 5 treatment selection, 6 , 7 antiviral therapy monitoring, 8 shortening of the window period, 9 , 10 and isolation of infected patients.
PCR‐based techniques have been developed over the last two decades to facilitating the sensitive early detection of viruses. 11 Before the development of nucleic acid testing (NAT), Cell culture, electron microscope, complement fixation, agglutination assay, and immune‐based tests have been considered, as routine methods, for virus detection. 12 , 13 , 14 These tests are time‐consuming and have low sensitivity and precision, and have been widely replaced by NAT‐based tests such as PCR.
Digital PCR is a new generation of traditional quantitative polymerase chain reaction (qPCR), which can be used for absolute quantification of target nucleic acids (Figure 1). In a digital assay, the sample is compartmentalized into several small bioreactors, each of which has either zero or one or two (or three, four, etc.) copies of the target nucleic acids. Each droplet has a particular encapsulated area that prevents cross‐contamination between micro bioreactors. Several methods have been developed to partition samples, including microwell formats, microfluidics chambers, and droplets. 15 Microwells or chips suffer from a limited number of micro bioreactors, partition differences in volume, and often cross‐contamination. The microfluidic can generate millions of such micro bioreactors in a cost‐effective manner. 15
Figure 1.
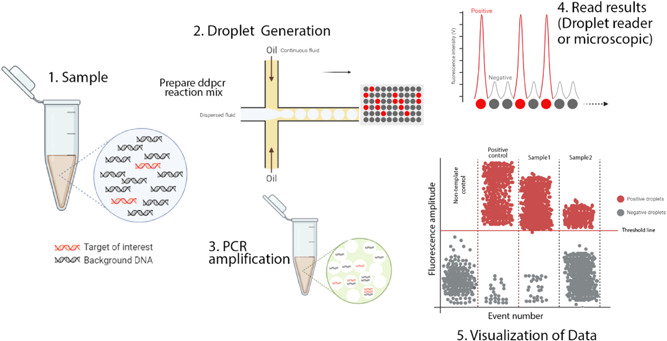
Schematic illustration of ddPCR. This figure illustrates the digital PCR droplet principle and how a single sample containing the target sequence is partitioned. PCR amplified produces thousands of copies that can be detected and interpreted by a detection system. In a typical ddPCR workflow, a single sample includes target and nonspecific sequences (DNA or RNA), real‐time PCR primers and fluorescent‐labeled probes, and standard real‐time PCR master mixes. 1 A sample partitioned into thousands of single nanoliter droplets with the generation of water‐in‐oil emulsions. A proportion of droplets contain no template molecules, while others contain one or more targets. The generation of droplets from the sample is achieved in several ways. Standard methods include T‐junction and flow‐focusing geometry. Only the flow‐focusing geometry is shown here. For more details, see the text. 2 traditional end‐point PCR is then performed to amplify the target sequence. 3 Target sequence droplets exhibit higher fluorescence intensity and are known to be positive droplets. Empty or no targets show low and negative fluorescent intensity. This fluorescence intensity versus time is plotted on a graph. The number of targets per partition will follow the normal Poisson distribution encapsulation of the DNA or RNA that occurs randomly. Various methods can interpret the fluorescent intensity of droplets. The most popular of these is a fluorescent microscope or a droplet reader. 4 The acquired data is visualized in a graph by different software. The threshold value indicates the intensity of fluorescence as the positive particles are separated from the negative. Although this value is set automatically by the software, it can be adjusted manually. ddPCR, droplet‐based digital PCR
Droplet‐based digital PCR (ddPCR) is a type of digital PCR that employs one immiscible fluid (i.e., the so‐called dispersed fluid) in oil (i.e., the so‐called continuous fluid) to generate submicroliter droplets at kilohertz rates. 16 , 17 Nucleic acids, such as DNA, RNA, and complementary DNA (cDNA), may be encapsulated stochastically inside the droplets as reaction chambers. A small percentage of the droplets contain one or fewer copies of the DNA template (many of the droplets do not include any of the target DNA) and are then clonally amplified in each microdroplet. After routine PCR amplification, concentrations are determined based on the proportion of nonfluorescent partitions by Poisson distribution. 18 , 19 Several commercial PCR droplet platforms have recently been developed to accelerate the clinical application of ddPCR, such as BioMark HD dPCR (Fluidigm), OpenArray, QuantStudio 12K Flex dPCR (Life Technologies), RainDropTM (RainDance Technologies), Bio‐Rad QX200TM Droplet Digital, and NAICATM.
Traditional qPCR assay is a well‐established method for measuring viral nucleic acid, but the drawbacks should be considered. 18 , 20 , 21 A calibration curve is needed for viral acid nucleic quantification by qPCR. In this regard, cell lines, plasmids, and other calibrators are widely used to generate a calibration curve. 22 The external calibrator often is not stable and has been known to have a day to day variability; however, a reliable calibrator improved reproducibility between virus laboratories. 23 ddPCR does not rely on a standard calibration curve; therefore, it is exempt from calibration curve limitations. Busby et al. 22 proved that on average ddPCR values were 60% of qPCR values of the 8E5 calibration standard due to the loss of human immunodeficiency virus (HIV) DNA from the 8E5 cell calibrant. 24
Here, we review recent advances in ddPCR and how these micro‐reactor droplets are built to accelerate the development of viral detection methods. This review paper addresses recent advances, ranging from microfluidics to detecting viruses by ddPCR techniques, including the review of microfluidics devices, droplet generation, and various aspects of droplet formation and geometry. We also summarize the application of ddPCR in identifying some clinically important viruses and the advantages and disadvantages of the PCR method compared with the ddPCR method. In the last section, we discuss the future challenges of ddPCR and how ddPCR integrates with emerging upstream and downstream techniques. To date, only some of the viruses have been covered in the review studies about ddPCR, such as Li and et al., 25 who consider HIV and hepatitis. 26 Rutsaert et al. 27 only reviewed the performance of digital PCR in quantification and characterization of the persistent HIV reservoir. 28 according to our knowledge, there has been no general review of the use of ddPCR in various clinically significant viruses.
1.1. Microfluidics and droplet‐based microfluidic methods
Microfluidic technology is a multidisciplinary field that deals with the science of manipulating fluids, usually in the submicron scale. In recent years, microfluidic technology has made noteworthy progress in improving diagnostic test performance. This versatile technology is a promising approach for many areas of biology, including pathogen detection, cancer cell isolation, drug screening, in vitro diagnostic devices, single‐cell analysis, analytical chemistry, point‐of‐care diagnostic tests (POCTs), genome sequences and organ‐on‐chip, and also nucleic acid (NAT) reactions. 23 , 24 , 25 , 27 , 29 , 30 , 31
The miniaturization of biological assays on microfluidic makes it possible to control body fluids, cells, tissues, and also pathogens using a low amount of reagents and samples (pL, nL) that dramatically reduces costs. 32 , 33 Microfluidic systems' have some other advantages: faster processing times, high performance, precision, reliability, portable, multifunction integration, parallelization, and flexibility. 31 , 34 , 35 , 36 Various microfluidic systems such as lateral flow devices, 37 centrifugal devices, 38 paper‐based devices, 39 digital microfluidics 40 and, droplet‐based 41 have been developed.
The behavior of fluids differs significantly between macroscale and micro‐scale. Fluid flow in microfluidic channels is laminar due to the low Reynold number. 42 As the fluid behavior is more predictable in the micro‐scale, a new level of control over biological reactions arises in fluid engineering. Disadvantages such as cross‐contamination, Taylor diffusion, dilution of the sample and reagents, and prevention of reagents' absorption on the channel walls have arisen, despite a few laminar flow advantages in microfluidic. 42 , 43 The droplet‐based microfluidic systems address this disadvantage.
The field of droplet‐based microfluidics has been a rapidly promising technology over the last few years owing to the generation of reproducible discrete monodisperse droplets, facilitate the production of droplets in comparison to traditional methods, fast mass transfer, fast heat transfer, accurate liquid handling, low cost and reduced cross‐contamination. 15 , 19
1.2. Droplet generation
In general, each droplet‐based assay can be divided into three steps 1 : droplet compartmentalization, 2 droplet manipulation, and 3 droplet analysis. 17 , 44
The production of droplets in the microfluidic system is based on emulsions. An emulsion is a colloid system containing a mixture of two immiscible liquids. One is dispersed (dispersed phase) throughout the other (continuous phase) in small droplets. Two immiscible fluids (e.g., water and oil) can form an oil‐in‐water (O/W) or water‐in‐oil (W/O) emulsion, depending on the continuous phase. 43 , 45 The continuous fluid is typically organic oil, and the dispersed fluid is generally an aqueous liquid. The dispersed phase partitions into uniform microdroplets, so microdroplets can be handled, collected, incubated, split, sorted, and combined. The aqueous phase can include macromolecules, such as DNA, RNA, single cells, or even pathogens. Microfluidic‐based generated droplets can act as a chamber for the PCR reaction, called ddPCR.
Many microfluidic geometries have been developed for the compartmentalization of droplets. Droplet manipulation and generation techniques may be passive or active, where the former produces micro‐droplets without external forces, the latter requires an external force to produce micro‐droplets such as gravitational, centrifugal, fluid velocity, thermal, electrical, Di‐electrophoresis, and Electrowetting‐on‐dielectric. 44 T‐junction and flow‐focusing geometry are the two primary production methods of droplets in microfluidic applications among passive techniques. 44 Also, coflow, step emulsification, and parallel devices are the other passive methods used to generate droplets. 17 The fabrication of polydimethylsiloxane (PDMS)‐based devices via the conventional soft‐lithotherapy method is a well‐developed and straightforward process (Figure 2). 43 , 46 The most common material used in microfluidic devices is PDMS, a transparent, nontoxic, minimally ultraviolet fluorescent, high gas permeability biocompatible polymer. 47 Alternative materials used to fabricate droplet‐based microfluidic devices are glass and polymethyl methacrylate (PMMA). 48 Micro‐droplet manipulation strategies are broad and include passive and active droplet fission and mixing, droplet sorting, phase changing in the droplet, polymerization, and barcoding of droplets. The acquisition of data from droplets is a necessary step in the implementation of droplet‐based microfluidic devices. Various methods of detecting and measuring droplets have been developed, including fluorescence, mass spectrometry, Raman spectrometry, electrical measurement, electrophoresis, and chemiluminescence. 49
Figure 2.
