Abstract
Background
COVID‐19 safety measures and possibly SARS‐CoV‐2 antibody testing may alter blood donor demography, which has the potential to alter blood safety. We characterized pre‐pandemic and pandemic rates of donor infectious disease marker (IDM) reactivity which reflect the residual risk of transfusion‐transmitted infections (TTIs) undetectable by current testing.
Methods
This cross‐sectional analysis of allogeneic blood donor presentations and successful donations in a large national US blood collector identifies changes in self‐reported behavioral risk factors and IDM reactivity. Data on allogeneic blood donor presentations and successful donations from March 1 through August 31, 2020 and the same period in 2019 were retrieved from the blood center's computer system. Donor demographics and deferrals for reported behavioral risk factors and confirmed‐positive IDMs were compared in pre‐pandemic and pandemic periods.
Results
With increasing mobile blood drive cancellations, pandemic donors were more likely than 2019 donors to be female, over age 30, non‐Hispanic Whites, and have a post‐secondary degree. First‐time donations (at highest risk for confirmed‐positive IDMs) did not substantially increase. Pandemic donors reported fewer behavioral risks and IDMs declined among these donors. Mid‐pandemic introduction of screening for SARS‐CoV‐2 antibodies did not affect IDM rates.
Conclusions
Unlike disasters, which tend to bring out more first‐time donors with increased IDM reactivity and TTI residual risk, COVID‐19 donors had lower IDM rates which were not affected by SARS‐CoV‐2 antibody testing. Already‐low TTI residual risk is likely to have declined as a result.
Keywords: blood donors, blood safety, coronavirus, COVID‐19
1. INTRODUCTION
Measures to curb the spread of Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) have altered many aspects of everyday life, including blood donation. 1 , 2 , 3
Vitalant, the second largest blood provider in the United States, collects just under 1.4 million red blood cells and 340,000 platelet units annually in 26 states. Restrictions on public gatherings and stay‐at‐home orders resulted in the unexpected cancellation of ~8550 mobile blood drives from March through August 2020, amounting to a shortfall of almost 215,800 red blood cell collections. Of these, 39% had been scheduled at high schools or colleges. (By way of comparison, high school‐ and college‐age donors provided ~13% of the red cell supply, March through August in 2019.) Efforts to replace lost collections involved rescheduling of mobile blood drives and increased emphasis on fixed site collections where robust donor safety measures were in place. Supply–demand imbalances, as hospitals first paused, then resumed elective surgical procedures prompted public health announcements by FDA and the US Surgeon General encouraging blood donation during public gathering restrictions.
On March 27, 2020, the US Food and Drug Administration (FDA) announced an emergency pathway to collect Coronavirus Disease 2019 (COVID‐19) Convalescent Plasma (CCP) from recovered donors for transfusion to hospitalized patients. It took more than a month to set up a program and begin to collect to need. On June 1, 2020, Vitalant began testing all donors for antibodies to SARS‐CoV‐2 to further enlarge the small volunteer CCP donor pool.
The donor demographic impact of donation site changes, blood shortage messaging, and donor testing for COVID‐19 exposure could alter blood product residual risk for transfusion‐transmissible infections (TTIs). Screening infectious disease marker (IDM) reactivity rates are used to estimate the likelihood of infectious disease window‐period donations, during which blood‐borne agents are not identifiable by testing. 4 First‐time donors (FTDs) are known to have a 2.5‐ to 3.5‐fold greater residual risk for TTIs than repeat donors. 5 Thus, changes in the balance of first‐time versus repeat donors or an increase in higher‐risk demographic presentations could affect overall blood safety. We sought to characterize pre‐pandemic and pandemic rates of infectious disease marker reactivity and correlate them with observed donor demographic changes.
2. METHODS
Blood donors routinely give IRB‐approved consent for the research use of anonymized information. Data were retrieved for all allogeneic donor presentations and successful donations from March 1 through August 31, 2020 and the same six‐month, pre‐COVID period in 2019 from Vitalant centers using the eProgesa (MAK System, Paris, France) blood establishment computer system. Two separate 2019 and 2020 sub‐periods were considered: March–May (in 2020, prior to SARS‐CoV‐2 antibody testing) and June–August (in 2020, after implementation of antibody testing). Donation demographics included donor age, sex, race/ethnicity, highest education level, and experience (first‐time, active [prior presentation within 2 years], or reengaged [prior presentation >2 years ago]), as well as collection site type (fixed site or mobile drive). Encoded donor identifiers were used to derive donor‐level data. Within each of the four discrete time periods, test results from individual donors' first presentation were used to characterize experience and report IDM rates by donor.
