Abstract
Background
With coronavirus disease 2019 (COVID‐19) convalescent plasma (CCP) offering an early treatment option for COVID‐19, blood collectors needed to quickly overcome obstacles to recruiting and qualifying eligible donors. We provide attributes of CCP donors and products and compare to standard donors and products.
Study Design and Methods
Information on CCP donors was gathered from the American Red Cross qualification website through product collection. Data from 2019 for standard plasma/platelet apheresis (SA) and whole blood (WB) donor demographics and SA donations including product disposition and reactions were used for comparison.
Results
Of almost 59 000 donors registering on the website, 75% reported an existing COVID‐19 diagnostic polymerase chain reaction or an antibody test. The majority (56.2%) of 10 231 CCP donors were first‐time donors in contrast to SA or WB donor populations, which were only 3.0% and 30.6%, respectively, first‐time donors. The number of female donors was 12% higher than SA donors. Older (≥ 65 years) and younger (16‐19 years) were comparatively underrepresented in CCP donors. Deferral (10.2%) and Quantity Not Sufficient rates (6.4%) for presenting CCP donations were higher than SA (8.2% and 1.1%, respectively). Human leukocyte antigen antibody reactivity was the highest cause of product loss for CCP donations vs SA donations (9.6% vs 1.3%). Acute adverse events also occurred at a higher rate among both first‐time and repeat CCP donations compared to SA.
Conclusions
CCP donors were more likely to be first‐time and female donors than WB or SA donors. CCP donations had a higher rate of donor adverse reactions, deferrals, and product loss than SA donations.
Keywords: COVID‐19 convalescent plasma, donor demographics
Short abstract
Abbreviations
- ARC
American Red Cross
- CCP
COVID‐19 convalescent plasma
- FDA
Food and Drug Administration
- HLA
human leukocyte antigen
- LOC
loss of consciousness
- NPD
Nonproductive Donation
- PAS3
platelet additive solution 3
- PCR
polymerase chain reaction
- PD
Productive Donations
- QNSd
Quantity Not Sufficient at the collection site
- QNSm
Quantity Not Sufficient at manufacturing
- SA
standard apheresis
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WB
whole blood
1. INTRODUCTION
On 11 March 2020, the World Health Organization declared coronavirus disease 2019 (COVID‐19), the disease caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a global pandemic. 1 On 24 March, the US Food and Drug Administration (FDA) announced emergency approval for compassionate use of convalescent plasma to treat patients with COVID‐19. 2 With no known therapies or vaccines available, demand for COVID‐19 convalescent plasma (CCP) paralleled the increase in affected individuals. 1 , 2 , 3
As the virus continues to spread and case counts continue to rise worldwide, 3 CCP currently remains the most accessible viral‐specific therapy for hospitalized patients. 1 , 4 , 5 , 6 Understanding demographic and other collection characteristics of individuals who registered and qualified for CCP donation is important to maintaining a viable inventory. This report characterizes these CCP donor characteristics, major reasons for donor deferral, and donor adverse events, as well as product loss following collections at the American Red Cross (ARC). Importantly, our study compares CCP donors to standard whole blood (WB) and standard apheresis (SA) donors from calendar year 2019 to understand the unique attributes of this donor population. Additionally, the donation characteristics of CCP and SA donors, including productive donation rate, product loss rates, and donor adverse event rates, are compared. Improved knowledge of CCP donors and donations could be used to guide donor retention strategies and in‐production planning.
Establishing a CCP program included multiple operational challenges, but the largest hurdle was donor recruitment and qualification. While a significant amount of analysis has been performed on the characteristics of donor demographics for WB and SA donors, there are currently few equivalent data for CCP donors, 7 , 8 and information on how these donors compare to WB and SA donors is not readily available. Though CCP should contain a certain level of neutralizing antibody, as the product was being launched, the clinically optimal titer was not known and high throughput testing for neutralizing antibody titer was not available to screen potential donors. The ARC focus was directed to recruiting individuals who had been clinically symptomatic and had received a confirmatory diagnosis via either a viral polymerase chain reaction (PCR) or antibody test. 9 The most immediate challenge was that potential donors had to be sufficiently recovered so the donorʼs own health was not jeopardized by the collection 9 and so as not to pose an infectious risk to collections staff and other donors, potentiating viral transmission. Because CCP was to be collected via apheresis, collections were limited to donors who were in proximity to and/or had relatively easy access to a brick‐and‐mortar collection site, as our blood center does not perform mobile plasma apheresis collections.