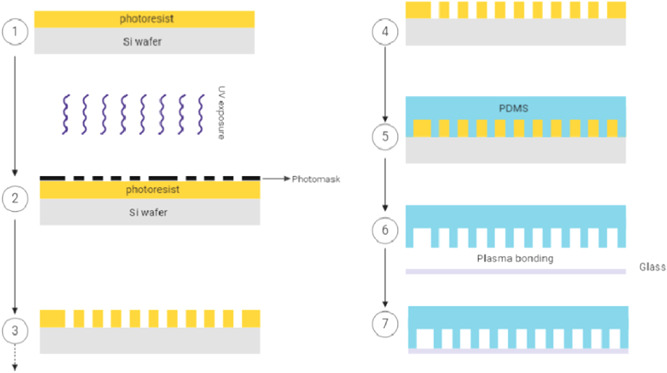
Schematic diagram of the SU‐8 soft‐lithography process for the manufacture of droplet‐based microfluidic devices. There are many methods of making microfluidic devices, but soft lithography is the most common process. In the soft lithography workflow, mold making, often created in SU‐8 negative photoresist, is needed for the replicating of PDMS microfluidic features. a small quantity of the SU‐8 was initially added to the silicon wafer (Si wafer) to produce the SU‐8 molds than to the spin‐coating according to the manufacturer's procedure. 1 The SU‐8 was pre‐baked. A mask with the desired design was used for fexposure of the spin‐coated wafer. SU‐8 photoresist undergoes a crosslinking reaction when exposed to UV light exposure. 2 , 3 A postexposure bake followed the exposure of the SU‐8 wafer. The next step was to eliminate the unexposed region with the developer. 4 Subsequently, the result is a micropatterned, formed mold. PDMS is poured over the SU‐8 master. 5 The molded PDMS is cured and bonded PDMS to glass by the plasma bonding technique. 6 , 7 ddPCR, droplet‐based digital PCR; PDMS, polydimethylsiloxane; UV, ultraviolet
1.3. Droplet digital PCR versus conventional qPCR methods
Droplet digital PCR provides many benefits over conventual qPCR. In a qPCR method, amplicons are calculated at the end of each step using DNA binding dyes. The intensity of fluorescent dyes attached to dsDNA is proportional to the PCR amplicons. 50 A standard curve is needed for the specific absolute quantification of the viral nucleic acid by qPCR. These standard curves are obtained by dilution of the sample with a known concentration. Standard curve production is closely impressed by both lab‐to‐lab and day‐to‐day errors. 51 As absolute quantification by ddPCR does not require a calibration curve, it does not have its limitations. It should be noted that RNA quantification with RT‐dPCR should be standardized to a reference sample. 52
ddPCR uses the same primers, probes, Taq polymerase, and reagents as conventual PCR to amplify the target DNA fragment, but its sensitivity and repeatability are more significant. 53 This superior performance stems from the PCR droplet method's two distinct features: (1) compartmentalization and (2) data acquisition from the end‐point reaction.
Compartmentalization of target DNA improves the signal to noise ratio by amplifying target gene signals relative to other genes. 16 In normal bulk PCR reactions, inhibitors or excess background DNA (noise) can affect amplification effectiveness. In ddPCR, as a binary system, the absolute quantification is acquired by counting the number of positive and negative fluorescence droplets, however in qPCR, as an analog system, fluorescence signal increases proportion to the amount of replicated DNA. As droplet PCR is an end‐point measurement, it is less dependent on the reaction efficiency than qPCR methods. 54 Thanks to the end‐point reaction of ddPCR, droplets are often explicitly labeled as positive or negative forms of fluorescence, and the identification of droplets containing DNA/RNA viruses is much less error‐prone.
The tolerance to PCR inhibitors is enhanced in ddPCR. 19 it can help evaluate viral infection in some inhibition‐prone samples, including stool, sputum, and tissue, 55 as well as in the direct quantification of viral acid nucleic without the need an RNA isolation. Direct virus quantification without the need for nucleic acid purification will minimize costs and improve the identification of viruses even in the presence of inhibitors. Jernej Pavsic et al. 56 have demonstrated that direct quantification of viruses is in good correlation with accurate viral load in clinical samples. 57 It showed that ddPCR or cytomegalovirus (CMV) virus detection is more tolerant and reliable for quantification in the presence of inhibitors such as SDS and heparin in sample DNA compared with qPCR. 58
Viral burden, particularly in the early period of infection, is often below the detection limit of routine viral infection detection methods. 59 , 60 , 61 Besides this, the concentration of viral particles in the blood can be smaller than that (1–10 copies). The initial target template concentration in qPCR is comparatively higher (at least 50 µg or approximately 15,000 copies). If the target DNA quantity is lower than the method detection limit, the false‐negative outcome can be increased, particularly in inadequate efficiencies. For example, one ng of HPV RNA is needed to detect HPV E6 and E7 oncoproteins by ddPCR accurately. On the other hand, 20–50 ng RNA per reaction is usually required to conduct qPCR from head and neck squamous cell carcinoma (SCC), which is about 20–50 times higher than ddPCR. 62
ddPCR shows higher sensitivity and specificity than qPCR for detecting viral nucleic acids. A microfluidic droplet assay was developed to measure low viral load samples with an HBV DNA detection limit of eight copies/ml. In comparison, the COBAS TaqMan assay has a lower quantification limit of 169 HBV copies/ml and a lower detection limit (LLOD) of 58 copies/ml. 63 One field of interest is the quantification of the RNA virus by ddPCR in latent infections. Strain and et al. showed that ddPCR had a significant increase in accuracy, particularly in less than 300 copies/106 cells, with an average fivefold decrease in coefficient of variation of target copy numbers relative to qPCR. 64
Viral infections are more likely to spread during the window period. The window period is the interval between the onset of the infection and when the test will accurately detect the organism. The shedding of viral particles is hinting at the infection before symptoms begin. The virus's window period depends on the type of technique; a high‐performance test identifies the virus faster than conventional methods; it reduces the gap between the detection and the virus's emergence. Compared with the qPCR assay, the ddPCR assay had a shorter window period. 65
One challenging issue in both ddPCR and qPCR assays is the misestimation of viral DNA/RNA, which could introduce inaccuracy into the viral load. Misestimation could account for contradictory reports. Various technical errors can cause underestimation or overestimation in these methods. Misestimation in qPCR can arise from two issues, including 1 Inherent pitfalls in the generation of the standard curve and 2 PCR amplification efficiency, but misestimation in ddPCR stems from different sources. In 2012, it was reported that undigested fragments could not be effectively packed into droplets, leading to the underestimation of HIV‐1 DNA copies by ddPCR. 66 Restriction endonuclease digestion of plasmids can improve efficiency and overcome underestimation in undigested fragments DNA. The underestimation of DNA copy number and 2‐LTR circles was also seen when the input sample has more than 75,000 copies of DNA 66 ; hence, the input DNA should be adjusted before a ddPCR assay. Mu et al. 67 have demonstrated that ddPCR has a good sensitivity ranging from 1 to 105 copies of HBV DNA. 68 It is important to note that this underestimation is possible in the measurement of RNA, in the one‐step RT‐digital PCR methods, 52 and the measurement of cell‐associated (CA) HIV RNA. 69 The Instability of external calibration, which was used to quantify HIV DNA by qPCR, has been shown in the 8E5 cell line. 22 Failure to amplify the target sequence is another disadvantage that affects low target copy number estimates in ddPCR. 70 On the other hand, an overestimation of the tissue‐based viral reservoir's size may have been seen due to truncated genomes' detection. 56
A mismatched primer in qPCR significantly reduced amplification efficiency, while ddPCR has a higher resistance to mismatches. 71 In qPCR, due to mismatches in the priming/probe template, viral DNA/RNA could be underestimated. 69 The detection of HBA DNA has shown, where there is more mismatch between the primer and the target sequence, the underestimation copy number is enhanced. 72
1.4. Applications of ddPCR for detection of viral pathogens
Recently, the growth rate of ddPCR has risen in the diagnosis of viral diseases. ddPCR is a reliable and scalable method for managing virus infections. 73 ddPCR is expected to be a powerful diagnostic method in clinical laboratories, such as identifying and detecting viruses, viral load assay, single‐copy viral genome analysis, single‐nucleotide polymorphisms, and virus‐host interactions. 74 , 75 In addition, ddPCR applicable for CNS malignancy, 76 viral drug resistance, 77 Viral Vectors, 78 , 79 and quantification of World Health Organization (WHO) standards. 80 , 81 Although ddPCR is still in the early stages of routine clinical implementation, performance is reliable and sensitive compared to other routine methods. The number of clinically significant viruses is addressed in the following section that ddPCR assay has been performed to detect DNA or RNA of these viruses.
1.5. Cytomegalovirus
CMV, like other herpesviruses, can cause a latent infection for a long time. According to the latency period of the CMV virus, the amount of virus DNA in the blood can be much lower than the limited detection of standard techniques.6 ddPCR is a reproducible method, particularly at lower CMV DNA concentrations. 82
Various experiments have been performed to assess the sensitivity of qPCR and ddPCR techniques in identifying CMV positive patients with low viral load. 83 , 84 , 85 Results from earlier studies have shown that qPCR results somewhat have a lower level of detection limit (LOD) (3 log10 vs. 4 log10 copies/ml and IU/ml for NIST and WHO standards, respectively) than ddPCR in WHO and the National Institute of Standards and Technology (NIST) CMV quantitative standards. 20 In contrast to earlier studies, experiments have indicated that qPCR's sensitivity and specificity are less than or equal to ddPCR. 83 Guojun Cao and colleagues make the point that the frequency of CMV virus detection was higher in Posner‐Schlossman Syndrome patients with ddPCR than qPCR (400–100 copies/ml, respectively). 86
ddPCR platforms for measuring CMV's viral load revealed a good correlation at high virus concentrations. 87 Inter‐laboratory assessment of different dPCR platforms for the quantification of human CMV DNA indicates that digital PCR assays offer completely repeatable (e.g., inside the instrument) and reproducible (e.g., between instruments, stages, and research centers) measurements of viral DNA. It was demonstrated that in a digital PCR method, the reagents and platform difference leads to result variation in samples with a low CMV. 88
Subclinical herpesviruses' coinfection is highly prevalent with people living with HIV (PLWH). 89 ddPCR is an appropriate technique for studying latent viruses such as EBV and CMV in AIDS patients. 90 Aaron Christensen‐Quick et al. 91 indicated that ddPCR could measure the frequency of total HIV DNA and CMV DNA in PLWH patients. 92 Early and accurate diagnosis of low CMV levels in PLWH patients can be helpful during treatment with antiretroviral therapy.