Reported rates (per 10,000) include only confirmed‐reactive results using FDA‐approved supplemental tests after a reactive screening test for Human Immunodeficiency Virus (HIV), Human T‐cell Lymphotrophic Virus (HTLV), Hepatitis B surface Antigen or Nucleic Acid Testing (HBsAg/NAT), syphilis, and Hepatitis C Virus (HCV). Because Hepatitis B core Antibody (HBcAb) reactivity in the absence of HBsAg or NAT positivity cannot be confirmed with supplemental testing (>99% represent resolved hepatitis B or false‐positive test results), this result was reported separately. Reactive screening test results trigger a 12‐month deferral for syphilis and indefinite deferral for all the other IDMs.
Rates at which presenting donors reported immediately deferrable behavioral risk factor(s) for HIV and other infections at health history were calculated using previously described criteria, 6 in addition to incarceration, HIV prophylaxis use, hepatitis exposure, transplant recipients and individuals tattooed in unregulated establishments. Vitalant had a minimum 12‐month deferral period in place for reported high‐risk behaviors throughout all but the last 2 weeks of the study. As allowed by FDA, some high‐risk 12‐month deferrals were shortened to 3 months on August 16, 2020.
Chi‐square testing was used to compare IDM rates and reported behavioral risk factors between corresponding periods in 2019 and 2020 and between the two periods in 2020. A p value <.05 was considered significant.
3. RESULTS
Pandemic donors were more likely than 2019 donors to be female, over age 30, non‐Hispanic Whites, and have a post‐secondary degree (Table 1). The 2019 fixed site‐to‐mobile ratio of 46% to 54% was reversed in 2020 with 65% fixed site/35% mobile drive collections. First‐time donations during the 6‐month periods rose slightly from 15.2% in 2019 to 15.9% in 2020, while reengaged donors increased 50% from 8.8% in 2019 to 13.3% in 2020. Apheresis donors are more likely to be repeat donors with lower IDM rates, but collections did not appear to change substantively during the pandemic. From March through August 2019, 13.3% of donations were from apheresis (1.91 donations per donor). In the same months of 2020, apheresis collections were 13.5% of donations (1.90 donations per donor). In these same 2019 and 2020 periods, whole blood and double red cell donors gave 1.07 and 1.09 times, contributing 86.7% and 86.5% of collections, respectively.
TABLE 1.
Donor demography, March–August 2019 and 2020
| Mar‐May 2019 | Jun‐Aug 2019 | Mar‐May 2020 | Jun‐Aug 2020 | ||
|---|---|---|---|---|---|
| Successful Donations | 237,115 | 232,786 | 203,269 | 258,967 | |
| Demographic factors | % | % | % | % | |
| Sex | Female | 46.4 | 47.0 | 49.8 | 50.9 |
| Male | 53.6 | 53.0 | 50.2 | 49.1 | |
| Age | 16–18 | 11.4 | 2.9 | 3.8 | 1.6 |
| 19–22 | 5.7 | 4.5 | 3.4 | 2.9 | |
| 23–29 | 8.8 | 10.2 | 7.9 | 7.8 | |
| 30–64 | 57.7 | 64.7 | 65.1 | 68.8 | |
| ≥65 | 16.3 | 17.8 | 19.8 | 18.9 | |
| Race/Ethnicity | Asian/Pacific Islander, NH | 2.8 | 2.7 | 2.0 | 2.2 |
| Black, NH | 2.9 | 2.5 | 1.5 | 1.4 | |
| Hispanic | 16.8 | 16.0 | 11.0 | 11.5 | |
| Native American/Alaskan, NH | 0.9 | 0.8 | 0.6 | 0.6 | |
| Other/Mixed, NH | 0.8 | 0.8 | 0.7 | 0.8 | |
| White, NH | 73.7 | 74.8 | 81.0 | 80.2 | |
| Unknown | 2.2 | 2.5 | 3.2 | 3.3 | |
| Type | Active | 75.8 | 76.2 | 68.4 | 71.9 |
| First‐Time | 16.5 | 13.8 | 17.0 | 15.5 | |
| Reengaged | 7.7 | 10.0 | 14.7 | 12.6 | |
| Education | Some or No HS | 13.7 | 6.0 | 6.0 | 3.8 |
| HS Graduate | 11.9 | 13.2 | 10.1 | 10.2 | |
| Some College/Tech School | 28.3 | 30.5 | 26.1 | 26.9 | |
| Assoc. or Bachelor's Degree | 25.5 | 28.1 | 29.4 | 30.6 | |
| Graduate Degree | 12.0 | 12.7 | 15.3 | 15.5 | |
| Unknown | 8.7 | 9.6 | 13.1 | 13.1 | |
| Site | Fixed | 44.1 | 47.5 | 67.3 | 62.9 |
| Mobile | 55.9 | 52.5 | 32.7 | 37.1 | |
Normally, confirmed IDM reactivity rates rise in the summer when schools with lower‐risk donors are out of session (Figure 1). The 2019 pattern was not evident in 2020, since many schools had canceled blood drives at the onset of the pandemic. A significant decrease in confirmed‐reactive IDM results was evident in 2020 compared with both periods in 2019, unchanged before and after the June 1 initiation of SARS‐CoV‐2 antibody testing. HBcAb reactivity declined in 2020 as well, with only the Jun‐Aug 2020 period reaching statistical significance.