2. MATERIALS AND METHODS
2.1. CCP donor demographics and comparative information
Potential CCP donors registered on the ARC web‐based CCP donor registration portal. The registration portal opened before donors were able to donate at ARC sites. Donors were qualified based on a positive diagnostic SARS‐CoV‐2 PCR or serologic test and were scheduled for apheresis collection at the nearest donation site at least 28 days after complete resolution of symptoms. From 7 April to 26 April 2020, donor retention tubes were collected and stored for future antibody testing. On 27 April 2020, ARC began CCP donor qualification with the VITROS Anti‐SARS‐CoV‐2 Total (Ortho Clinical Diagnostics, New Jersey) assay. Units from eligible donors with a signal‐to‐cutoff ratio of 1.0 or greater (reactive per package insert) were labeled as CCP units. 10 All CCP donors were also required to meet traditional allogeneic blood donor criteria per the Code of Federal Regulations (21 CFR 630.10 and 630.15). 11 At time of plasma collection, donors consented to use of deidentified donor information and test results for research purposes. During the study period, CCP donors were permitted to donate every 28 days. SA plasma donors are also recruited every 28 days, and SA platelet donors are recruited every 14 days.
Qualified CCP donors presenting for collection between 7 April and 15 July 2020, and their associated collections, were analyzed and compared with WB and SA donors from calendar year 2019 to evaluate donor and collection characteristics. Donors were characterized by sex, age, race, and donor status (first‐time and repeat). Repeat donors were further characterized as active donors (donated at least once within the past 12 months), inactive donors (>12‐24 months since last donation), and lapsed donors (>24 months since last donation).
2.2. CCP donor complications
Donor complications were captured at the time of donation and documented by collections staff into the electronic blood donation record. Reaction types were stratified according to the ARCʼs 15 standardized definitions associated with apheresis collection: prefaint, short loss of consciousness (LOC) (<1 minute), long LOC (≥1 minute and/or complicated by loss of bladder/bowel control), LOC with injury, prolonged recovery of prefaint/LOC symptoms, small hematoma (≤2 in.), large hematoma (>2 in.), nerve irritation, suspected arterial puncture, minor citrate, major citrate, minor allergic, major allergic, minor other, and major other. 12 Minor complications such as prefaint, minor citrate, or small hematoma identified on‐site were assigned by collections staff. Major complications underwent further evaluation and follow‐up by Donor and Client Support Center staff and regional medical directors.
2.3. CCP donation and product disposition
Collections from CCP donors between 7 April and 15 July 2020 were retrospectively analyzed. Presenting donors were annotated by staff as “Convalescent Plasma Evaluation” in our Blood Establishment Computer System (eProgesa, MAK‐System, Brussels, Belgium). Those who were deferred though health history or physical examination, were categorized as On‐Site Deferral. Deferral rate was defined as the total number of donors deferred at time of collection out of total number of presenting donors. The SA comparison group for this portion of the study was all platelet and plasma apheresis collections from calendar year 2019.
Donations were then categorized according to the outcome of their attempted collection into the following groups: Productive Donations (PD), which resulted in CCP product; and Nonproductive Donations (NPD), which included No Blood Collected, Quantity Not Sufficient at the collection site (QNSd) or Contaminated. The PD rate was defined as the number of successful donation events out of total presenting donors. Collections considered PD by collections staff may later be compromised during manufacturing or testing. Units were also discarded if they did not pass standard infectious disease screening, were human leukocyte antigen (HLA) antibody positive, were determined to have incorrect volume (underweight or Quantity Not Sufficient at manufacturing [QNSm]) and/or if the collection container broke. These were aggregated into Discarded Donations. The Discarded Donation rate is defined as the number of discarded donations over the total number of productive donations. Donation characteristics were compared with total platelet and plasma apheresis donations from the 2019 calendar year.
3. RESULTS
3.1. CCP donor website registration
Between 27 March and 15 July 2020, 58 965 potential donors registered via the ARC CCP website. Overall, 44 496 (75%) acknowledged an existing COVID‐19 diagnostic PCR test, but that percentage varied over time, as percentage of donors with a PCR diagnostic test rose from an average of only approximately 40% in the first 3 weeks to greater than 60% after week 4 and approximately 80% by week 12. The ARC chose to qualify only those donors who registered with both a diagnostic test and a history of symptoms consistent with COVID‐19 per FDA guidance. 9 During this time period, donors were scheduled to donate by apheresis at the nearest donation site at least 28 days after symptom resolution. The number of presenting CCP donors to the website increased markedly between April and May by approximately 8‐fold but then remained steady through mid‐July.