The nested PCR method was previously used to measure CMV's viral load in less reliable blood samples. In 2016, H. Parry and colleagues showed that cytomegalovirus's viral load could be quantified with high precision by ddPCR in monocyte cells. 93 In other approaches, a multiplex method is investigated to advance the efficiency of a droplet‐based PCR. Multiplex digital PCR test for CMV, HHV‐6A, HHV‐6B, and EBV viral DNA in low‐and high‐grade astrocytoma was conducted to investigate these viruses' oncogenic role. 76 ddPCR has also been used for other aspects of the CMV virus, including the T‐cell response to CMV, 94 validated CMV standard reference, 21 , 95 and tumorigenesis. 96
1.6. Human immunodeficiency virus
The WHO reported that 37.9 million people were diagnosed with HIV worldwide, and 62% (23.3 million) of those were undergoing antiretroviral treatment at the end of 2018. According to the WHO guideline, antiviral therapy aims to reduce the number of copies of the virus to less than 1000 copies/ml in the reservoir. 97 Viral load monitoring is the recommended screening method to validate the failure of antiretroviral therapy. 98
Recently, the development of ddPCR has created an enormous opportunity for progress in HIV research, diagnosis, and care, including the following: quantification of proviral HIV‐1 DNA 66 , 99 , 100 , 101 , 102 , 103 and RNA, 28 , 71 , 101 optimize monitoring of ART‐treated patients 104 HSCs stem‐cell transplantation, 91 , 105 monitoring of ART concentrations, 106 detection of clinically relevant rare viral variants, test novel antiviral drugs, single‐cell assays of HIV‐infected cell lines, 107 applicable in HIV models, 108 , 109 vaccine 110 and dynamic of HIV provirus. 73 , 111 The advances in the field of ddPCR provide the opportunity to analyze viral proviral DNA (DNA‐based viral load test) and CA viral RNA (RNA‐based viral load test) for the enumeration and monitoring of host immune response to HIV.
The interaction between ddPCR and other PCR methods for HIV nucleic acid was acceptable. 112 Bosman et al. 113 have shown that various ddPCR platforms, Quantstudio 3D (Life Technologies) and QX100 (Bio‐Rad), and semi‐nested qPCR have been able to measure 2.5 HIV DNA copies of serial HIV DNA dilutions and DNA extracted from PBMCs of ART‐suppressed patients. ddPCR and qPCR have similar dynamic ranges for linear and episomal DNA calibrators as well as HIV‐1‐DNA and 2‐LTR circles. 66 Lada et al., 114 combined pulsed‐field gel electrophoresis and digital droplet PCR to improve the sensitivity of proviral integrated HIV DNA measurement. 115 The combination of different methods can open up new horizons to identify HIV DNA, which is extensively reviewed by Gibellini. 116
Strain et al. 73 have demonstrated that ddPCR is more accurate than qPCR in diagnosing very rare HIV DNA during combination antiretroviral therapy. 64 Virus DNA and episomal 2‐LTR were analyzed in 300 clinical samples using the Bio‐Rad QX‐100 emulsification machine. The findings revealed that the coefficient of variation decreased by an average of fivefold the number of copies and by more than 20‐fold the 2‐LTR circles' accuracy. This high precision goes beyond quantification's advantages without the need for external standards and relative insensitivity to primer and probe sequence mismatches that are common due to the heterogeneity sequence of proviral DNA. 64 The findings reported by Strain et al. 73 were consistent with the results of other studies. 66 However, in some studies, the sample size used is not adequate.
CA HIV RNA is a sensitive marker for measuring viral reservoirs and monitoring ATR therapy. 69 The challenge of quantifying the RNA with ddPCR appears to be greater than the DNA, as qPCR, external control, and cDNA synthesis are required. Besides this, the number of copies of RNA within tissues or cellular reservoirs can vary from cell to cell and from time to time. Conventional bulk methods such as RT‐qPCR cannot distinguish between the number of copies of RNA as well as cellular phenotype and single‐cell transcriptome profiling. Single‐cell analysis using ddPCR will overcome their drawbacks. 117
Recently, experiments have been performed on the study of HIV transcripts using RT‐ddPCR. This experiment has shown that HIV latency CD4+ T cells may occur due to numerous mechanisms. 118 The number of CD4+ T cells that produce unspliced (us)RNA and multiply spliced (ms)RNA caused by either a histone deacetylase inhibitor (romidepsin) or T cell receptor (TCR) stimulation in single HIV‐1 infected cells has shown that single‐cell dPCR assay can reproducibly determine the numbers of transcriptionally active cells from individuals on ART.
ddPCR is a sensitive technique for detecting rare mutations in the presence of a predominant wild‐type gene, particularly in cancer‐causing mutations. Sedlak et al. 82 demonstrated that ddPCR rapidly quantifies a range of indel mutants in the endonuclease‐treated HIV proviral with detection of as low as 0.02% mutant in a wild‐type background. 119
Conventional qPCR assays use short, conserved subgenomic amplicons that do not distinguish intact and defective proviruses. 120 The quantitative viral outgrowth assay (QVOA) was used to demonstrate the HIV reservoir for the first time in resting CD4+ T cells. QVOA assay underestimated the latent reservoir as not all proviruses are induced to grow. 121 Besides this, total HIV DNA quantification overestimates the reservoir as more than 95% of the proviruses are defective and will not lead to HIV rebound if therapy was interrupted. 122 Researchers have implemented an intact proviral DNA assay (IPDA) to tackle these faults, which measures intact HIV proviruses to separately quantifying intact and defective proviruses by ddPCR. 123 IPDA has shown a more reliable high‐throughput DNA assay than routine methods for measuring intact HIV proviruses. 120 Recently, nested RT‐ddPCR has shown that the persistence of HCV RNA in PBMCs is not common in HIV/HCV coinfected patients. 124
The application of ddPCR in HIV viral load is controversial. Nested qPCR increases the sensitivity of the HIV viral load assays. Kiselinova et al. have shown that semi‐nested real‐time quantitative qPCR of CA HIV‐1 RNA has better quantitative linearity, accuracy, and sensitivity in the quantification of calibrators than ddPCR, especially in the lower quantification ranges 112 ; however, it must be cross‐validated by several laboratories before it can be commonly used in diagnostic laboratories. In another study, the QX100 (Bio‐Rad) ddPCR platform showed high precision, reliability, quantitative linearity, and minimal bias compared to the semi‐nested qPCR method that displayed false‐positive results. The semi‐nested qPCR method was proposed to detect HIV DNA at low concentrations, 113 while most HIV experiments show high levels of ddPCR reproducibility.
False‐positive results are the major disadvantages of digital PCR to accurately measure the proviral DNA/RNA reservoir. 99 There are also a variety of technical limitations, which should be considered when using ddPCR. The software's automated threshold is usually too low and falsely considered a positive droplet, regardless of whether it has low fluorescence. These limitations in sensitivity may be related to the technique of droplet digital PCR assays. 125 It has been shown that poorly handled droplets, rough pipetting, interference caused by the phenomenon of "rain" in the adjusting of a threshold, the copy number of input DNA, adjusting the cycling conditions, and read‐out for any sample being lower than 10,000 droplets 125 and patient‐to‐patient mismatches in the target sequence 100 which alter the amount of HIV viral load, also reduce the sensitivity and efficiency of the ddPCR‐based test.
1.7. Hepatitis
ddPCR had provided a new horizon for identifying and quantifying Hepatitis virus nucleic acids. The use of ddPCR has provided a promising method for monitoring hepatitis‐infected individuals taking combination therapy.
ddPCR is a robust tool that can be measured as little as one copy of the HBV DNA. 126 , 127 The LLOD and quantitation limit (LLOQ) for ddPCR are 0.8 copies/105 cells and 3.8 copies/105 cells. These indices were 19.1 copies/105 and 71.1 copies/105 for qPCR. According to EASL 2017 clinical practice guidelines, the virological response during treatment is defined as undetectable HBV DNA with a limit of 10 IU/ml. 128 This is the lowest real value that can be detected by conventional assays.
Covalently closed circular DNA (cccDNA) of the hepatitis B virus has become a new prognostic biomarker for occult hepatitis B virus infection (OBI) and Hepatocellular Carcinoma. 129 Direct purification of DNA followed by ddPCR may help cccDNA quantification. All HBV RNA molecules are derived from the cccDNA, which presents in the hepatocellular of chronic hepatitis B virus individuals. Purification of DNA with standard methods phenol, chloroform, or ethanol slightly reduced the cccDNA of HBV in anti‐HBC‐positive liver donors. 130 Besides this, contaminants were found in formalin‐fixed paraffin‐embedded (FFPE) hepatocellular carcinoma tissue samples without purification. 114 ddPCR is more sensitive to cccDNA HBV with low copy numbers than qPCR. 68 , 116 , 131 Gian Paolo Caviglia et al. demonstrated that ddPCR is 10–100‐fold more sensitive than qPCR‐based for intrahepatic HBV cccDNA quantitation. 130
The measurement of HBV pregenomic RNA and HEV RNA by ddPCR 132 is more sensitive than conventional qPCR assays. 133 Recently, RT‐ddPCR has been reported to have a relatively high sensitivity among the HBe Ag‐negative group with low viral loads. 134 Despite the high sensitivity of the ddPCR method for HBV and HBC DNA measurement, similar to PCR, this method cannot detect HBV DNA in HBV patients‐derived HUMSCs. 127
ddPCR can increase the DNA detection limit of the HBV DNA with a high degree of linearity. 126 , 131 As seen in other viruses, the detection of HBV RNA copy numbers by RT‐ddPCR and RT‐qPCR demonstrated a high degree of linearity and quantitative similarity. One of the critical difficulties in measuring HBV DNA infection is OBI. Low viral replication (<200 IU/ml, approximately 1000 copies/ml) in the absence of hepatitis B surface antigen is an essential feature of OBI. 129 The relationship between ddPCR and qPCR tests appears to be conflicted. However, there was only a mild correlation between ddPCR and qPCR results (R 2 = .6037). Yang et al. 136 reported an acceptable agreement between these methods. 126
There is a growing body of evidence to suggest that ddPCR is a highly accurate technique with precision and reproducibility comparable to the traditional qPCR method. Further optimization of this method is still required to determine this method's performance for low viral load clinical samples, especially in occult hepatitis B virus infection and cell therapy approaches.
1.8. Influenza
Detection of the influenza virus with ddPCR is well established. 136 , 137 , 138 , 139 Defective interfering particles are massively inner deletion mutants of the virus genome produced by most families of RNA viruses. The ddPCR provides accurate and sensitive quantification of the prevalence of deficient interference RNAs in the population of influenza A. 137 YongYan and his colleagues have shown that RT‐ddPCR, compared to RT‐PCR, is more sensitive and reliable to estimate influenza H7N9(A) viral load without using a calibrator. 138 A cost‐effective six‐plex ddPCR was recently developed to detect influenza A (H1, H3, and M) and influenza B (Yamagata HA, Victoria HA, and M) segments in a single reaction mixture. 67
This assay's precision and simplicity will make it easier to compare gene editing approaches and finally boost progress in this fast‐moving area. Some mutations in the human influenza A virus (H1N1) may alter the response of the neuraminidase inhibitor oseltamivir (Tamiflu). In 2016, the ddPCR method was used to quantify these mutations. 75 Sensitive virology techniques can adjust the decision on seroconversion by reducing the amount of cutoff. ddPCR achieved 30 fold higher sensitivity and ten times higher accuracy than the qPCR to quantify the resistant strains of the H1N1 virus. 140
1.9. Severe acute respiratory syndrome coronavirus 2
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in Wuhan, China, at the end of 2019. 141 Early identification of persons infected with SARS‐CoV‐2 helps to monitor the spread of the pandemic. COVID‐19 RT‐qPCR is prescribed for the detection of SARS‐CoV‐2.