FIGURE 1.
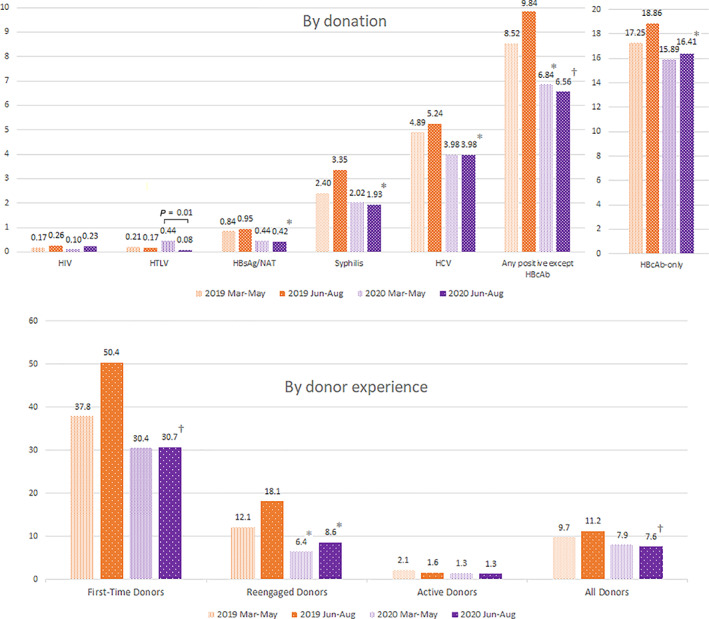
IDM confirmed reactivity rates by donation and by donor (per 10,000), March–August 2019 and 2020 (*p < .05 compared with same period 2019; †p < .0001 compared with same period 2019; there were no significant differences between Mar‐May 2020 and Jun‐Aug 2020 for any group except by‐donation HTLV testing) [Color figure can be viewed at wileyonlinelibrary.com]
All donors' confirmed IDM reactivity declined in Jun‐Aug 2020 compared with Jun‐Aug 2019 (Figure 1). There was a significant decline in IDMs in FTDs between Jun‐Aug 2019 and Jun‐Aug 2020. Reengaged donor rates declined in both 2020 periods compared to similar periods in 2019. No significant change was seen between rates in the two 2020 periods for any donor group. There was a drop in reported high‐risk behaviors in 2020 donors compared with the same periods in 2019; in 2020, a significant decrease was also observed between the two 2020 periods (Figure 2). The significant decrease in Jun‐Aug 2020 compared with Mar‐May 2020 was minimally affected by a relaxation of deferral criteria in the last 16 days (17%) of the Jun‐Aug period whereby behaviors triggering deferral were only considered in the preceding 3 instead of 12 months [or ever for injectable drug use]). The 31% relative decrease in reported behaviors exceeds that expected with even complete cessation of 12‐month high‐risk deferrals in those 16 days in Jun‐Aug.
FIGURE 2.