3.2. Comparative characteristics of CCP compared to SA and WB donors
From 7 April 2020‐15 July 2020, approximately 27 000 units of CCP were generated from 14 272 CCP donations from 10 231 unique donors. CCP donor characteristics and comparative WB and SA donor characteristics are provided in Table 1. Females comprised the majority of CCP donors (56.9% vs 43.1%), the opposite of SA donors, who were 44.5% female and 55.5% male. WB donors also had more female representation (55.0% vs 45.0%). The majority (82.4%) of CCP donors were White. Black and Hispanic CCP donors comprised a slightly lower percentage than SA or WB donors. Overall, the racial demographics were similar to regular WB and SA blood donors. Older donors (>65) and high school–aged donors were underrepresented among CCP donors. Almost 83% of CCP donors were between ages 25 and 64. By contrast, the percentage of SA donors aged 65 and older were almost 2‐fold higher compared to CCP donors (19.1% vs 9.8%). Only 1% of the CCP donors were 19 years of age or younger, a population comprising 16.1% of WB donors and 3.2% of SA donors. Almost half (43.8%) of the CCP donors had prior experience donating blood, and of those, approximately 36% were repeat donors who were lapsed or inactive and only 7% were active, repeat donors. Only a small minority (3.0%) of SA donors were first‐time donors, in contrast to almost 56.2% of CCP donors being first‐time donors. By contrast, SA donors were overwhelmingly composed of repeat, active donors (84%), with approximately 13% being repeat lapsed or inactive. WB donors were 30.6% first‐time donors and 17% active repeat donors, with 52% being repeat lapsed or inactive donors. Of the CCP donors who were active donors before March 2020, the overwhelming majority (90%) were WB donors, and only 10% were SA donors, which was similar to our general donor base, as expected (95% WB, 5% SA).
TABLE 1.
Donor demographic characteristics for CCP 7 April 2020‐15 July 2020 (n = 10 231), SA donors (n = 125 095) and WB donors (n = 2 348 832) in calendar year 2019 to the ARC
| Donor characteristics | CCP donors | SA donors | WB donors |
|---|---|---|---|
| Frequency, n (%) | Frequency, n (%) | Frequency, n (%) | |
| Age, y | |||
| 16‐19 | 106 (1.0) | 4049 (3.2) | 378 295 (16.1) |
| 20‐24 | 692 (6.8) | 8565 (6.9) | 185 792 (7.9) |
| 25‐34 | 2124 (20.8) | 19 754 (15.8) | 338 077 (14.4) |
| 35‐44 | 1846 (18.0) | 16 335 (13.1) | 327 568 (13.9) |
| 45‐54 | 2224 (21.7) | 21 601 (17.3) | 368 368 (15.7) |
| 55‐64 | 2245 (21.9) | 30 775 (24.6) | 426 126 (18.1) |
| 65‐74 | 866 (8.5) | 20 294 (16.2) | 257 446 (11.0) |
| 75+ | 128 (1.3) | 3722 (2.9) | 67 160 (2.9) |
| Sex | |||
| Female | 5822 (56.9) | 55 688 (44.5) | 1 291 364 (55.0) |
| Male | 4409 (43.1) | 69 407 (55.5) | 1 057 468 (45.0) |
| Race/Ethnicity | |||
| White | 8429 (82.4) | 105 624 (84.4) | 1 938 737 (82.5) |
| Hispanic | 744 (7.3) | 6711 (5.4) | 148 408 (6.3) |
| Asian | 452 (4.4) | 4636 (3.7) | 81 421 (3.5) |
| Black | 279 (2.7) | 4156 (3.3) | 107 689 (4.6) |
| Native American | 17 (0.2) | 430 (0.3) | 9506 (0.4) |
| Multiple | 131 (1.3) | 2184 (1.8) | 37 368 (1.6) |
| Other | 125 (1.2) | 1039 (0.8) | 18 340 (0.8) |
| Prefer not to answer | 54 (0.5) | 315 (0.3) | 7363 (0.3) |
| Donor status | |||
| First‐time | 5751 (56.2) | 3731 (3.0) | 719 549 (30.6) |
| Repeat | 4480 (43.8) | 121 364 (97.0) | 1 629 283 (69.4) |
Abbreviations: ARC, American Red Cross; CCP, COVID‐19 convalescent plasma; SA, standard platelet/plasma apheresis; WB, whole blood.
