Abstract
Background
Coronavirus disease 2019 (COVID‐19) convalescent individuals carry antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that, through a plasma donation, can be used as a potential therapeutic either in direct transfusion or for the manufacture of hyperimmune globulin (HIG). The success of such interventions depends on the antibody potency in such plasma donations, but little information on the collection of potent units is currently available.
Study Design and Methods
A total of 8749 plasma units, collected from April until September 2020 from first‐time U.S. COVID‐19 convalescent plasma donors, were characterized for SARS‐CoV‐2 immunoglobulin G (IgG) antibodies by Abbott chemiluminescent microparticle immunoassay (CMIA). The period between COVID‐19 onset until donation and donor age, ethnicity, sex, and COVID‐19 severity were evaluated against the obtained signal (index S/C).
Results
A marked decrease in mean index S/C was seen over the plasma collection period surveyed, which was significantly correlated to decreases in mean plasma donor age (p < .0001; R2 = .726) and percentage of donations obtained from COVID‐19 convalescent patients who had been hospitalized (p = .001; R2 = .4426). The highest titer plasma units were obtained soon after convalescence from COVID‐19 patients who required hospitalization, from advanced age donors, and from Black/African/Hispanic American versus White/Caucasian ethnicities, whereas there was no effect of donor sex on the values obtained with the Abbott CMIA.
Conclusion
Since the onset of the pandemic, the average SARS‐CoV‐2 IgG values of first‐time U.S. COVID‐19 convalescent plasma donations have significantly dropped, mainly due to donations from progressively younger aged donors who tend to experience less severe COVID‐19.
Keywords: Abbott CMIA, convalescent donor selection, CoVIg‐19, COVID‐19, hyperimmune globulin, index S/C, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The ongoing Coronavirus disease 2019 (COVID‐19) pandemic has sparked an unprecedented global effort to identify and develop effective medications for the treatment and prevention of the disease. One of the first therapies that became available was COVID‐19 convalescent plasma (CP), donated by persons who recovered from infection by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus causing the disease. These donations contain antibodies specific for SARS‐CoV‐2, which are known to exert an antiviral effect. CP has previously been shown to be effective in the treatment of viral infections that cause severe pneumonia, for example, against the influenza pandemic of 1918 or SARS. 1 , 2 There is some evidence that patients hospitalized with COVID‐19 benefit from CP transfusion, especially early in disease, 3 , 4 , 5 , 6 which was sufficient to justify Food and Drug Administration (FDA) Emergency Use Authorization for COVID‐19 CP. 7 Efficacy of treatment with CP is affected by variations in the quality of plasma donations, 3 a factor that can be eliminated through the manufacture of hyperimmune globulin (HIG) from large pools of these CP units, thereby equalizing antibody titer variability. Another important benefit of treatment with an HIG preparation based on an established immunoglobulin manufacturing process is the viral safety afforded by the manufacturing pathway of such a plasma‐derived medicinal product. The Gammagard Liquid pathway, for example, entails three dedicated virus clearance steps, which ensure substantial safety margins. 8 Soon after the outbreak of SARS‐CoV‐2, major plasma fractionators united to jointly engage in the production of a COVID‐19 HIG, termed CoVIg‐19, 9 enriched with SARS‐CoV‐2 antibodies as a treatment option for patients with COVID‐19, which is currently in clinical trials. 10 Both treatment modalities, CP transfusion and HIG, rely on the collection of high antibody titer CP units. Little is currently understood with respect to high antibody potency in CP and different donor characteristics, information which may allow for a more targeted collection of potent units.
Here, we report the characterization of 8749 CP units that were collected in the United States from first‐time COVID‐19 convalescent donors for SARS‐CoV‐2 IgG antibody content and a correlation with donor characteristics in an effort to identify factors that directly influence potency.