As a gold standard test, the qPCR approach has high false results 142 , 143 due to poor sensitivity of current kits, low viral load, and delay in RNA extraction. ddPCR have been developed for COVID‐19 assays. 135 , 144 , 145 , 146
In the first attempt, it was reported that the qPCR method was sensitive and precise; however, ddPCR worked well to measure low‐viral samples. 135 The results of the study conducted by Dong et al. 147 showed that the ddPCR method was approximately 93% effective in the identification of SARS‐CoV‐2 and could detect as few as two copies of viral DNA per reaction. 146 The results reveal that ddPCR was 500 times more sensitive to SARS‐CoV‐2 than RT‐PCR in low‐viral throat swabs. In this research, 26 samples were positive for ddPCR using the same samples from COVID‐19 outpatients with RT‐PCR negative. In other words, the NPV (negative predictive values) of ddPCR (63%, 36–83) is slightly higher than that of RT‐PCR (16%, 13–19). 144 The rate of false‐negative results in ddPCR is less common than that of other methods. 144 , 146 Digital PCR shows improved low detection limit, sensitivity, and accuracy, allowing COVID‐19 to be detected with fewer false results than RT‐qPCR, particularly for low‐viral load samples. 141 ddPCR demonstrates higher performance for detecting COVID‐19 infection in false‐negative low‐viral specimens. 148 , 149 ddPCR offers qPCR advantages for SARS‐CoV‐2 viral load measurement with higher sensitivity when using directly crude lysate rather than purified RNA as input. 150
This pandemic is still underway, and the various characteristics of the epidemic have not yet been identified. Much of the findings came from a study that has not yet been peer‐reviewed. Therefore, the data and performance of ddPCR should be interpreted with caution.
1.10. Application of ddPCR in detection of other virus
The ddPCR ultrasensitive technique is a versatile method that can be used to detect other viruses. Like HIV, The ddPCR has comprehensively characterized defective and intact HTLV‐1 proviruses in infected individuals. 151 The ddPCR assay provides rapid and reliable laboratory identification of chromosomally incorporated form of the virus (ciHV‐6) from conveniently extracted cell samples. 152 The presence of a ciHV‐6 is a technical drawback in detecting the number of copies of the HHV‐6 virus in whole blood or plasma by typical RT‐qPCR. 152 , 153 CiHHV‐6 should be distinguished from active HHV‐6 infections. As conventional HHV‐6 plasma PCR tests identify both forms (ciHV‐6, active HHV‐6), patients with cIHHV‐6 are often confused with active HHV‐6.
The efficacy of ddPCR could help determine virus‐related cancers. Recently, ddPCR has been an ultra‐sensitive nucleic acid detection tool for a tumor‐associated virus biomarker study. In the case of multiple tumor suppressor 1 (p16) immunohistochemistry (IHC), the sensitivity and specificity of ddPCR HPV E6/E7 for p16 positivity detection is 91.3% and 100%, respectively. However, HPV‐16 E6 and E7 were negative with ddPCR in both noncancerous and nonoropharyngeal SCC (OPSCC) tumors, and two patients with IHC had false‐positive findings. The qPCR analysis found that the sensitivity of the combined saliva and plasma pretreatment status of HPV‐16 DNA–HPV‐16 tumor status was 76%. 154
Measurement of vector copy number with ddPCR has been considered in recent years. 155 , 156 Titrations of the genome by ddPCR were shown 5‐, 1.9‐ and 2.3‐fold higher than those determined using standard qPCR, optimized qPCR, and agarose gel assays, respectively single‐stranded and self‐complementary AAV genomes. 157 Cell‐based enumeration by ddPCR can provide the required precision and sensitivity to normalize drug concentrations in both the upstream and downstream pharmacokinetic studies. 158
ddPCR can also be used to standardize standard RT‐qPCR methods. The One‐Step RT‐dPCR approach was used in one study to measure the reference materials for the Ebola RNA virus. 159 The ddPCR showed a better method for the detection of a low‐copy number of Merkel cell polyomavirus in FFPE biopsies. 84 Studies have shown that ddPCR can be an effective method to classify clinical outcomes in cancer patients. Quantification of HPV viral load by ddPCR may be informative for further stratifying clinical outcomes in HPV positive oropharyngeal cancer patients. 160 A list of some of the viruses detected by ddPCR is shown in Table 1.
Table 1.
List of some of the viruses detected by ddPCR
| Method | Virus | Finding | References |
|---|---|---|---|
| One‐step RT‐ddPCR | SVA | LOD is 10 times lower than that of an RT‐qPCR. | 161 |
| ddPCR | HTLV‐1 | – | 151 |
| Two‐step RT‐ddPCR | HPeV3 | Two‐step RT‐ddPCR was less variable and more specific than one‐step RT‐ddPCR. | 162 |
| RT‐ddPCR | JEV | ddPCR had a high degree of linearity, high specificity, and JEV RT‐ddPCR was more sensitive than real‐time RT‐PCR. | 163 |
| ddPCR | RSV | – | 139 |
| Micro RT‐ddPCR | Zika Virus | ddPCR has outstanding accuracy and sensitivity for samples of low concentrations. | |
| RT‐ddPCR | dengue virus serotype 2 | RT‐ddPCR assay developed had similar specificity to the routine RT‐qPCR assay. | 164 |
| RT‐ddPCR | Zika virus | – | 73 , 166 |
| ddPCR | Anelloviruses | – | 166 |
| ddPCR | HCMV, herpes simplex virus, EBV, and varicella‐zoster virus | The results of ddPCR were consistent with that of next‐generation sequencing. Compared with qPCR, results of ddPCR showed better consistency with the validity of clinical treatment. | 86 |
Abbreviations: EBV, Epstein‐Barr virus; HCMV, human cytomegalovirus; HPeV3, human par echovirus type 3; HTLV‐1, human T‐cell leukemia virus, type 1; JEV, Japanese encephalitis virus; LOD, limit of detection; RSV, respiratory syncytial virus; SVA, seneca virus A.
1.11. Challenges and future perspectives
Microfluidics has a remarkable ability to enhance novel approaches for detecting viral infections. Although ddPCR opens a new frontier for viral detection, virologists have not adopted the ddPCR approach well.
Current ddPCR platforms need automation and cost reduction. The ddPCR technique has a higher throughput than the qPCR, at a similar cost per sample, but the instrument purchase initial costs were not yet cost‐effective. 51 besides this, it requires trained personnel to perform and interpret results. The availability of microfluidic technologies is a critical barrier. Many reagents and equipment are not available in underdeveloped countries where they are more vulnerable to viral infections.
Owing to the droplets closed environment, it is also hardly possible to exchange reagents or perform washing steps. Some drawbacks of PCR‐based approaches have been inherited. As ddPCR is a PCR‐based test method, only known sequences can be amplified.
In the future, ddPCR may face a few challenges as methods are developed. By developing the method, some of them will get eliminated or reduced. For example; one of the known drawbacks of ddPCR is the observation of false‐positive partitions in no template control (NTC) well. 69 , 71 , 112 However, the origin of the false‐positives droplet in NTC samples has not yet been determined, but some simple considerations, such as adjusting the fluorescence threshold with software adjusting 71 and bioinformatic pipelines, 103 can significantly reduce biases.
As the ddPCR instrument parts, including the droplet generation instruments, thermocyclers, and droplet reader, usually have a complex structure, a single device for digital PCR detection has been designed with solar power for absolute quantitative detection of the hepatitis B virus in human serum. 167 Portable rapid methods are one of the growing fields of interest in molecular detection. Affordable POCT devices are effective tools to monitor epidemics such as the coronavirus outbreak of 2019. Recently, one‐dollar microwell array‐based dPCR has amplified cDNA for the H7N9 influenza virus at a concentration range of 1000–100,000 copies/μl.
ddPCR can integrate with other superior technologies such as single‐cell analysis methods and CRISPR gene editing (Figure 3). Chinese researchers have introduced a PCR‐based CRISPR‐Cas13a detection system (referred to as PCR‐CRISPR) to detect HBV DNA and drug resistance mutations. 168
Figure 3.
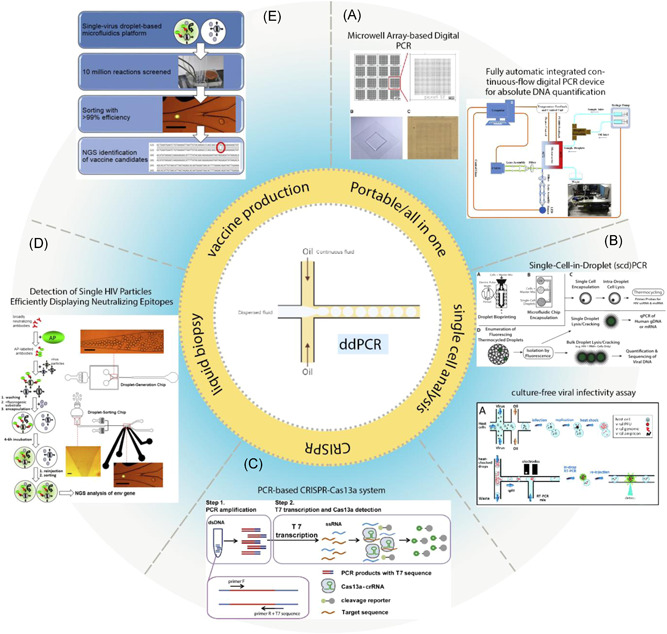
Integration of droplet‐based microfluidic in various biological methods. (A) This figure shows a microwell array‐based digital PCR made by conventional soft lithography, Illustrated by a fluorescent microscope. The use of the microwell Array reduces generation, thermal cycling, and reading of droplets cost. 169 Schematic diagram of a fully automatic integrated digital PCR device based on continuous‐flow digital PCR works with solar power. (B) Schema of the single‐cell encapsulation, lysis, Tat‐Rev spliced or unspliced cell‐associated HIV‐1 RNA detection, and rescue of cellular genomic DNA and mRNA in droplets. Droplets are created by one of the bio print methods or microfluidic chips. CD4+ T‐cells were isolated from peripheral blood mononuclear cells (PBMCs). CD4+ T cells were washed and mixed 1:1 with ddPCR master mix and cell lysis agent. Droplets generated, and cells are lysed inside the droplets, followed by PCR amplification of Tat‐Rev spliced or unspliced cell‐associated HIV‐1 RNA. 170 The schematic diagram shows a rapid, targeted, and culture‐free viral infectivity assay in drop‐based microfluidics. murine norovirus strain MNV‐1 that infect host cells, coencapsulated in droplets. 147 (C) PCR‐based CRISPR‐Cas13a system. 171 (D, E) A highly efficient microfluidic droplet‐based platform to screen single virus particles for optimum vaccine candidate antigenic compounds. Like the standard ELISA method, HIV‐1 particles were incubated with alkaline phosphatase (AP)‐labeled broadly neutralizing antibody PGT128. Next, HIV‐1 particles were bonded to AP were encapsulated separately in droplets, together with a fluorogenic substrate for AP (FDP). Finally, the fluorescent intensity of each droplet was measured and sorted by another microfluidic device. Viruses were recovered, and NGS of the env genes was performed after RT‐PCR using specific primers for HIV‐1 env. 175 172 ELIS, enzyme‐linked immunosorbent assay; mRNA, messenger RNA
The ddPCR technique has been extended to single‐cell manipulation, setting the stage for integrating DNA/RNA measurements with upstream sample handling, such as single‐cell manipulation. High mutation rates and segmented genome viruses are significant factors in the reduction of antiviral therapeutic efficacy. The heterogeneity of the genome segments of incomplete influenza A virus is analyzed at a single cell level by ddPCR. 173 Tao et al. 174 have developed a new viral infectivity assay using droplet‐based microfluidics. 147 As a model, noroviruses (MNV) are incubated in many picolitre droplets with host cells for one viral replication cycle followed by ddPCR.