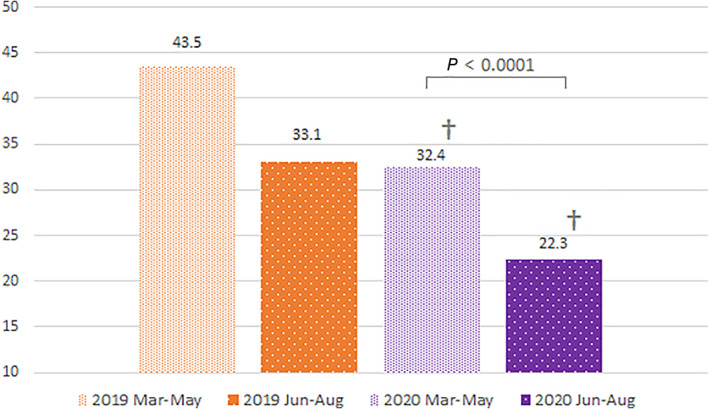
High risk deferral rate per 10,000 presentations, March–August 2019 and 2020 (†p < .0001 compared with same period 2019; a significant difference was observed between Mar‐May 2020 and Jun‐Aug 2020) [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Our data show that the shift from convenient community drives to a limited number of fixed sites, accompanied by intensified recruitment messaging resulted in noticeable donor demographic changes with a corresponding decline in confirmed‐reactive and HBcAb IDM rates. Donor deferrals for reported high‐risk behavior fell significantly from 38.35 per 10,000 presentations in Mar‐Aug 2019 to 26.79 per 10,000 in the corresponding months of the pandemic in 2020. The confirmed‐reactive IDM rate dropped in parallel from 9.17 per 10,000 in Mar‐Aug 2019 to 6.68 per 10,000 in the same months of 2020.
Unlike the reported 5.2‐fold increase in first‐time donations (to 46%) seen after the September 11, 2001 tragedy in the U.S., 7 the fraction of FTDs did not dramatically increase during the pandemic. Akin to the September 11th response though, we observed a relative increase in female donations. Relative increases in various groups by age, race/ethnicity, and educational level were not reported in that study, with all subgroups donating 1.8 to 3.0 times more in the week after September 11th as in the preceding four. 7 Confirmed HIV, HCV, and HBsAg reactivity increased 2‐ to 3‐fold in the weeks after the tragedy, driven primarily by the overall increase in FTDs, whose IDM reactivity rates were individually unchanged before and after September 11th. Messaging around the critical need for blood was directed to and resonated with donors who had not presented within the last 2 years, as these previously committed individuals presented in higher numbers during the pandemic.
Pandemic donations are clearly subject to different phenomena, driven by changes in blood drive accessibility and individual perception of the risk of SARS‐CoV‐2 infection in public places. There appeared to be no adverse effect of SARS‐CoV‐2 antibody testing on presenting donor risk behaviors or consequent IDM reactivity. The generalizability of these results from 14 of 17 states in the western half of the U.S. is limited to this geography. Further characterization of the “pandemic donor” will be helpful for the future, especially as these donors appear to be different from “disaster donors.” Already‐low residual risks for TTIs have presumably decreased during the pandemic, and there was no discernable effect of offering donor SARS‐CoV‐2 antibody testing on donor IDM reactivity.
CONFLICT OF INTEREST
We declare no competing interests.
Vassallo RR, Bravo MD, Kamel H. Pandemic blood donor demographics – Do changes impact blood safety? Transfusion. 2021;61:1389–1393. 10.1111/trf.16320
REFERENCES
- 1. Courtemanche C, Garuccio J, Le A, Pinkston J, Yelowitz A. Strong social distancing measures in the United States reduced the COVID‐19 growth rate. Health Aff. 2020;39(7):1237–46. [DOI] [PubMed] [Google Scholar]
- 2. Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, van der Westhuizen HM, et al. Face masks against COVID‐19: An evidence review. Preprints. 2021;118:e2014564118. 10.20944/preprints202004.0203.v4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juneau CE, Pueyo T, Bell M, Gee G, Collazzo P, Potvin L. Evidence‐based, cost‐effective interventions to suppress the COVID‐19 pandemic: A systematic review. MedRxiv. 2020. 10.1101/2020.04.20.20054726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, et al. A new strategy for estimating risks of transfusion‐transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45(2):254–64. [DOI] [PubMed] [Google Scholar]
- 5. Zou S, Stramer SL, Dodd RY. Donor testing and risk: Current prevalence, incidence, and residual risk of transfusion‐transmissible agents in US allogeneic donations. Transfus Med Rev. 2012;26(2):119–28. [DOI] [PubMed] [Google Scholar]
- 6. Williams AE, Thomson RA, Schreiber GB, Watanabe K, Bethel J, Lo A, et al. Estimates of infectious disease risk factors in US blood donors. Retrovirus epidemiology donor study. JAMA. 1997;277(12):967–72. [PubMed] [Google Scholar]
- 7. Glynn SA, Busch MP, Schreiber GB, Murphy EL, Wright DJ, Tu Y, et al. Effect of a national disaster on blood supply and safety – The september 11 experience. JAMA. 2003;289(17):2246–53. [DOI] [PubMed] [Google Scholar]