2. MATERIALS AND METHODS
2.1. COVID‐19 plasma donations
BioLife US started COVID‐19 CP collection in March 2020. For eligibility, donors had to have a status of 14 days postrecovery and provide a positive test result (either Polymerase Chain Reaction [PCR] or serological analysis) for COVID‐19 to be accepted for the program. A total of 8749 COVID‐19 donations, collected from the end of April until mid‐September, that is, between week 18 and week 38, 2020 (21 weeks), were included in the analysis. These donations originated from first‐time COVID‐19 donors, that is, persons who gave plasma for the first time after convalescence from COVID‐19 but who might have already donated plasma prior to disease. The plasma volumes collected in the COVID‐19 program were similar to volumes collected during non‐COVID‐19 plasmapheresis. Patient demographic characteristics (sex, age, ethnicity) and information regarding onset and severity of COVID‐19 (hospitalization vs. nonhospitalization) were recorded.
2.2. SARS‐COV‐2 antibody testing
The Abbott SARS‐CoV‐2 IgG chemiluminescent microparticle immunoassay (CMIA; Abbott Laboratories, Abbott Park, IL, USA; reference 06R8620), granted FDA Emergency Use Authorization, was used for the detection of SARS‐CoV‐2 IgG antibodies in CP. This assay qualitatively detects IgG antibodies against the SARS‐CoV‐2 nucleocapsid protein, with an assay specificity of 99.6%–99.9% and sensitivity of 100%. 11 , 12 The dose: response reactivity was evaluated in plasma serial dilution experiments. Highly significant (p < .0001) correlation was seen (R2 = .998) for the obtained index S/C values (9.10–1.73) against the log2‐transformed dilution series (Figure S1), indicating that some quantitation is possible with the Abbott CMIA.
Testing was done in laboratories certified under the Clinical Laboratory Improvement Amendments on ARCHITECT i2000SR equipment (Abbott, IL, USA) with a sample volume of 75 μL and following the manufacturers' instructions. The amount of IgG antibodies to SARS‐CoV‐2 in each sample was determined automatically through comparison of the measured chemiluminescent relative light unit (RLU) to the calibrator RLU, which is reported as index S/C. The positivity cutoff defined by the manufacturer is 1.40 (S/C). 12
2.3. Data analysis
A data plausibility check was performed, and donations for which a time period of more than 30 days between onset of symptoms and positive PCR test was stated were excluded from analysis. Due to underrepresentation of the greater than 65 years age group, data from this cohort were included in the descriptive analysis but excluded from inferential statistical analyses. Multiple regression analysis was employed to evaluate the effects of age, donation delay (i.e., time from symptom onset to plasma collection), sex, and disease severity on the index S/C. To avoid undue leverage from unusual observations, the dataset was further filtered for the central 90% for donation delay (20–95 days; i.e. data from extreme conditions were excluded). Confounding effects between the two strongest contributors, age and donation delay, were reduced by randomly sampling balanced subsets (n = 398) from nine subgroups generated by dividing both factors into three bins each. For the purpose of ethnicity analyses, a similar sampling strategy was applied, ensuring that, in each of the ethnic groups with sufficiently meaningful size, the same age structure was maintained. Ethnic groups below 2% were excluded from the analysis because of poor statistical reliability. The described sampling techniques were not applied for analyses regarding association with hospitalization due to poor repeatability of statistical results. All representative data were used; the effect of potential confounding phenomena is discussed in connection with the results and in Supplementary Statistical Analysis. Statistical analyses and visualization were performed in Minitab v. 17.3.1 with GraphPad Prism v. 8.1.1.
3. RESULTS
3.1. COVID‐19 plasma donations
Of the 8749 first‐time COVID‐19 CP donors, 55.8% were female, and most (98.0%) did not require hospitalization. Donor ages ranged from 18 to 83 years, with more than 40% of donors younger than the 28 years of age, which represents a somewhat younger plasma donor population compared to non‐COVID‐19 plasma donors (data not shown). Information on ethnicity was available for 53.6% of donors and included White/Caucasian (38.2%), Hispanic (9.8%), and Black/African (2.5%) Americans, as well as American/Alaskan Indian and Asian/Pacific Islander (1.6%) and donors who stated multiple ethnicities (1.6%).