Droplet‐based single‐cell transcriptome analyses (scRNA‐Seq) systems such as Drop‐seq, inDrop, and Chromium 10Xis are the most common methodologies in the emerging scRNA‐seq technique. 175 The transcriptome of single human primary fibroblasts by drop‐seq procedure during the first hours of HSV‐1 lytic infection gave a deep insight into biological processes involving the early stages of HSV‐1 infection. 176 scseq‐RNA is a useful tool for virus‐host analysis. Fluorescence‐activated cell sorting and magnetic‐activated cell sorting allow a biased selection of the cell population based on cell size or shape. Microdroplet‐based technologies enable unbiased isolation of single cells. Today, ddPCR is used to generate cDNA libraries in various ScRNA‐Seq workflows to understand virus‐host interactions. 177
Indirect noninvasive detection of disease via biomarkers often referred to as “liquid biopsy,” has become more preferred than traditional invasive biopsies. Many of these biomarkers may be identified before the onset of the main disease's clinical symptoms. ddPCR may be beneficial in detecting circulating tumor DNA (ctDNA), microRNAs, and/or viral DNAs/miRNAs in liquid biopsies for prostate cancer diagnostic purposes, especially at the early stage of diseases. 178 Cell‐free DNA (cfDNA) is a promising biomarker for both transplant rejection and tissue response monitoring. This provides an excellent opportunity for the physician to benefit from personalized immunosuppression.
The performance of ddPCR appeared sufficient to minimize the duration of hospitalization as well as risk stratification. The application of ddPCR in noninvasive monitoring of graft integrity after liver transplantation (LTx) has been considered in HCV patients. The plasma‐derived cfDNA (GcfDNA) of 115 adults post‐LTx was measured by ddPCR. The study results revealed that GcfDNA results compared to assess conventional liver function enzymes had a high sensitivity. It reduced the duration of discrimination of LTx patients with acute rejection between 7 and 14 days. 179 Huang et al. 149 showed that measuring cccDNA copy number in formalin‐fixed paraffin‐embedded hepatocellular carcinoma tissue is sensitive and specific. 114 The ddPCR method has been evaluated for the diagnosis of HPV DNA in serum. The high‐sensitivity ddPCR detects HPV E7 DNA in 61/70 (87%) serum specimens of HPV patients associated with invasive carcinomas, regardless of the initial tumor sites. 180 The ability of ddPCR to measure HPV ctDNA to monitor the efficacy of immune control point inhibitors has been demonstrated in another case report study. 181
DdPCR involved in the production of vaccines for emerging viruses and antiviral drug development, especially in the single‐virus droplet. 172 Although the application of the technique in the production of vaccines is limited today, recent results have shown that ddPCR is an effective and sensitive method to validation of vaccines 182 and contamination detection in attenuated vaccines. 183 , 184 Due to the acceptable sensitivity of the test, ddPCR is implemented with automation in support of multiple upstream and downstream process development efforts for influenza vaccine manufacturing. 185
In the dengue virus workflow, the RT‐ddPCR assay will provide absolute quantification of all four serotypes in a single multiplex assay without the need for a standard curve. The assay also required less time and reagent use and reduced technical error probability. 186 Droplet‐based amplification technologies considerably can tolerate mismatch between the primers and target sequence. It has raised expectations for diagnostic testing development, especially in viruses such as Ebola, which has a high mutation rate. 174 High‐throughput ddPCR has shown speedy results in the bacteriophage field compared to conventional counts of plaque‐forming units (PFUs) for enumerating phages, trace bacteria, and phage concentrations. 187
2. CONCLUSIONS
The emerging ddPCR technique has been promising compared to conventional methods. ddPCR has a high reproducibility and is sensitive and accurate. ddPCR improves our knowledge of how the virus works inside our host cell as well as developing precise virus detection platforms. Although ddPCR improves on the inherent limitations of conventional PCR, it requires the adoption of rigorous quality control plans.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Mahdieh Farzanehpour and Amir Asri were involved in planning and supervised the work and proof outline. Amir Asri, Hadi Esmaeili, Ruhollah Dorostkar, Ali Jafarpour, Masoumeh Bolandian, Majid Mirzaei drafted the manuscript and designed the figures. All authors discussed the results and commented on the manuscript.
Kojabad AA, Farzanehpour M, Galeh HEG, et al. Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives. J Med Virol. 2021;93:4182–4197. 10.1002/jmv.26846
REFERENCES
- 1. UNAIDS . Global HIV and AIDS statistics 2019 Fact sheet. Glob HIV AIDs ststistics, World AIDS day 2019 Fact Sheet. 2019.
- 2. WHO . Hepatitis C. Fact sheet no. 164. World Health Organization Media Centre. 2018.
- 3. Kukka CM. Hepatitis B Fact Sheet. Hepatitis C Support Project. 2010.
- 4. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV‐1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665‐708. [DOI] [PubMed] [Google Scholar]
- 6. Speck SH, Ganem D. Viral latency and its regulation: lessons from the γ‐Herpesviruses. Cell Host and Microbe. 8. Cell Press; 2010:100‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein‐Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374‐7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen LS, Normann P, Thykier‐Nielsen S, Sørensen JH, de Stricker K, Rosenørn S. Analysis of the epidemiological dynamics during the 1982‐1983 epidemic of foot‐and‐mouth disease in Denmark based on molecular high‐resolution strain identification. J Gen Virol. 2005;86:2577‐2584. [DOI] [PubMed] [Google Scholar]
- 9. Why early detection of outbreaks is so important . Can Fam Physician. 2017. [PMC free article] [PubMed]
- 10. Zou X, Wu J, Gu J, Shen L, Mao L. Application of aptamers in virus detection and antiviral therapy. Front Microbiol. 2019;10:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma V, Denise Dowd M, Slaughter AJ, Simon SD. Effect of rapid diagnosis of influenza virus type A on the emergency department management of febrile infants and toddlers. Arch Pediatr Adolesc Med. 2002;156:41. [DOI] [PubMed] [Google Scholar]
- 12. Nafa S. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology. 2000;32:1078‐1088. [DOI] [PubMed] [Google Scholar]
- 13. Stramer SL, Krysztof DE, Brodsky JP, et al. Comparative analysis of triplex nucleic acid test assays in United States blood donors. Transfusion. 2013;53:2525‐2537. [DOI] [PubMed] [Google Scholar]
- 14. Galel SA, Simon TL, Williamson PC, et al. Sensitivity and specificity of a new automated system for the detection of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus nucleic acid in blood and plasma donations. Transfusion. 2018;58:649‐659. [DOI] [PubMed] [Google Scholar]
- 15. Boonham N, Kreuze J, Winter S, et al. Methods in virus diagnostics: from ELISA to next generation sequencing. Virus Res. 2014;186:20‐31. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Qu S, Xu L. Progress in the study of virus detection methods: the possibility of alternative methods to validate virus inactivation. Biotechnol Bioeng. 2019;116:2095‐2102. [DOI] [PubMed] [Google Scholar]
- 17. Souf S. Recent advances in diagnostic testing for viral infections. Biosci Horizons. 2016;9:hzw010. [Google Scholar]
- 18. Valones MAA, Guimarães RL, Brandão LAC, De Souza PRE, De Albuquerque Tavares Carvalho A, Crovela S. Principles and applications of polymerase chain reaction in medical diagnostic fields: a review. Brazilian J Microbiol. 2009;40:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor SC, Laperriere G, Germain H. Droplet digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Sci Rep. 2017;7:2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayden RT, Gu Z, Ingersoll J, et al. Comparison of droplet digital PCR to real‐time PCR for quantitative detection of cytomegalovirus. J Clin Microbiol. 2013;51:540‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powell EA, Babady NE. Digital PCR in the clinical microbiology laboratory: another tool on the molecular horizon. Clin Microbiol Newsl. 2018;40:27‐32. [Google Scholar]
- 22. Busby E, Whale AS, Ferns RB, et al. Instability of 8E5 calibration standard revealed by digital PCR risks inaccurate quantification of HIV DNA in clinical samples by qPCR. Sci Rep. 2017;7:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yager P, Edwards T, Fu E, et al. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412‐418. [DOI] [PubMed] [Google Scholar]
- 24. Mastiani M, Mosavati B, Kim M. Numerical simulation of high inertial liquid‐in‐gas droplet in a T‐junction microchannel. RSC Adv. 2017;7:48512‐48525. [Google Scholar]
- 25. Li H, Bai R, Zhao Z, et al. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci Rep. 2018;38:BSR20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casadevall i Solvas X, De Mello A. Droplet microfluidics: recent developments and future applications. Chem Commun. 2011;47:1936‐1942. [DOI] [PubMed] [Google Scholar]
- 27. Rutsaert S, Bosman K, Trypsteen W, Nijhuis M, Vandekerckhove L. Digital PCR as a tool to measure HIV persistence. Retrovirology. 2018;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basova EYu, Foret F. Droplet microfluidics in (bio)chemical analysis. Analyst (Lond). 2015;140:22‐38. [DOI] [PubMed] [Google Scholar]
- 29. Gach PC, Iwai K, Kim PW, Hillson NJ, Singh AK. Droplet microfluidics for synthetic biology. Lab Chip. 2017;17:3388‐3400. [DOI] [PubMed] [Google Scholar]
- 30. Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper‐based analytical devices. Anal Chem. 2010;82:3‐10. [DOI] [PubMed] [Google Scholar]
- 31. Bhatia SN, Ingber DE. Microfluidic organs‐on‐chips. Nature Biotechnol. 2014;32:760‐772. [DOI] [PubMed] [Google Scholar]
- 32. DeMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature. 2006;442:394‐402. [DOI] [PubMed] [Google Scholar]
- 33. Srinivasan V, Pamula VK, Fair RB. An integrated digital microfluidic lab‐on‐a‐chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004. [DOI] [PubMed] [Google Scholar]
- 34. Brouzes E, Medkova M, Savenelli N, et al. Droplet microfluidic technology for single‐cell high‐throughput screening. Proc Natl Acad Sci USA. 2009;106:14195‐14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiu DT, deMello AJ, Di Carlo D, et al. Small but perfectly formed? successes, challenges, and opportunities for microfluidics in the chemical and biological sciences. Chem. 2017;2:201‐223. [Google Scholar]
