Abstract
Background
The study aimed to estimate the prevalence of active or previous SARS‐CoV‐2 infection in asymptomatic adults admitted for elective surgery in Australian hospitals. This surveillance activity was established as part of the National Pandemic Health Intelligence Plan.
Methods
Participants (n = 3037) were recruited from 11 public and private hospitals in four states (NSW, Vic, SA and WA) between 2 June and 17 July 2020, with an overall 66% participation rate. Presence of SARS‐CoV‐2 viral RNA was assessed by Reverse Transcriptase ‐ Polymerase Chain Reaction (RT‐PCR) analysis of nasopharyngeal swabs taken after induction of anaesthesia. Presence of anti‐SARS‐CoV‐2 antibodies was assessed by analysis of serum collected at the same time using a novel dual‐antigen ELISA assay.
Results
No patient (0/3010) returned a positive RT‐PCR result. The Bayesian estimated prevalence of active infection of 0.02% (95% probability interval 0.00–0.11%), with the upper endpoint being 1 in 918. Positive serology (IgG) was observed in 15 of 2991 patients, with a strong positive in five of those individuals (Bayesian estimated seroprevalence 0.16%; 95% probability interval 0.00–0.47%).
Conclusion
These results confirm that during periods of low community prevalence of SARS‐CoV‐2 elective surgery patients without fever or respiratory symptoms had a very low prevalence of active SARS‐CoV‐2 infection.
Keywords: elective surgery, health policy, infection control, COVID 19
During the COVID 19 pandemic decisions regarding elective surgery cessation had significant impact on patients and practitioners. This study formed part of the National Pandemic Health Intelligence Plan, and finds that in areas where community transmission of SARS Cov‐2 is low, the risk of an asymptomatic patient undergoing elective surgery having SARS CoV‐2 is very low.
Introduction
The first cases of SARS‐coronavirus‐2 (SARS‐CoV‐2) disease (COVID‐19) were reported in late 2019 in Wuhan, China, and rapidly spread to the rest of the world. 1 , 2 As of 4 September 2020, the number of cases had exceeded 26 million globally, with 868 983 deaths, of which 26 049 confirmed cases and 678 deaths had occurred in Australia.
In the first 6 months of the pandemic, Australian case numbers had been kept low through federal‐state government cooperation, decisive and timely border closures, social distancing and quarantining, and other positive actions. 3 A National Emergency Plan 4 for COVID‐19 derived from previous whole of government responses such as the National Communicable Diseases Plan and the Australian Health Management Plan for Pandemic Influenza, was enacted Australia‐wide to curb the ‘first wave’ of COVID‐19. As part of this plan, non‐urgent elective surgery in public and private hospitals ceased across the country on 2 April 2020 for a period of 4 weeks in order to increase intensive care and hospital bed capacity for patients with COVID‐19.
During the first few weeks, most identified cases were among international arriving passengers and were successfully quarantined. Very little community transmission was apparent in Australia, and the number of active cases peaked at 5000 and then halved by mid‐April. Mindful that delays in necessary surgery harms patients' health and wellbeing, on the National Cabinet determined that elective surgery undergo a staged resumption commencing 27 April.
There continued to be concerns, however, about the risks COVID‐19 posed to elective surgical patient outcomes 5 and to exposed healthcare workers. 6 When cases of community transmission became evident in Melbourne in July the Department of Health and Human Services in Victoria mandated routine pre‐operative COVID‐19 testing prior to elective surgery from 15 July, and some medical representative organizations such as the Australian Society of Anaesthetists actively advocated for similar policies in other states and territories.