3.2. COVID‐19 plasma collection and SARS‐COV‐2 antibodies OVER time
Analysis of the 8749 COVID‐19 plasma donations showed a pronounced decrease in mean index S/C over the 21 weeks of plasma collection, from index S/C 5.3 ± 0.3 (mean ± standard error of the mean [SEM]) at the end of April to index S/C 3.4 ± 0.1 in mid‐September (Figure 1(A)). A similar decline was seen in average donor age over this time period, from 38.4 ± 1.2 (mean ± SEM) to 32.6 ± 0.8 (Figure 1(B)) years, which significantly (p < .0001) correlated (R2 = .726) with the decrease in mean index S/C (Figure S2(A)). Likewise, the percentage of plasma donations given from convalescent individuals following hospitalization decreased over time (Figure 1(C)), an observation that significantly (p = .001) correlated (R2 = .4426) with the drop in mean index S/C (Figure S2(B)). These findings are fully in line with the changing age distribution of persons affected by COVID‐19 in the United States, where a shift in median age from 46 years in May, 2020 to 37–38 years in July–August, 2020 was reported. 13 The trend of more younger‐aged persons being affected by COVID‐19 is reflected in generally milder courses of disease as less severe COVID‐19 has been reported in younger persons, 14 , 15 which in turn results in lower antibody levels, as reported after mild disease. 16 , 17 , 18 , 19
FIGURE 1.
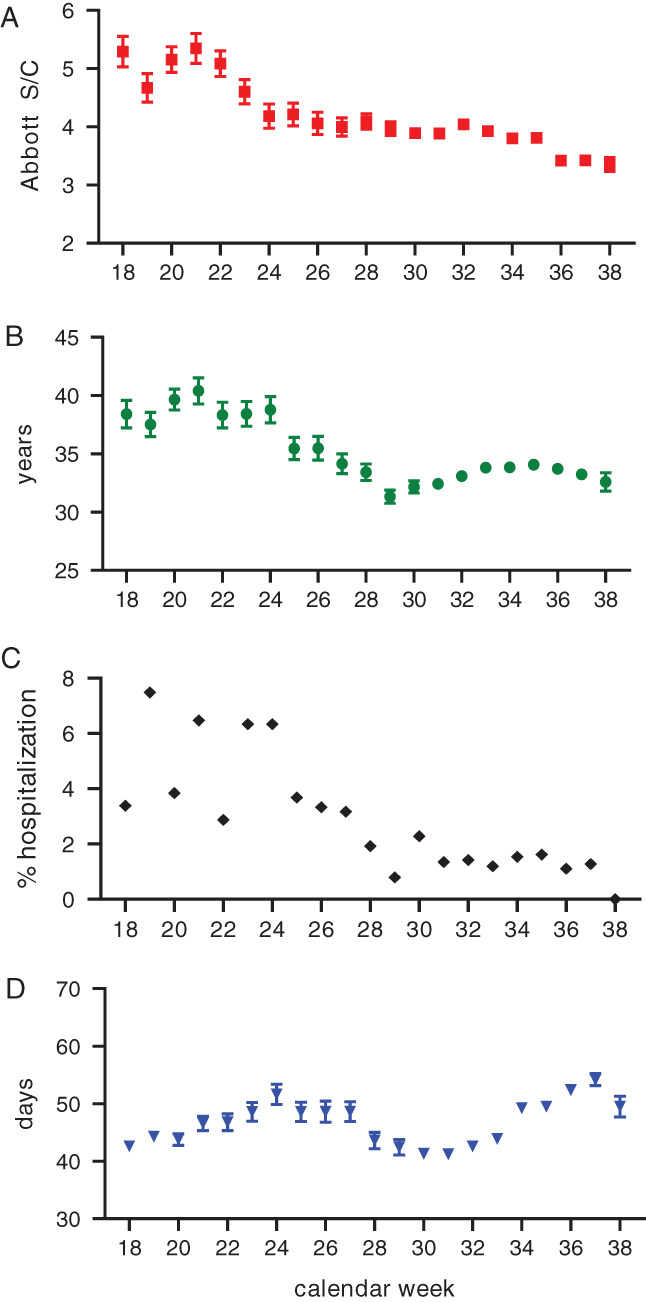
Characterization of 8749 first‐time Coronavirus disease 2019 (COVID‐19) convalescent plasma donations collected at US BioLife Centers between week 18 (end of April) and week 38 (mid‐September) 2020 for (A) severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglobulin G (IgG) values measured by Abbott chemiluminescent microparticle immunoassay (mean ± standard error of the mean [SEM] index S/C), (B) donor age (mean ± SEM years), (C) frequency (%) of donations from COVID‐19 convalescent patients who had been hospitalized, and (D) donation delay (mean days between symptom onset and donation ± SEM) [Color figure can be viewed at wileyonlinelibrary.com]
Donation delay (i.e. period between COVID‐19 onset until donation) did not change over time (Figure 1(D)) and was not significantly (p = .098) correlated to the decline in index S/C values (R2 = .1373) (Figure S2(C)).