- 36. Whitesides GM. The origins and the future of microfluidics. Nature [Internet]. 2006;442(7101):368‐373. Available from http://www.ncbi.nlm.nih.gov/pubmed/16871203 [DOI] [PubMed] [Google Scholar]
- 37. Convery N, Gadegaard N. 30 years of microfluidics. Micro and Nano Engineering. 2019;2:76‐91. [Google Scholar]
- 38. Simpson C, Lee SS, Lee CS, Yamauchi Y. Microfluidics: an untapped resource in viral diagnostics and viral cell biology. Current Clinical Microbiology Reports. 2018;5:245‐251. [Google Scholar]
- 39. Verpoorte E. Microfluidic chips for clinical and forensic analysis. Electrophoresis. 2002;23:677‐712. [DOI] [PubMed] [Google Scholar]
- 40. Yeo LY, Chang HC, Chan PPY, Friend JR. Microfluidic devices for bioapplications. Small. 2011;7:12‐48. [DOI] [PubMed] [Google Scholar]
- 41. Halldorsson S, Lucumi E, Gómez‐Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218‐231. [DOI] [PubMed] [Google Scholar]
- 42. Carrell C, Kava A, Nguyen M, et al. Beyond the lateral flow assay: a review of paper‐based microfluidics. Microelectron Eng. 2019;206:45‐54. [Google Scholar]
- 43. Gorkin R, Park J, Siegrist J, et al. Centrifugal microfluidics for biomedical applications. Lab Chip. 2010;10:1758. [DOI] [PubMed] [Google Scholar]
- 44. Akyazi T, Basabe‐Desmonts L, Benito‐Lopez F. Review on microfluidic paper‐based analytical devices towards commercialisation. Anal Chim Acta. 2018;1001:1‐17. [DOI] [PubMed] [Google Scholar]
- 45. Samiei E, Tabrizian M, Hoorfar M. A review of digital microfluidics as portable platforms for lab‐on a‐chip applications. Lab Chip. 2016;16:2376‐2396. [DOI] [PubMed] [Google Scholar]
- 46. Theberge AB, Courtois F, Schaerli Y, et al. Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angewandte Chemie ‐ International Edition. 2010;49:5846‐5868. [DOI] [PubMed] [Google Scholar]
- 47. Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices microfluidics toward a Lab‐on‐a‐Chip. Annu Rev Fluid Mech. 2004;36:381‐411. [Google Scholar]
- 48. Shang L, Cheng Y, Zhao Y. Emerging droplet microfluidics. Chem Rev. 2017;117:7964‐8040. [DOI] [PubMed] [Google Scholar]
- 49. Kaminski TS, Scheler O, Garstecki P. Droplet microfluidics for microbiology: Techniques, applications and challenges. Lab Chip. 2016;16:2168‐2187. [DOI] [PubMed] [Google Scholar]
- 50. Quan PL, Sauzade M, Brouzes E. DPCR: a technology review. Sensors (Switzerland). 2018;18:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teh SY, Lin R, Hung LH, Lee AP. Droplet microfluidics. Lab Chip. 2008;8:198. [DOI] [PubMed] [Google Scholar]
- 52. Baret JC. Surfactants in droplet‐based microfluidics. Lab Chip. 2012;12:422‐433. [DOI] [PubMed] [Google Scholar]
- 53. Zhu P, Wang L. Passive and active droplet generation with microfluidics: a review. Lab Chip. 2017;17:34‐75. [DOI] [PubMed] [Google Scholar]
- 54. Shembekar N, Chaipan C, Utharala R, Merten CA. Droplet‐based microfluidics in drug discovery, transcriptomics and high‐throughput molecular genetics. Lab Chip. 2016;16:1314‐1331. [DOI] [PubMed] [Google Scholar]
- 55. Sahore V, Doonan SR, Bailey RC. Droplet microfluidics in thermoplastics: device fabrication, droplet generation, and content manipulation using integrated electric and magnetic fields. Anal Methods. 2018;10:4264‐4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pavšič J, Žel J, Milavec M. Digital PCR for direct quantification of viruses without DNA extraction. Anal Bioanal Chem. 2016;408:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vyawahare S, Griffiths AD, Merten CA. Miniaturization and parallelization of biological and chemical assays in microfluidic devices. Chem Biol. 2010;17:1052‐1065. [DOI] [PubMed] [Google Scholar]
- 58. Zhu Y, Fang Q. Analytical detection techniques for droplet microfluidics‐A review. Anal Chim Acta. 2013;787:24‐35. [DOI] [PubMed] [Google Scholar]
- 59. Basu AS. Digital assays part I: partitioning statistics and digital PCR. SLAS Technol. 2017;22(4):369‐386. [DOI] [PubMed] [Google Scholar]
- 60. Kralik P, Ricchi M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front Microbiol. 2017;8.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boulter N, Suarez FG, Schibeci S, et al. A simple, accurate and universal method for quantification of PCR. BMC Biotechnol. 2016;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barea JS, Lee J, Kang DK. Recent advances in droplet‐based microfluidic technologies for biochemistry and molecular biology. Micromachines. 2019;10(6):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao Y, Xia Q, Yin Y, Wang Z. Comparison of droplet digital PCR and quantitative PCR assays for quantitative detection of xanthomonas citri Subsp. Citri. PLoS One. 2016;11:e0159004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Veach AJ, Beard C, Porter F, Wilson M, Scorza FB. Digital droplet PCR for influenza vaccine development. Procedia Vaccinol. 2015;9:96‐103. [Google Scholar]
- 65. Campomenosi P, Gini E, Noonan DM, et al. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanders R, Mason DJ, Foy CA, Huggett JF. Evaluation of digital PCR for absolute RNA quantification. PLoS One. 2013;8:e75296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mu D, Yan L, Tang H, Liao Y. A sensitive and accurate quantification method for the detection of hepatitis B virus covalently closed circular DNA by the application of a droplet digital polymerase chain reaction amplification system. Biotechnol Lett. 2015;37:2063‐2073. [DOI] [PubMed] [Google Scholar]
- 68. Sedlak RH, Kuypers J, Jerome KR. A multiplexed droplet digital PCR assay performs better than qPCR on inhibition prone samples. Diagn Microbiol Infect Dis. 2014;80:285‐286. [DOI] [PubMed] [Google Scholar]
- 69. Long S, Berkemeier B. Maximizing viral detection with SIV droplet digital PCR (ddPCR) assays. PLoS One. 2020;15:e0233085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dingle TC, Sedlak RH, Cook L, Jerome KR. Tolerance of droplet‐digital PCR vs real‐time quantitative PCR to inhibitory substances. Clin Chem. 2013;59:1670‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Biron VL, Kostiuk M, Isaac A, et al. Detection of human papillomavirus type 16 in oropharyngeal squamous cell carcinoma using droplet digital polymerase chain reaction. Cancer. 2016;122:1544‐1551. [DOI] [PubMed] [Google Scholar]
- 72. Liu Y, Cathcart AL, Delaney WE, Kitrinos KM. Development of a digital droplet PCR assay to measure HBV DNA in patients receiving long‐term TDF treatment. J Virol Methods. 2017;249:189‐193. [DOI] [PubMed] [Google Scholar]
- 73. Strain MC, Lada SM, Luong T, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One. 2013;8(4):e55943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kurosaki Y, Martins DBG, Kimura M, et al. Development and evaluation of a rapid molecular diagnostic test for Zika virus infection by reverse transcription loop‐mediated isothermal amplification. Sci Rep. 2017;7:13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mukaide M, Sugiyama M, Korenaga M, et al. High‐throughput and sensitive next‐generation droplet digital PCR assay for the quantitation of the hepatitis C virus mutation at core amino acid 70. J Virol Methods. 2014;207:169‐177. [DOI] [PubMed] [Google Scholar]
- 76. Whale AS, Bushell CA, Grant PR, et al. Detection of rare drug resistance mutations by digital PCR in a human influenza a virus model system and clinical samples. J Clin Microbiol. 2016;54:392‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hill JA, HallSedlak R, Magaret A, et al. Efficient identification of inherited chromosomally integrated human herpesvirus 6 using specimen pooling. J Clin Virol. 2016;77:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Handa N, Kato M, Ishihama Y, Masuda G. Reduction in the window period of an acute infection by chemiluminescence enzyme immunoassay of HIV‐1 p24 antigen. Rinsho Byori. 1999;47:881. [PubMed] [Google Scholar]
- 79. Beer NR, Hindson BJ, Wheeler EK, et al. On‐chip, real‐time, single‐copy polymerase chain reaction in picoliter droplets. Anal Chem. 2007;79:8471‐8475. [DOI] [PubMed] [Google Scholar]
- 80. Hijano DR, Brazelton de Cardenas J, Maron G, et al. Clinical correlation of influenza and respiratory syncytial virus load measured by digital PCR. PLoS One. 2019;14:e0220908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leibovitch EC, Brunetto GS, Caruso B, et al. Coinfection of human herpesviruses 6A (HHV‐6A) and HHV‐6B as demonstrated by novel digital droplet PCR assay. PLoS One. 2014;9:e92328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sedlak RH, Liang S, Niyonzima N, et al. Digital detection of endonuclease mediated gene disruption in the HIV provirus. Sci Rep. 2016;6:20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taylor SC, Carbonneau J, Shelton DN, Boivin G. Optimization of droplet digital PCR from RNA and DNA extracts with direct comparison to RT‐qPCR: clinical implications for quantification of oseltamivir‐resistant subpopulations. J Virol Methods. 2015;224:58‐66. [DOI] [PubMed] [Google Scholar]
- 84. Ahn SM, Chan JYK, Zhang Z, et al. Saliva and plasma quantitative polymerase chain reaction‐based detection and surveillance of human papillomavirus‐related head and neck cancer. JAMA Otolaryngol—Head Neck Surg. 2014;140:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morgan RA, Unti MJ, Aleshe B, et al. Improved titer and gene transfer by lentiviral vectors using novel, small β‐globin locus control region elements. Mol Ther. 2019;28(1):328‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lin HT, Okumura T, Yatsuda Y, Ito S, Nakauchi H, Otsu M. Application of droplet digital PCR for estimating vector copy number states in stem cell gene therapy. Hum Gene Ther Methods. 2016;27:197‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lock M, Alvira MR, Chen SJ, Wilson JM. Absolute determination of single‐stranded and self‐complementary adeno‐associated viral vector genome titers by droplet digital PCR. Hum Gene Ther Methods. 2014;25:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dyavar SR, Ye Z, Byrareddy SN, et al. Normalization of cell associated antiretroviral drug concentrations with a novel RPP30 droplet digital PCR assay. Sci Rep. 2018;8:3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin CTM, Leibovitch EC, Almira‐Suarez MI, Jacobson S. Human herpesvirus multiplex ddPCR detection in brain tissue from low‐ and high‐grade astrocytoma cases and controls. Infect Agent Cancer. 2016;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Košir AB, Cvelbar T, Kammel M, Grunert H‐P, Zeichhardt H, Milavec M. Digital PCR method for detection and quantification of specific antimicrobial drug‐resistance mutations in human cytomegalovirus. J Virol Methods [Internet]. 2020;281:113864. https://linkinghub.elsevier.com/retrieve/pii/S0166093420301166 [DOI] [PubMed] [Google Scholar]