Routine preoperative testing is not without significant burden to patients, however, who may be required to isolate between the test and admission for their procedure, and to the health system in terms of the cost of conducting, processing and following up on the tests. In order to inform this debate, and to better quantify the risk to patients and staff when community transmission rates are relatively low, we conducted a multicentre prospective study of prevalence of SARS‐CoV‐2 viral RNA and serology in asymptomatic elective surgery patients across Australia. This study also informed Australia's National Pandemic Health Intelligence Plan. 7
Methods
Study setting, sponsorship and governance
Eleven public and private hospitals in New South Wales, South Australia, Victoria and Western Australia participated, selected on the basis of high elective patient throughput and perioperative research experience (Appendix S1). The study was coordinated by investigators at the Australian National University (ANU), and the Australian and New Zealand College of Anaesthetists Clinical Trials Network located at Monash University. The study was sponsored by the Australian Government Department of Health and the Medibank Better Health Foundation and was approved by the Alfred Hospital Ethics Committee (64 558, local ref. 339/20) under the National Mutual Acceptance Scheme and by local Ethics Committees as shown (Appendix S1).
Participants and recruitment
Consecutive patients admitted for elective surgery at each of the participating hospitals were approached during a six‐week period (2 June to 17 July 2020; Fig. 1), if they were adult (≥18 years) admitted for elective surgery under general anaesthesia. Eligible patients were informed that they would have a nasopharyngeal swab under anaesthesia for SARS‐CoV‐2 testing as part of their care, and all consented to surgery. Exclusion criteria included symptoms or signs of SARS‐CoV‐2 or any other respiratory tract infection, or recent COVID‐19 contact. We expected the prevalence of pre‐ or asymptomatic SARS‐CoV‐2 infection in elective surgical patients to parallel that seen in the community (less than 1 in 1000) and therefore aimed to enrol at least 3000 patients. Using exact binomial confidence intervals, with an upper 99% confidence limit as an arbitrary worst‐case scenario and no diagnostic test inaccuracy, no detected cases with this sample size would rule out a true prevalence of 0.18% or more, or less than 2 per 1000.
Fig 1.
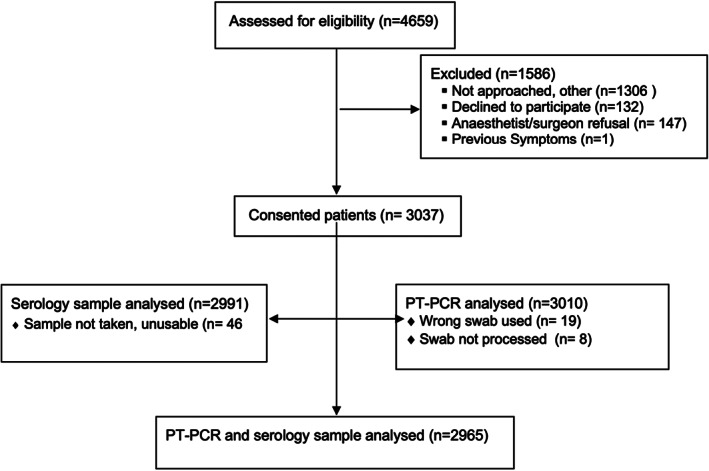
Flow diagram.
Sample collection and testing for SARS‐CoV2 RNA and antibodies
Deep nasopharyngeal swabs were collected after induction of anaesthesia and were tested for SARS‐CoV‐2 by RT‐PCR using TGA‐approved kits at a local public health laboratory under the supervision of a specialist in infectious diseases. As per national guidance on personal protective equipment use, standard surgical personal protective equipment (PPE) was used for the sample collection.
Serum was separated locally and stored frozen, then shipped as a batch to ANU. All samples were heated to 56°C for 1 h prior to analysis. An automated ELISA assay, based on Amanat et al., 8 was used on a high‐throughput platform to measure levels of IgG antibodies to SARS‐CoV2 Spike protein receptor‐binding domain (RBD) and nucleocapsid protein (N). 9
Data handling and analysis
Electronic study data were collected and managed using REDCap electronic data capture tools hosted at Monash University.