4. EFFECT OF PLASMA DONOR DEMOGRAPHICS ON SARS‐COV‐2 ANTIBODIES
Hospitalization had a significant effect (p < .0001) on SARS‐CoV‐2 antibody content, with mean index S/C values of 5.86 versus 3.88 for donors who were hospitalized for COVID‐19 (n = 171) versus donors who were not hospitalized (n = 8578), respectively (Figure 2(A)). Here, a confounding variable was age, where significant (p = .0003) correlation (R2 = .8246) was seen for donor age and hospitalization frequency for COVID‐19, with advanced age donors more likely to have been hospitalized (Figure S3). Similar findings of higher antibody levels in older persons and in those affected by severe COVID‐19 have recently been reported from a large‐scale study of humoral response to SARS‐CoV‐2. 18
FIGURE 2.
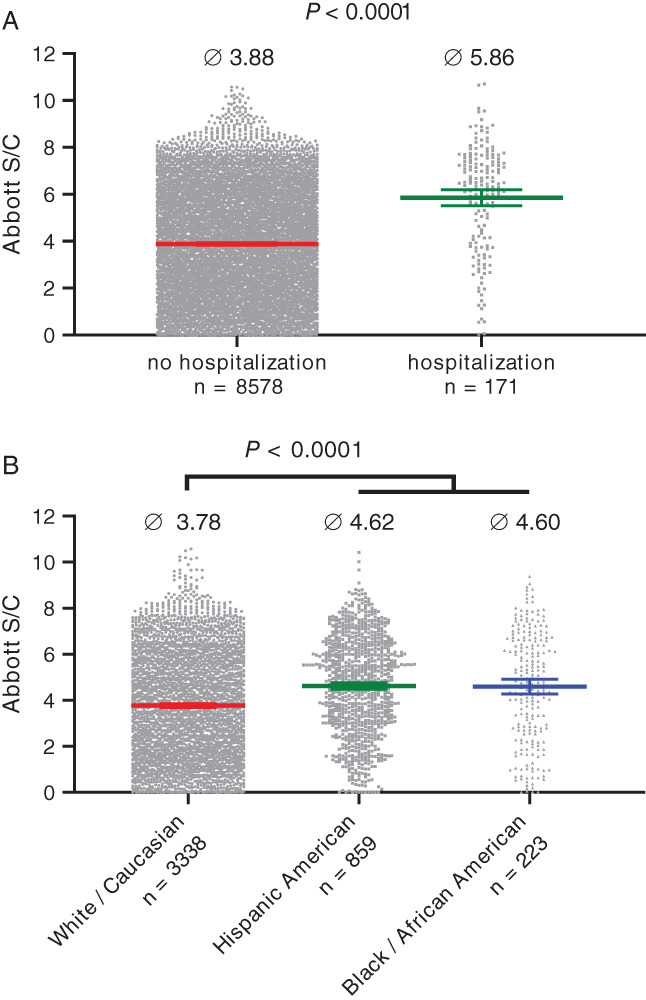
Effect of donor demographics on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglobulin G (IgG) levels in plasma, as measured by the Abbott chemiluminescent microparticle immunoassay. (A) Significantly (p < .0001; two‐sample t‐test) higher indexes S/C were determined for plasma from first‐time donors who had been hospitalized for COVID‐19 and (B) significantly higher (p < .0001; Tukey's multiple comparisons test) indexes S/C were seen for first‐time plasma donations from Hispanic or Black/African Americans versus plasma donations from White/Caucasian Americans. Bars indicate mean ± 95% confidence interval [Color figure can be viewed at wileyonlinelibrary.com]
Donor ethnicity had a significant influence on index S/C values. Donations from Hispanic Americans and Black/African Americans, which did not differ in mean index S/C values from each other (4.62, n = 859 vs. 4.60, n = 223, respectively), had significantly (p < .0001) higher mean Abbott values than White/Caucasian Americans (3.78, n = 3338) (Figure 2(B)). Further analysis of data subsets with similar age distribution across the three ethnicity groups (Pearson's Chi‐square test) revealed a marginally significant (p = .04) trend toward more frequent hospitalization for Hispanic or Black/African American plasma donors in comparison