When the adverse events data were segregated by first‐time vs repeat CCP donors (Figure 1), reaction rates remained higher than SA donors for both donor types. The overall pattern of reaction rates between both first‐time and repeat CCP and SA donors were similar, with small hematomas and prefaint reactions being the more common reactions. Not unexpectedly, the overall reaction rates in first‐time donors, both CCP and SA, were higher than repeat CCP and SA donors (1.3‐ vs 1.7‐fold, respectively). The increase between first‐time CCP as compared to first‐time SA donor overall reaction rates was modest, 1.2‐fold higher (1527.9 vs 1292.5 per 10 000 donations, respectively); similar‐fold increase in specific donor reactions were seen in first‐time CCP vs first‐time SA donors within the subcategories of prefaint, major/minor hematoma/bruising, and minor citrate. Interestingly, the overall difference in total reactions between repeat CCP vs SA donors was more pronounced than the difference seen among first‐time donors (1.5 vs 1.2‐fold). While small/large hematomas were 1.3‐fold higher (754.8 vs 568.6 per 10 000) and citrate reactions were 1.6‐fold higher (104.1 vs 63.8 per 10 000) in repeat CCP donors vs repeat SA donors, prefaint reactions were greater than 2‐fold higher (312.5 vs 134/10000 donations, respectively). These reactions rates were not adjusted for volume collected, starting blood volume of donors, or saline replacement, which may shed greater insight into the observed differences.
FIGURE 1.
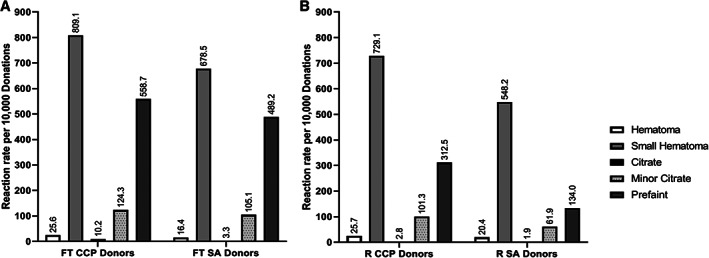
Reaction rates for 10 000 donations for first‐time CCP and SA donors (A) and repeat CCP and SA donors (B). Rates are expressed as numbers per 10 000 donations, with specific numbers denoted above each bar. The types of reactions captured are denoted
3.3. CCP productive donation rates compared to SA donations
Collection and product manufacturing characteristics from CCP collections were examined between the time period of 7 April to 15 July 2020, and data were compared to characteristics of SA donations from 2019 (Table 2, SA calendar year 2019 baseline). CCP donors were collected either on the ALYX machine (plasma only) or on the Amicus machine with a platelet product in platelet additive solution 3 (PAS3; Fresenius Kabi, Lake Zurich, IL) collected concurrently. The plasma products collected per procedure on the ALYX were similar between CCP and SA (2.9% for both). However, the plasma products collected per procedure on the Amicus were slightly higher for CCP vs SA collections (1.9 vs 1.3) due to the fact that for some of our SA collections, platelets were collected in plasma rather than in PAS3 and a concurrent plasma product was intentionally not generated for some platelet donors. Productive donations for CCP donors were in the range of 79% to 83% over the time period compared to 90.4% for SA donors over calendar year 2019. The deferral rate at collection site for CCP donors was much higher (12.6% vs 8.2%) in April, but that rate slowly declined to 7.7% by July, comparable to SA donor rates from 2019. This is likely because we improved our process for identifying and deferring ineligible donors over time before their arrival at the collection center. Repeat donors over time would reduce the number of deferrals as well. The QNSd rate for CCP donors (6.4%) was nearly 6‐fold higher for CCP donors compared to SA donors (1.1%) and accounted for the largest portion of NPD, which were also 6‐fold higher in the CCP donors (8.5%) compared with SA donors (1.4%). These rates did not change much over the time frame of data collection.
TABLE 2.