Prevalence of SARS‐CoV‐2 viral RNA using TC‐PCR, and anti‐SARS‐CoV‐2 antibodies by serology were each estimated using a Bayesian approach because it requires consideration of the imperfect sensitivity and specificity of the diagnostic testing regimens employed. This approach incorporates uncertainty about the values of sensitivity and specificity, in the form of prior distributions for these parameters. Guided by existing data, 10 , 11 , 12 the following prior distributions were adopted:
For RT‐PCR
Specificity prior distribution: Beta(19.9, 0.1), which has its mean at 99.5%, 2.5th percentile at 95.1%, and 97.5th percentile at 100%, meaning specificity was assumed to be between 95% and 100% with 95% probability. Sensitivity prior distribution: Beta(19,1), which has its mean at 95%, 2.5th percentile at 82%, and 97.5th percentile at 99.9%, and reflects greater uncertainty than that for specificity. A secondary analysis was done using a lower and more dispersed sensitivity prior distribution Beta(15,5), which has its mean at 75%, 2.5th percentile at 54% and 97.5th percentile at 91%.
For serological testing, the performance of the automated ELISA testing platform was assessed using a library of 184 plasma samples collected pre 2020 and a panel of 43 convalescent sera from individuals previously diagnosed with SARS‐CoV2 by viral RNA testing. 9 This resulted in a sensitivity of 43/43 = 100% and specificity 182/184 = 98.9% using the average of the IgG responses to the Spike‐RBD and nucleocapsid antigens. These data were incorporated as additional inputs into the Bayesian model, with uniform (Beta(1)) prior distributions placed on the sensitivity and specificity parameters so as to let the observed diagnostic accuracy data be the major determinant of the uncertainty of their values.
For both RT‐PCR and serological testing, the prior distribution for prevalence of Covid‐19 infection was assumed to be uniform over 0% to 100% (Beta(1)) thereby allowing the data to dictate this parameter's value without influence of imposing prior opinion. Statistical analysis was conducted using RStan Version 2.21.1, implementing 20 000 draws across four chains. Convergence and appropriate mixing of chains was confirmed with diagnostic plots and all l R‐hat values being <1.001. Prevalence estimates are reported as the median of the posterior distribution, and 95% probability intervals use the highest posterior density. Data were otherwise summarized as crude and relative (%) frequencies, giving mean (standard deviation) or median (interquartile range) for numerical data.
Results
Participant demographics and clinical characteristics
A total of 4659 patients were assessed for eligibility, and the overall participation rate was 66% resulting in a final cohort of 3037 participants (Fig. 1). It comprised 1558 women and 1479 men with mean age 54 (range 37–81) and other characteristics shown in Table 1. Reasons for non‐enrolment primarily related to patients assessed as eligible not being enrolled prior to anaesthesia. Travel overseas was reported by 203 (6.7%), five of them in the previous 2 weeks. Contact with a confirmed case of SARS‐CoV2 was reported by 15 individuals (0.5%), two in the previous 2 weeks. Participants had presented for a wide variety of elective surgeries (Table 1) in NSW (n = 1017), South Australia (n = 549), Victoria (n = 830) and Western Australia (n = 641). The majority of patients (1912) had at least one overnight stay (63% overnight stay, 37% day cases), with the median length of stay for these patients of 2 days, interquartile range 1–4 days. No participants developed COVID‐19 symptoms or tested positive post‐operatively. There were no reports of SARS‐CoV2 infections in healthcare workers involved in these patients' care.
Table 1.