to White/Caucasian American plasma donors (Table S1), despite the underrepresentation of elderly donors in this subpopulation. These results are consistent with previous reports of more severe disease associated with higher antibody levels 16 , 17 and approximately four times higher rates of hospitalization for COVID‐19 for Hispanic and Black persons versus non‐Hispanic white persons. 20 An additional contributing factor to the observed differences in antibody levels between the different ethnicities to consider is the generally higher IgG levels in persons of Black/African background. 21 , 22 , 23
After the cohort (n = 8697) had been corrected for underrepresentation of the greater than 65 years age group, the marked increase in SARS‐CoV‐2 IgG levels that was observed with advancing donor age was similar for both sexes, except for the 50–55 years age group, where a trend for higher index S/C values was seen for plasma from male versus female donors (Figure 3(A)). Although higher fatality rates and severe COVID‐19 are consistently reported for men, 24 there was no significant difference in serum IgG levels across sex in mild COVID‐19, 25 , 26 fully consistent with the results obtained here. In a previous study, we reported significantly higher neutralization titer for CP units from male donors. 17 This discrepancy might be due to differences in the methodologies that were used for the detection of SARS‐CoV‐2 antibodies. In the case where significant sex differences were seen, the “gold‐standard” neutralization assay was used, which detects functional IgG molecules, as well as immunoglobulin A (IgA) and immunoglobulin M (IgM), with specificities against all immunogenic epitopes. A recent evaluation of SARS‐CoV‐2 antibody titers in 1215 COVID‐19 convalescent patients in Iceland evaluated six different binding assays and reported significant sex differences when assays specific for protein S1 were used but no difference in antibody levels between males and females for assays that were specific for the nucleoprotein, 18 similar to the Abbott assay used in the current study.
FIGURE 3.