Productive donation rate for CCP donors by month (7 April 2020‐15 July 2020) at collection site compared to overall rate of SA donors, CY19
| Productive donation rate | SA CY19 baseline | CCP Apr‐20 | CCP May‐20 | CCP Jun‐20 | CCP Jul‐20 MTD (7/15) | CCP CY20 YTD Total |
|---|---|---|---|---|---|---|
| Presenting donors, n | 157 498 | 727 | 6129 | 5443 | 1973 | 14 272 |
| Productive donations | 142 351 | 574 | 4928 | 4454 | 1644 | 11 600 |
| On‐site deferral | 12 872 | 92 | 685 | 521 | 152 | 1450 |
| Nonproductive donations | 2275 | 61 | 516 | 468 | 177 | 1222 |
| Productive donation rate, % | 90.4 | 79.0 | 80.4 | 81.8 | 83.3 | 81.3 |
| Deferral rate, % | 8.2 | 12.6 | 11.2 | 9.6 | 7.7 | 10.2 |
| Nonproductive donation rate, % | 1.4 | 8.4 | 8.4 | 8.6 | 9.0 | 8.5 |
Abbreviations: CCP, COVID‐19 convalescent plasma; CY19, calendar year 2019; MTD, month to date; SA, standard platelet/plasma apheresis; YTD, year to date.
3.4. CCP donation and product losses compared to SA donations
Table 3A provides information on CCP product discard rate, which was over 2.3‐fold higher than the loss rate of SA products (19.5% vs 8.4%), and the top reasons for the discards. By far, the largest driver of CCP product loss was HLA antibody reactivity (9.6%) followed by QNSm collection (4.4%). Loss of SA products due to HLA antibody reactivity and QNSm was 1.3% and 2.1%, respectively. As expected, the loss due to broken units was comparable. As 56.2% of CCP donors were first‐time donors compared to 3.0% of SA donors, not surprisingly, the rate of hepatitis B core antibody reactivity was higher for CCP donors (between 0.6% and 1.1% over the time period studied) compared to 0.04% for baseline SA donors over 2019. Table 3B provides the actual numbers of units lost for each of these categories. HLA antibody reactivity accounted for 49.0% of all CCP units discarded but only for 15.6% of all SA product discards. The loss due to QNSm and broken units were comparable between CCP donors and baseline SA donation products. The loss rates in the various categories for CCP donations remained steady over the time period of study. Hepatitis B core antibody positivity rate was also steady. No additional infectious disease markers resulted as positive for CCP donations during the study period.
TABLE 3.
A and B: Product discard and discard rates for CCP productive donations by month (7 April 2020‐15 July 2020) compared to overall rates of SA donation products for CY19
| CCP apheresis plasma ‐ losses and loss rates | SA CY19 baseline | CCP Apr‐20 | CCP May‐20 | CCP Jun‐20 | CCP Jul‐20 MTD (7/15) | CCP Total |
|---|---|---|---|---|---|---|
| A | ||||||
| CCP donation discard rate, % | 8.4 | 14.5 | 21.5 | 19.9 | 14.1 | 19.5 |
| HLA antibody positive | 1.3 | 7.3 | 10.9 | 9.5 | 6.6 | 9.6 |
| Incorrect volume—under weight or QNSm | 2.1 | 3.1 | 4.6 | 4.8 | 2.9 | 4.4 |
| Hepatitis B Core antibodies test | 0.0 | 0.5 | 0.9 | 1.0 | 0.5 | 0.9 |
| B | ||||||
| Count of CCP donations discarded, n | 12 016 | 83 | 1060 | 888 | 232 | 2263 |
| HLA antibody positive | 1876 | 42 | 535 | 424 | 109 | 1110 |
| Incorrect volumeunder weight or QNSm | 3059 | 18 | 226 | 216 | 48 | 508 |
| Hepatitis B Core antibodies test | 50 | 3 | 46 | 44 | 9 | 102 |
| HLA antibody positive loss rate, % | 15.6 | 50.6 | 50.5 | 47.7 | 47.0 | 49.0 |
| QNSm loss rate, % | 25.5 | 21.7 | 21.3 | 24.3 | 20.7 | 22.4 |
| Hepatitis B core antibody+ test loss rate, % | 0.4 | 3.6 | 4.3 | 5.0 | 3.9 | 4.5 |
Abbreviations: CCP, COVID‐19 convalescent plasma; CY19, calendar year 2019; HLA, human leukocyte antigen; QNSm, Quantity Not Sufficient at manufacturing; MTD, month to date; SA, standard platelet/plasma apheresis.