- 91. Christensen‐Quick A, Massanella M, Frick A, et al. Subclinical cytomegalovirus DNA is associated with CD4 T cell activation and impaired CD8 T cell CD107a expression in people living with hiv despite early antiretroviral therapy. J Virol. 2019;93(13):e00179‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dobnik D, Kogovšek P, Jakomin T, et al. Accurate quantification and characterization of adeno‐associated viral vectors. Front Microbiol. 2019;10:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Y, Bergelson S, Feschenko M. Determination of lentiviral infectious titer by a novel droplet digital PCR method. Hum Gene Ther Methods. 2018;29:96‐103. [DOI] [PubMed] [Google Scholar]
- 94. Bateman AC, Greninger AL, Atienza EE, Limaye AP, Jerome KR, Cook L. Quantification of BK virus standards by quantitative real‐time PCR and droplet digital PCR is confounded by multiple virus populations in the WHO BKV international standard. Clin Chem. 2017;63:761‐769. [DOI] [PubMed] [Google Scholar]
- 95. Greninger AL, Bateman AC, Atienza EE, et al. Copy number heterogeneity of JC virus standards. J Clin Microbiol. 2017;55:824‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang K, Lin G, Li J. Quantitative nucleic acid amplification by digital PCR for clinical viral diagnostics. Clin Chem Lab Med. 2016;54:1427‐1433. [DOI] [PubMed] [Google Scholar]
- 97. Sedlak RH, Cook L, Cheng A, Magaret A, Jerome KR. Clinical utility of droplet digital PCR for human cytomegalovirus. J Clin Microbiol. 2014;52:2844‐2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Arvia R, Sollai M, Pierucci F, Urso C, Massi D, Zakrzewska K. Droplet digital PCR (ddPCR) vs quantitative real‐time PCR (qPCR) approach for detection and quantification of Merkel cell polyomavirus (MCPyV) DNA in formalin fixed paraffin embedded (FFPE) cutaneous biopsies. J Virol Methods. 2017;246:15‐20. [DOI] [PubMed] [Google Scholar]
- 99. Urso C, Pierucci F, Sollai M, Arvia R, Massi D, Zakrzewska K. Detection of Merkel cell polyomavirus and human papillomavirus DNA in porocarcinoma. J Clin Virol. 2016;78:71‐73. [DOI] [PubMed] [Google Scholar]
- 100. Hayden RT, Gu Z, Sam SS, et al. Comparative performance of reagents and platforms for quantitation of cytomegalovirus DNA by digital PCR. J Clin Microbiol. 2016;54:2602‐2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Leng SX, Margolick JB. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol. 2020;348:104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nixon G, Garson JA, Grant P, Nastouli E, Foy CA, Huggett JF. Comparative study of sensitivity, linearity, and resistance to inhibition of digital and nondigital polymerase chain reaction and loop mediated isothermal amplification assays for quantification of human cytomegalovirus. Anal Chem. 2014;86:4387‐4394. [DOI] [PubMed] [Google Scholar]
- 103. Cao G, Tan C, Zhang Y, et al. Digital droplet polymerase chain reaction analysis of common viruses in the aqueous humour of patients with Posner‐Schlossman syndrome in Chinese population. Clin Exp Ophthalmol. 2019;47:513‐520. [DOI] [PubMed] [Google Scholar]
- 104. Gianella S, Anderson CM, Var SR, et al. Replication of human herpesviruses is associated with higher HIV DNA levels during antiretroviral therapy started at early phases of HIV infection. J Virol. 2016;90:3944‐3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Christensen‐Quick A, Massanella M, Frick A, et al. Subclinical cytomegalovirus DNA is associated with CD4 T cell activation and impaired CD8 T cell CD107a expression in people living with HIV despite early antiretroviral therapy. J Virol. 2019;93:e00179‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Parry HM, Zuo J, Frumento G, et al. Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing. 2016;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tavenier J, Margolick JB, Leng SX. T‐cell immunity against cytomegalovirus in HIV infection and aging: relationships with inflammation, immune activation, and frailty. Med Microbiol Immunol. 2019;208:289‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Haynes RJ, Kline MC, Toman B, et al. Standard reference material 2366 for measurement of human cytomegalovirus DNA. J Mol Diagn. 2013;15(2):177‐185. [DOI] [PubMed] [Google Scholar]
- 109. Falcinelli SD, Ceriani C, Margolis DM, Archin NM. New frontiers in measuring and characterizing the HIV reservoir. Front Microbiol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. World Health Organization . HIV Prevention, Diagnosis, Treatment and Care for Key Populations (2016 Update). World Heal Organ. 2016;29‐30. [Google Scholar]
- 111. WHO . What's new in treatment monitoring: viral load and CD4 testing. HIV Treat care. 2017;1(1):107‐120. [Google Scholar]
- 112. Jones M, Williams J, Gärtner K, Phillips R, Hurst J, Frater J. Low copy target detection by Droplet Digital PCR through application of a novel open access bioinformatic pipeline, “definetherain. J Virol Methods. 2014;202:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bosman KJ, Wensing AM, Pijning AE, et al. Development of sensitive ddPCR assays to reliably quantify the proviral DNA reservoir in all common circulating HIV subtypes and recombinant forms. J Int AIDS Soc. 2018;21(9):e25185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lada SM, Huang K, VanBelzen DJ, Montaner LJ, O′Doherty U, Richmana DD. Quantitation of integrated HIV provirus by pulsed‐field gel electrophoresis and droplet digital PCR. J Clin Microbiol. 2018;56:e01158‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Henrich TJ, Gallien S, Li JZ, Pereyra F, Kuritzkes DR. Low‐level detection and quantitation of cellular HIV‐1 DNA and 2‐LTR circles using droplet digital PCR. J Virol Methods. 2012;186:68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gibellini L, Pecorini S, De Biasi S, et al. Exploring viral reservoir: the combining approach of cell sorting and droplet digital PCR. Methods. 2018;134‐135:98‐105. [DOI] [PubMed] [Google Scholar]
- 117. Van Hecke C, Trypsteen W, Malatinkova E, et al. Early treated HIV‐1 positive individuals demonstrate similar restriction factor expression profile as long‐term non‐progressors. EBioMedicine. 2019;41:443‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rutsaert S, Bosman K, Trypsteen W, Nijhuis M, Vandekerckhove L. Digital PCR as a tool to measure HIV persistence. Retrovirology [Internet]. 2018;15(1):16. https://retrovirology.biomedcentral.com/articles/10.1186/s12977-018-0399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Alteri C, Scutari R, Stingone C, et al. Quantification of HIV‐DNA and residual viremia in patients starting ART by droplet digital PCR: their dynamic decay and correlations with immunological parameters and virological success. J Clin Virol. 2019;117:61‐67. [DOI] [PubMed] [Google Scholar]
- 120. Gupta RK, Peppa D, Hill AL, et al. Evidence for HIV‐1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem‐cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. 2020;7:e340‐e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rothenberger M, Wagner JE, Haase A, et al. Transplantation of CCR5∆32 homozygous umbilical cord blood in a child with acute lymphoblastic leukemia and perinatally acquired HIV infection. Open Forum Infect Dis. 2018;5(5):ofy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lee SA, Telwatte S, Hatano H, et al. Antiretroviral therapy concentrations differ in Gut vs. lymph node tissues and are associated with HIV Viral transcription by a novel RT‐ddPCR assay. J Acquir Immune Defic Syndr. 2020;83(5):530‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Telwatte S, Morón‐López S, Aran D, et al. Heterogeneity in HIV and cellular transcription profiles in cell line models of latent and productive infection: Implications for HIV latency. Retrovirology. 2019;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Myerski A, Siegel A, Engstrom J, McGowan I, Brand RM. The use of droplet digital PCR to quantify HIV‐1 replication in the colorectal explant model. AIDS Res Hum Retroviruses. 2019;35:326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Andersen AHF, Nielsen SSF, Olesen R, et al. Humanized nog mice for intravaginal hiv exposure and treatment of hiv infection [Internet]. J Visualized Exp. 2019;2020. http://www.ncbi.nlm.nih.gov/pubmed/32065160 [DOI] [PubMed] [Google Scholar]
- 126. van den Berg FT, Makoah NA, Ali SA, et al. AAV‐mediated expression of broadly neutralizing and vaccine‐like antibodies targeting the HIV‐1 envelope V2 region. Mol Ther—Methods Clin Dev. 2019;14:100‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Anderson EM, Simonetti FR, Gorelick RJ, et al. Dynamic shifts in the HIV proviral landscape during long term combination antiretroviral therapy: implications for persistence and control of HIV infections. Viruses. 2020;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bejarano DA, Puertas MC, Börner K, Martinez‐Picado J, Müller B, Kräusslich HG. Detailed characterization of early HIV‐1 replication dynamics in primary human macrophages. Viruses. 2018;10:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kiselinova M, Pasternak AO, De Spiegelaere W, Vogelaers D, Berkhout B, Vandekerckhove L. Comparison of droplet digital PCR and seminested real‐time PCR for quantification of cell‐associated HIV‐1 RNA. PLoS One. 2014;9:e85999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bosman KJ, Nijhuis M, Van Ham PM, et al. Comparison of digital PCR platforms and semi‐nested qPCR as a tool to determine the size of the HIV reservoir. Sci Rep. 2015;5:13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pasternak AO, Berkhout B. What do we measure when we measure cell‐associated HIV RNA. Retrovirology. 2018;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bertoldi A, D′Urbano V, Bon I, et al. Development of C‐TILDA: a modified TILDA method for reservoir quantification in long term treated patients infected with subtype C HIV‐1. J Virol Methods. 2020;276:113778. [DOI] [PubMed] [Google Scholar]
- 133. Yukl SA, Kaiser P, Kim P, et al. HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med. 2018;10:eaap9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hodel F, Patxot M, Snäkä T, Ciuffi A. HIV‐1 latent reservoir: size matters. Future Virol. 2016;11:785‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yang D, Hu T, Wu X, Li K, Zhong Q, Liu W. Droplet‐digital polymerase chain reaction for detection of clinical hepatitis B virus DNA samples. J Med Virol. 2018;90(12):1868‐1874. [DOI] [PubMed] [Google Scholar]