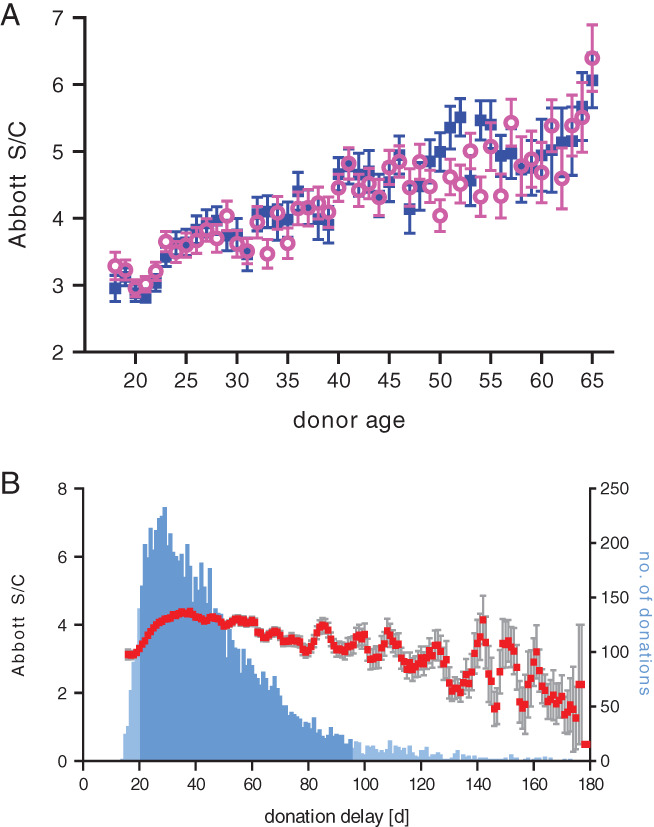
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglobulin G (IgG) content in 8697 first‐time COVID‐19 plasma donations (>65 year age group excluded) as measured by Abbott chemiluminescent microparticle immunoassay. (A) No significant difference in index S/C was seen between (■) male and (○) female donors. (B) Relation of index S/C and donation delay (days between symptom onset and plasma donation) illustrated as rolling average (mean (■) ± standard error of the mean [SEM]) over a 5‐day window; bars indicate the number of donations per day, with the central 90% of donations (days 20–95, n = 7852) indicated in darker shade [Color figure can be viewed at wileyonlinelibrary.com]
Most of the plasma (90%, n = 7852) was donated with a delay of 20–95 days following COVID‐19 (Figure 3(B)). Within this central dataset, an increase in SARS‐CoV‐2 IgG value was seen from day 20 (S/C 3.07) until day 36 (S/C 4.58) from where the values slowly declined until day 95 (S/C 3.25). A similar slow decline in SARS‐CoV‐2 IgG was seen over a 3‐month period in a recent study that measured IgG against three different epitopes, including the nucleocapsid protein, 27 as also detected in the Abbott CMIA used here, as well as in a study evaluating the decline of SARS‐CoV‐2 neutralizing antibody titers. 19
The regression models indicate four prime effects on index S/C, which are age, donation delay, hospitalization, and ethnicity. The collected information on the COVID‐19 plasma donations was sufficiently large to randomly subselect a dataset (n = 398) balanced for the factors donor age and donation delay for multiple regression analysis (see also Supplementary Statistical Analysis). The resulting curvilinear model (Figure 4) confirmed that the highest index S/C values can be expected in plasma from advanced age donors and donations collected soon after COVID‐19 recovery.
FIGURE 4.
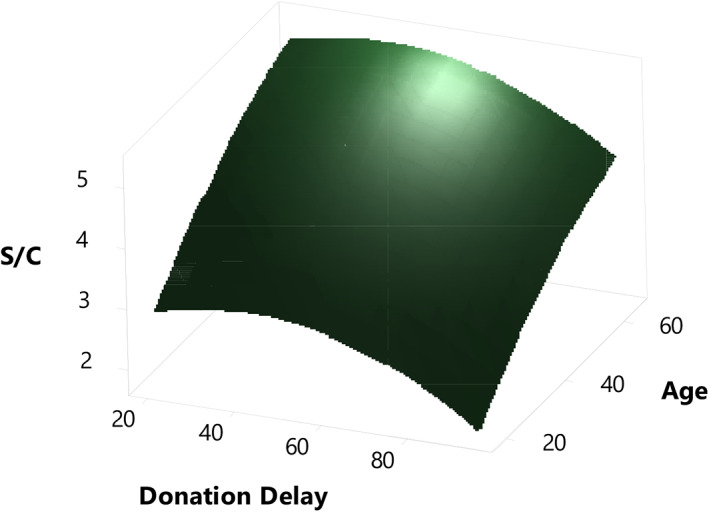
Surface plot of a curvilinear model of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) immunoglobulin G (IgG) content (Abbott index S/C) as a function of donor age and donation delay. A representative plasma donation subset (n = 398) was used for multiple regression analysis [Color figure can be viewed at wileyonlinelibrary.com]
Obesity has been associated with increased COVID‐19 severity 28 , 29 , 30 and with greater IgG response, 31 and while the current study did not evaluate donor body mass index in relation to SARS‐CoV‐2 antibody levels in plasma donations, these might be additional factors to be considered in CP collection.