4. DISCUSSION
COVID‐19 has caused an unprecedented global pandemic affecting more than seven million patients in the United States alone, resulting in more than 488 432 deaths to date. 3 For blood collectors, the pandemic ushered in a great deal of uncertainty surrounding the blood supply caused in part by the loss of nearly 50 000 sponsored blood drives causing nearly 1.5 million units to go uncollected, as well as the challenges of absence due to quarantine or illness of crucial staff, affecting all aspects of our operations. However, despite these pressures on normal blood collection, and the rapid progression of the pandemic with few effective treatments and medical preventive strategies, it became imperative that blood centers quickly develop a program to recruit, collect, and distribute CCP to meet the emergent need by hospitals for investigational use of this product.
Although convalescent plasma is not a novel therapeutic approach and has been used in the recent past, for example, to treat other respiratory infectious epidemics, including other coronaviruses (eg, severe acute respiratory syndrome, Middle East respiratory syndrome), 11 , 13 , 14 , 15 these efforts were relatively small on the order of 10 to 100 units. By contrast, the COVID‐19 pandemic has required an unprecedented scale‐up and deployment of CCP as reflected by the ARC itself having collected almost 27 000 units from over 14 000 donations (10 231 unique donors) as of July 15. Collections from donors have occurred at 170 collection sites across the country, and as such, the donor and donation characteristics presented are derived from our broad‐based, national donor base.
By 7 April 2020, the ARC had developed a website through which potential donors were encouraged to consider donating plasma by registering specific information such as date of diagnostic tests and last day of symptoms. Although the middle‐aged and older adults have been disproportionately affected by COVID‐19, with those over 50 accounting for 4‐fold or higher comparative hospitalizations and higher positive rate by testing 10 than the 18‐ to 29‐year‐old age group, they did not constitute the majority of our CCP donors. Whereas 44% of our SA donors were 55 years of age or older, only 32% of CCP donors were within that age group. Almost 70% of CCP donors were in the 20‐ to 54‐year‐old age range vs 53% of SA donors, suggesting that CCP donors represented a younger cohort. Despite higher infection, hospitalization, and death rates in Black and Hispanic populations, 16 this was not reflected in CCP donors, as White donors made up even a slightly higher percentage than SA donors. Even with active focused recruitment efforts in ethnically and racially diverse communities, it is well recognized that these populations continue to be underrepresented even among regular WB and SA blood donors. 17 Lack of specific marketing as well as severely reduced collections in schools and universities may have contributed to poor CCP donor representation among minority populations despite increased numbers of eligible donors within minority communities.
Overall, the majority of CCP donors were first‐time donors, and degree of fold increase of first‐time CCP donors was particularly striking when compared to SA (19‐fold) as compared to WB (2‐fold) donors. Though it is not unusual for first‐time donors to be introduced directly to apheresis‐based collections, SA donors are commonly recruited from WB donors, whereas CCP donors were recruited due to a history of a COVID diagnosis, so the high rate of first‐time donors is not unexpected. This comparatively high relative increase in first‐time donors explained the higher deferral rate at.
0 collection sites and Quantity Not Sufficient rates, both QNSd and QNSm, which are typically higher in first‐time donors. 18 The major reason for discard of collected units was HLA reactivity. This was not unexpected in a population with a high number of first‐time donors. Moreover, even within the group of CCP donors who were return donors, the overwhelming majority (90%) were previously WB donors and, therefore, did not have prior HLA testing results available in our system. Interestingly, donor reaction rates among CCP donors were higher as compared to SA donors for both first‐time and repeat donor categories. We do not know why the rates in first‐time CCP donors may be higher than first‐time SA donors or why a relatively higher rate continued to be observed among repeat CCP donors as compared to repeat SA donors. One possibility is that CCP donors collected on Amicus machines, which collect a concurrent platelet, have a larger plasma‐products‐per‐procedure value than do SA donors. The number of collections per machine type was not evaluated in this study, so no definitive correlation can be made. A large proportion of the repeat CCP donors were lapsed or inactive (36%) vs SA (13%), which may account for the higher donor reaction rate among even repeat CCP donors compared to SA donors.
The studyʼs major limitation is that it represents data from a single blood collector, albeit one with a national footprint and large numbers of hospitals served (>2300). Nevertheless, blood centers employed different recruitment strategies, which likely impacted the donor mix of SA donors and thereby the comparator used for the study. Also, whereas the ARC required symptoms as well as a prior diagnostic test, not all blood collectors required the presence of symptoms. Since we used only apheresis technology, this may have also altered the donor mix and product disposition.