- 136. Peluso MJ, Bacchetti P, Ritter KD, et al. Differential decay of intact and defective proviral DNA in HIV‐1–infected individuals on suppressive antiretroviral therapy. JCI Insight. 2020;5(4):e132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV‐1 proviruses. Nature. 2019;566:120‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Frías M, Rivero‐Juárez A, Téllez F, et al. Evaluation of hepatitis C viral RNA persistence in HIV‐infected patients with long‐term sustained virological response by droplet digital PCR. Sci Rep. 2019;9:12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dong L, Meng Y, Wang J, Liu Y. Evaluation of droplet digital PCR for characterizing plasmid reference material used for quantifying ammonia oxidizers and denitrifiers. Anal Bioanal Chem. 2014;406:1701‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Persson S, Karlsson M, Borsch‐Reniers H, Ellström P, Eriksson R, Simonsson M. Missing the match might not cost you the game: primer‐template mismatches studied in different hepatitis A virus variants. Food Environ Virol. 2019;11:297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Whale AS, Cowen S, Foy CA, Huggett JF. Methods for applying accurate digital PCR analysis on low copy DNA samples. PLoS One. 2013;8:e58177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nolan DJ, Rose R, Rodriguez PH, et al. The spleen is an HIV‐1 sanctuary during combined antiretroviral therapy. AIDS Res Hum Retroviruses. 2018;34:123‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rowlands V, Rutkowski AJ, Meuser E, Carr TH, Harrington EA, Barrett JC. Optimisation of robust singleplex and multiplex droplet digital PCR assays for high confidence mutation detection in circulating tumour DNA. Sci Rep. 2019;9:12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 145. Liu W, Xie Y, Gao T, et al. Reflection and observation: cell‐based screening failing to detect HBV in HUMSCs derived from HBV‐infected mothers underscores the importance of more stringent donor eligibility to reduce risk of transmission of infectious diseases for stem cell‐based med. Stem Cell Res Ther. 2018;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397‐408. [DOI] [PubMed] [Google Scholar]
- 147. Dong L, Zhou J, Niu C, et al. Highly accurate and sensitive diagnostic detection of SARS‐CoV‐2 by digital PCR. medRxiv. 2020;224:121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Caviglia GP, Abate ML, Tandoi F, et al. Quantitation of HBV cccDNA in anti‐HBc‐positive liver donors by droplet digital PCR: a new tool to detect occult infection. J Hepatol. 2018;69:301‐307. [DOI] [PubMed] [Google Scholar]
- 149. Huang JT, Liu YJ, Wang J, et al. Next generation digital PCR measurement of hepatitis B virus copy number in formalin‐fixed paraffin‐embedded hepatocellular carcinoma tissue. Clin Chem. 2015;61:290‐296. [DOI] [PubMed] [Google Scholar]
- 150. Huang JT, Yang Y, Hu YM, et al. A highly sensitive and robust method for hepatitis B virus covalently closed circular DNA detection in single cells and serum. J Mol Diagn. 2018;20(3):334‐343. [DOI] [PubMed] [Google Scholar]
- 151. Tang H, Cai Q, Li H, Hu P. Comparison of droplet digital PCR to real‐time PCR for quantification of hepatitis B virus DNA. Biosci Biotechnol Biochem. 2016;80:2159‐2164. [DOI] [PubMed] [Google Scholar]
- 152. Nicot F, Cazabat M, Lhomme S, et al. Quantification of HEV RNA by droplet digital PCR. Viruses. 2016;8:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Inchauspé A, Locatelli M, Lebosse F, et al. Droplet digital PCR quantitation of HBV cccDNA pool and transcriptional activity in long‐term nucleos(t)ide analogue treated patients. J Hepatol. 2018;68(1):S481. [Google Scholar]
- 154. Limothai U, Chuaypen N, Poovorawan K, et al. Reverse transcriptase droplet digital PCR vs reverse transcriptase quantitative real‐time PCR for serum HBV RNA quantification. J Med Virol. 2020;92:3365‐3372. [DOI] [PubMed] [Google Scholar]
- 155. White MC, Tao H, Steel J, Lowen A CH5N8 and H7N9 packaging signals constrain HA reassortment with a seasonal H3N2 influenza A virus. Proc Natl Acad Sci U S A. 2019;116:4611‐4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schwartz SL, Lowen AC. Droplet digital PCR: a novel method for detection of influenza virus defective interfering particles. J Virol Methods. 2016;237:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yan Y, Jia X, Wang H, et al. Dynamic quantification of avian influenza H7N9(A) virus in a human infection during clinical treatment using droplet digital PCR. J Virol Methods. 2016;234:22‐27. [DOI] [PubMed] [Google Scholar]
- 158. Leong NKC, Chu DKW, Chu JTS, et al. A six‐plex droplet digital RT‐PCR assay for seasonal influenza virus typing, subtyping, and lineage determination. Influenza Other Respi Viruses. 2020;14:720‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lu R, Wang J, Li M, Wang Y, Dong J, Cai W. SARS‐CoV‐2 detection using digital PCR for COVID‐19 diagnosis, treatment monitoring and criteria for discharge. medRxiv. 2020. [Google Scholar]
- 160. Woloshin Steven, Patel Neeraj, Kesselheim Aaron S. False Negative Tests for SARS‐CoV‐2 Infection — Challenges and Implications. New England Journal of Medicine. 2020;383(6):e38. 10.1056/nejmp2015897 [DOI] [PubMed] [Google Scholar]
- 161. Provenzano M, Allayeh AK. Liquid biopsy to detect DNA/RNA based markers of small DNA oncogenic viruses for prostate cancer diagnosis, prognosis, and prediction. Front Oncol. 2020;10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Schütz E, Fischer A, Beck J, et al. Graft‐derived cell‐free DNA, a non‐invasive early rejection and graft damage marker in liver transplantation: a prospective, observational, multicenter cohort study. PLoS Med. 2017;14:e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schwarzenbach H, Hoon DSB, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426‐437. [DOI] [PubMed] [Google Scholar]
- 164. Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jeannot E, Becette V, Campitelli M, et al. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus‐associated invasive carcinoma. J Pathol Clin Res. 2016;2:201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cabel L, Bidard FC, Servois V, et al. HPV circulating tumor DNA to monitor the efficacy of anti‐PD‐1 therapy in metastatic squamous cell carcinoma of the anal canal: a case report. Int J Cancer. 2017;141:1667‐1670. [DOI] [PubMed] [Google Scholar]
- 167. Zhang S, Su X, Wang J, et al. Nucleic acid testing for coronavirus disease 2019: demand, research progression, and perspective. Crit Rev Anal Chem. 2020;49:1‐12. [DOI] [PubMed] [Google Scholar]
- 168. Suo T, Liu X, Feng J, et al. ddPCR: a more sensitive and accurate tool for SARS‐CoV‐2 detection in low viral load specimens. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wyler E, Franke V, Menegatti J, et al. Single‐cell RNA‐sequencing of herpes simplex virus 1‐infected cells connects NRF2 activation to an antiviral program. Nat Commun. 2019;10:4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cristinelli S, Ciuffi A. The use of single‐cell RNA‐Seq to understand virus–host interactions. Current Opinion in Virology. 2018;29:39‐50. [DOI] [PubMed] [Google Scholar]
- 171. Li CL, Ho MC, Lin YY, et al. Cell‐free virus‐host chimera DNA from Hepatitis B virus integration sites as a circulating biomarker of hepatocellular cancer. Hepatology. 2020;72:2063‐2076. [DOI] [PubMed] [Google Scholar]
- 172. Jiang Y, Manz A, Wu W. Fully automatic integrated continuous‐flow digital PCR device for absolute DNA quantification. Anal Chim Acta [Internet]. 2020;1125:50‐56. https://linkinghub.elsevier.com/retrieve/pii/S0003267020305936 [DOI] [PubMed] [Google Scholar]
- 173. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients. Clin Infect Dis. 2020;71:793‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tao Y, Rotem A, Zhang H, et al. Rapid, targeted and culture‐free viral infectivity assay in drop‐based microfluidics. Lab Chip. 2015;15:3934‐3940. [DOI] [PubMed] [Google Scholar]
- 175. Falzone L, Musso N, Gattuso G, et al. Sensitivity assessment of droplet digital PCR for SARS‐CoV‐2 detection. Int J Mol Med. 2020;46:957‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Dang Y, Liu N, Tan C, et al. Comparison of qualitative and quantitative analyses of COVID‐19 clinical samples. Clin Chim Acta [Internet]. 2020;510:613‐616. https://pubmed.ncbi.nlm.nih.gov/32858058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vasudevan H, Xu P, Servellita V, et al. Digital droplet PCR accurately quantifies SARS‐CoV‐2 viral load from crude lysate without nucleic acid purification. medRxiv [Internet]. 2020. Available from http://medrxiv.org/content/early/2020/09/03/2020.09.02.20186023.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Katsuya H, Islam S, Tan BJY, et al. The nature of the HTLV‐1 provirus in naturally infected individuals analyzed by the viral DNA‐capture‐seq approach. Cell Rep. 2019;29:724‐735. [DOI] [PubMed] [Google Scholar]
- 179. Sedlak RH, Cook L, Huang ML, et al. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin Chem. 2014;60:765‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mattiuzzo G, Ashall J, Doris KS, et al. Development of lentivirus‐based reference materials for Ebola virus nucleic acid amplification technology‐based assays. PLoS One. 2015;10:e0142751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Stevenson A, Wakeham K, Pan J, et al. Droplet digital PCR quantification suggests that higher viral load correlates with improved survival in HPV‐positive oropharyngeal tumours. J Clin Virol. 2020;129:104505. [DOI] [PubMed] [Google Scholar]
- 182. Stebbing J, Bower M, Cell‐free DNA. as a biomarker in the context of cancer, viruses, and methylation. J Infect Dis. 2012;205:1032‐1034. [DOI] [PubMed] [Google Scholar]
- 183. Cheng AP, Burnham P, Lee JR, et al. A cell‐free DNA metagenomic sequencing assay that integrates the host injury response to infection. Proc Natl Acad Sci USA. 2019;116:18738‐18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bernard‐Tessier A, Jeannot E, Guenat D, et al. Clinical validity of HPV circulating tumor DNA in advanced anal carcinoma: an ancillary study to the EPITOPES‐HPV02 trial. Clin Cancer Res. 2019;25:2109‐2115. [DOI] [PubMed] [Google Scholar]
- 185. Wang S, Li H, Kou Z, et al. Highly sensitive and specific detection of HBV DNA and drug resistance mutations utilizing the PCR‐based CRISPR‐Cas13a system. Clin Microbiol Infect. 2020. [DOI] [PubMed] [Google Scholar]
- 186. Jacobs NT, Onuoha NO, Antia A, Steel J, Antia R, Lowen AC. Incomplete influenza A virus genomes occur frequently but are readily complemented during localized viral spread. Nat Commun. 2019;10:3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen G, Ning B, Shi T. Single‐cell RNA‐seq technologies and related computational data analysis. Front Genet. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]