5. CONCLUSION
This study showed a marked decrease in SARS‐CoV‐2 IgG titers in U.S. source plasma over 21 weeks from April until September 2020. The decline in titers was most probably effected by the observed decrease in plasma donor age over time as younger persons tend to experience milder COVID‐19 14 , 15 and therefore develop lower levels of antibodies. 16 , 17 , 18 , 19 Modeling of the obtained data indicated that use of plasma from more advanced age donors soon after convalescence from moderate to severe COVID‐19 might be a strategy to ensure consistently high SARS‐CoV‐2 titers in CP for transfusion. Collection of such units should be done alongside careful medical monitoring of each individual in this most fragile donor population. The informed blending of multiple donations for HIG production will increase the probability of therapeutic success.
These findings inform the collection of CP for use in transfusion, as well as the development of medicines like HIG that are made from CP. The higher the potency of CP, the higher the potential therapeutic efficacy. Optimization of the CP collection strategy to obtain the most potent units may allow for better treatment and efficient manufacture of medicines as potentially less plasma is needed per dose for the medicine to be effective.
CONFLICT OF INTEREST
All authors are employees of Takeda and have Takeda stock interest.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The contributions of the Sample Logistics and Serology teams from BioLife (Takeda), most notably Lori Lewis, Markeith Smith, Dewana Daniel, Amos Brown, Derante Davis, Bhavesh Sureja, Adrienne Bowman, Javon Barker, and Juan Sevilla (sample processing and testing), are gratefully acknowledged. This study was funded by BioLife, USA and Baxter AG, Austria, now part of the Takeda group of companies.
Karbiener M, Farcet MR, Ilk R, et al. Longitudinal analysis of SARS‐CoV‐2 antibodies in 8000 U.S. first‐time convalescent plasma donations. Transfusion. 2021;61:1141–1147. 10.1111/trf.16291
REFERENCES
- 1. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu STH, Lin H‐M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: A propensity score–matched control study. Nat Med. 2020;26:1708–13. 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 4. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. A randomized clinical trial. JAMA. 2020;324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gharbharan A, Jordans CCE, Geurts van Kessel C, den Hollander JG, Karim F, Mollema FP, et al. Convalescent plasma for COVID‐19. A randomized clinical trial. medRxiv. 10.1101/2020.07.01.20139857. Posted July 03, 2020. [DOI] [Google Scholar]
- 6. Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: Initial three‐month experience. medRxiv. 10.1101/2020.08.12.20169359. Posted August 12, 2020. [DOI] [Google Scholar]
- 7. US Food and Drug Administration (FDA) Clinical memorandum. [cited 2020 Nov 11]. Available from: https://www.fda.gov/media/141480/download.
- 8. Poelsler G, Berting A, Kindermann J, Spruth M, Hämmerle T, Teschner W, et al. A new liquid intravenous immunoglobulin with three dedicated virus reduction steps: Virus and prion reduction capacity. Vox Sang. 2007;93:184–92. [DOI] [PubMed] [Google Scholar]
- 9. CoVIg‐19 Plasma Alliance . Working together to fight COVID‐19 with immunoglobulin (Ig) therapy. [cited 2020 Nov 11]. Available from: https://www.covig-19plasmaalliance.org/en-us#recruitment.
- 10. Inpatient Treatment With Anti‐Coronavirus Immunoglobulin (ITAC), ClinicalTrials.gov Identifier: NCT04546581. [cited 2020 Nov 11]. Available from: https://clinicaltrials.gov/ct2/show/NCT04546581.
- 11. Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance characteristics of the Abbott Architect SARS‐CoV‐2 IgG assays and seroprevalence in Boise, Idaho. J Clin Microbio. 2020;58(8):e00941–20. 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbott Package Insert . SARS‐CoV‐2 IgG for use with Architect. H14806R04. 2020. [cited 2020 Nov 11]. Available from: https://www.fda.gov/media/137383/download.