On 23 August, the FDA issued Emergency Use Authorization of CCP with additional guidance on titer levels to define high‐ vs low‐titer products, citing that the sum of the findings from the clinical studies provided safety assurances and a reasonable efficacy signal for hospitalized patients with COVID‐19. 1 , 19 Several clinical trials are underway as well as additional data analysis from the Mayo Clinic Expanded Access Program to further evaluate the efficacy of use as a treatment as well as for pre‐ and postexposure prophylaxis in adult and pediatric populations. Currently, CCP remains one of the only therapies that is believed to significantly improve mortality in the pre–intensive care unit patient with COVID‐19. 11 , 13 , 14 , 19 , 20 , 21 , 22 While we wait for more definitive data, demand for this product is anticipated to continue or perhaps grow. A better understanding of the CCP donors and disposition of donated products is necessary for blood collectors to maintain and grow this inventory to meet ongoing hospital demand.
CONFLICT OF INTEREST
P.Y. serves on the medical advisory board of Fresenius Kabi and Creative Testing Solutions. All other authors declare no conflicts of interests.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Lasky B, Goodhue Meyer E, Steele WR, Crowder LA, Young PP. COVID‐19 convalescent plasma donor characteristics, product disposition, and comparison with standard apheresis donors. Transfusion. 2021;61:1471–1478. 10.1111/trf.16286
REFERENCES
- 1. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FDA Guidance on INDs on CCP. [cited 2020 Mar 26 ]. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds.
- 3. JHU . Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering at Johns Hopkins [monograph on the internet]. The Center for Systems Science and Engineering (CSSE) at JHU; 2020. [cited 2021 Feb 21]. Available from: https://coronavirus.jhu.edu/map.html.
- 4. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19 — final report. N Engl J Med. 2020;383(19):1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazer MH, Vassallo R, Delaney M, Germain M, Karafin MS, Sayers M, et al. Trends in age and red blood cell donation habits among several racial/ethnic minority groups in the United States. Transfusion. 2017;57:1644–55. [DOI] [PubMed] [Google Scholar]
- 8. Patel EU, Bloch EM, Grabowski MK, Goel R, Lokhandwala PM, Brunker PA, et al. Sociodemographic and behavioral characteristics associated with blood donation in the United States: a population based study. Transfusion. 2019;59:2899–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FDA . Recommendations for Investigational COVID‐19 Convalescent Plasma [monograph on the internet]. 2020. [cited 2020 April 7]. Available from: https://www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/recommendations‐investigational‐covid‐19‐convalescent‐plasma.
- 10.Centers for Disease Control Cases DSAf. [cited 2020 Aug 12]. https://www.cdc.gov/coronavirus/2019‐ncov/covid‐data/covidview/index.html.
- 11. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann Intern Med. 2006;145:599–609. [DOI] [PubMed] [Google Scholar]
- 12. Eder AF, Dy BA, Kennedy JM, Notari EP IV, Strupp A, Wissel ME, et al. The American Red Cross donor hemovigilance program: Complications of blood donation reported in 2006. Transfusion. 2008;48:1809–19. [DOI] [PubMed] [Google Scholar]
- 13. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergman A, Sella Y, Agre P, Casadevall A. Oscillations in U.S. COVID‐19 incidence and mortality data reflect diagnostic and reporting factors. mSystems. 2020;5:e00544–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stokes J Jr, Wolman IJ, Carpenter H, Margolis J. Prophylactic use of parents' whole blood in anterior poliomyelitis: Philadelphia epidemic of 1932. Am J Dis Child. 1935;50:581–95. [Google Scholar]
- 16. Tai T, Shah A, Doubeni CA, Sia IG, Wieland ML. Disproportionate impact of COVID‐19 on Racial and Ethnic Minorities in the United States. Clin Infect Dis. 2020;72(4):702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaz B, Hillyer C. Minority donation in the United States: challenges and needs. Curr Opin Hematol. 2010;17:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Custer B, Schlumpf K, Simon TL, Spencer BR, Wright DJ, Wilkinson SL, et al. Demographics of successful, unsuccessful and deferral visits at six blood centers over a 4‐year period. Transfusion. 2012;52:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: Initial three‐month experience. medRxiv. 2020. 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 20. Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med. 2020;26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–7. [DOI] [PubMed] [Google Scholar]
- 22. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