- 13. Boehmer TK, DeVies J, Caruso E, van Santen KL, Tang S, Black CL, et al. Changing age distribution of the COVID‐19 pandemic – United States, may‐august 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Pan Z, Yue S, Yu F, Zhang J, Yang Y, et al. Disease severity dictates SARS‐CoV‐2‐specific neutralizing antibody responses in COVID‐19. Signal Transduct Target Ther. 2020;5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Mao B, Liang S, Yang JW, Lu HW, Chai YH, et al. Association between age and clinical characteristics and outcomes of COVID‐19. Eur Respir J. 2020;55:2001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krammer F, Simon V. Serology assays to manage COVID‐19. Science. 2020;368:1060–1. [DOI] [PubMed] [Google Scholar]
- 17. Jungbauer C, Weseslindtner L, Weidner L, Gänsdorfer S, Farcet MR, Gschaider‐Reichhart E, et al. Characterization of 100 sequential SARS‐CoV‐2 convalescent plasma donations. Transfusion. 2020;61:12–6. 10.1111/trf.16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral immune reponse to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383:1724–34. 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, et al. Dynamics of neutralizing antibody titers in the months after SARS‐CoV‐2 infection. J Infect Dis. 2020;223(2):197–205. 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention (CDC) , Coronavirus disease 2019 (COVID‐19) COVIDView updates for week 41, ending October 10, 2020. [cited 2020 Oct 28]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-10-16-2020.pdf.
- 21. Maddison SE, Stewart CC, Farshy CE, Reimer CB. The relationship of race, sex, and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull World Health Organ. 1975;52:179–85. [PMC free article] [PubMed] [Google Scholar]
- 22. Tollerud DJ, Weiss ST, Brown LM, Blattner WA, Maloney EM, Kurman CC, et al. Racial differences in serum immunoglobulin levels: Relationship to cigarette smoking, t‐cell subsets, and soluble interleukin‐2 receptors. JCLA. 1995;9:37–41. 10.1002/jcla.1860090107. [DOI] [PubMed] [Google Scholar]
- 23. McGowan JP, Shah SS, Small CB, Klein RS, Schnipper SM, Chang CJ, et al. Relationship of serum immunoglobulin and IgG subclass levels to race, ethnicity and behavioral characteristics in HIV infection. Med Sci Monit. 2006;12:CR11‐6. [PubMed] [Google Scholar]
- 24. Haitao T, Vermunt JV, Abeykoon J, Ghamrawi R, Gunaratne M, Jayachandran M, et al. COVID‐19 and sex differences. Mayo Clin Proc. 2020;95:P2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS‐CoV‐2 IgG antibody between male and female COVID‐19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–4. 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham NR, Whitaker AN, Strother CA, Miles AK, Grier D, McElvany BD, et al. Kinetics and isotype assessment of antibodies targeting the spike protein receptor‐binding domain of severe acute respiratory syndrome‐coronavirus‐2 in COVID‐19 patients as a function of age, biological sex and disease severity. Clin Transl Immunol. 2020;9:e1189. 10.1002/cti2.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. Persistence of serum and saliva antibody responses to SARS‐CoV‐2 spike antigens in COVID‐19 patients. Science Immunol. 2020;5:eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao F, Zheng KI, Wang X‐B, Sun QF, Pan KH, Wang TY, et al. Obesity is a risk factor for greater COVID‐19 severity. Diabetes Care. 2020;43:e1–3. 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 29. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID‐19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metab Clin Exp. 2020;108:154262. 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shields AM, Faustini SE, Perez‐Toledo M, Jossi S, Allen JD, Al‐Taei S, et al. Serological responses to SARS‐CoV‐2 following non‐hospitalised infection: Clinical and ethnodemographic features associated with the magnitude of the antibody response. medRxiv. 2020. 10.1101/2020.11.12.20230763. Posted November 16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