Demographic and clinical characteristics of cohort and SARS‐CoV‐2 transmission risk factors
| Factor | Value, n (%) | Missing, n (%) |
|---|---|---|
| n | 3037 | |
| Demographics | ||
| Age, mean (SD) [range] | 54.0 (17.5) [15,95] | 0 |
| Sex | 0 | |
| Female | 1558 (51.3) | |
| Male | 1479 (48.7) | |
| Location | ||
| Urban | 1983 (65) | 0 |
| Rural | 1054 (35) | |
| ASA physical status | 0 | |
| 1 | 717 (23.6) | |
| 2 | 1326 (43.7) | |
| 3 | 908 (29.9) | |
| 4 | 86 (2.8) | |
| Ethnicity | 0 | |
| White | 2607 (85.8) | |
| Asian | 203 (6.7) | |
| ATSI | 16 (0.5) | |
| Black/African | 19 (0.6) | |
| Other | 192 (6.3) | |
| IRSAD (%) | 0 | |
| 1–20 | 317 (10.4) | |
| 21–40 | 382 (12.6) | |
| 41–60 | 457 (15.0) | |
| 61–80 | 587 (19.3) | |
| 81–100 | 1294 (42.6) | |
| Medical history | ||
| Cardiovascular disease (HT, HF, CAD) | 1001 (33.0) | 0 |
| Treated diabetes | 337 (11.1) | 0 |
| COPD and/or Asthma | 483 (15.9) | 0 |
| Overseas travel in 2020 | 204 (6.7) | 1 (0.0) |
| When was travel | 0 | |
| ≤2 weeks | 5 (2.5) | |
| >2 weeks | 199 (97.5) | |
| Contact with an individual with confirmed COVID‐19 | 15 (0.5) | 1 (0.0) |
| When was contact | 0 | |
| ≤2 weeks | 2 (13) | |
| >2 weeks | 13 (87) | |
| Health worker | 167 (5.5) | 0 |
| Surgery type | 0 | |
| Orthopaedic | 553 (18.2) | |
| Urology | 432 (14.2) | |
| Gynaecological | 371 (12.2) | |
| Gastrointestinal | 356 (11.7) | |
| Plastics | 250 (8.2) | |
| Neurological | 245 (8.1) | |
| Cardiac | 105 (3.5) | |
| Vascular | 85 (2.8) | |
| Other | 640 (21.1) | |
| Hospital stay | 0 | |
| ≥ 1 night stay | 1913 (63.0) | |
| Day case | 1124 (37.0) | |
| Unplanned ICU/HDU admission | 31 (1.0) | 4† (0.1) |
| Number of nights in hospital, median (IQR) | 2.0 (1.0–4.0) | 4† (0.2) |
| In‐hospital mortality | 6 (0.2) | 4† (0.1) |
Patients still inpatients at 4 September 2020.
ASA, American Society of Anesthesiologists; ATSI, Aboriginal or Torres Strait Islander; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HDU, high dependency unit; HF, heart failure; HT, hypertension; ICU, intensive care unit; IRSAD, Index of Relative Socio‐economic Advantage and Disadvantage; IQR, interquartile range; SD, standard deviation.
SARS‐CoV2 viral RNA
None of 3037 samples were positive for viral RNA. The estimated prevalence of active infection was 0.02% (95% probability interval 0–0.11%), with a worst‐case value based on the 95% upper probability limit of 0.11% hence less than 1 in 918. With the lower and more dispersed assumptions about sensitivity, the estimated prevalence was 0.03% (95% probability interval 0–0.14%), leading to a worst‐case value of 0.14% hence less than 1 in 710.
SARS‐CoV2 serology
Serology (IgG) was strongly positive in 5 of 2991 samples, and a further 10 returned marginally positive results. Based on 15 individuals having positive serology, and assuming sensitivity 100% and specificity 98.9%, the estimated seroprevalence was 0.16%; 95% probability interval 0.00–0.47%.
One of the five (20%) strongly seropositive participants reported a history of contact with a confirmed case in the previous 2 weeks compared to 14 out of the remaining 2985 patients (0.5%, P = 0.025, Fisher's exact test). No overseas travel had been reported for any of the seropositive patients.
Discussion
Our prospective study of prevalence of SARS‐CoV‐2 among more than 3000 asymptomatic individuals presenting for elective surgery found that none had active SARS‐CoV‐2 infection by viral RNA testing, and that fewer than 0.1% had serologic evidence of prior infection. This is despite the study being conducted in winter, 4–5 months after the first cases were detected in Australia and coinciding with a new wave of community transmission in Victoria.
While the true sensitivity of the viral RNA test is unknown, it is reasonable to assume a high sensitivity in this study given the patients were under anaesthesia and a deep naso‐pharyngeal specimen was collected by experienced practitioners. For an average sensitivity of 95% (range from 82% to 99%) we estimate the true proportion of patients with COVID‐19 is 1 in 5000, and we would be confident that the true prevalence is less than 1 in 918 (95% probability). Even assuming the test sensitivity is among the poorest reported in the literature (average 75%, range from 54% to 91%) the estimate of the proportion of patients with COVID‐19 is 1 in 3333 and are confident the real prevalence is less than 1 in 710 (95% probability).
Our estimates of very low community prevalence in these otherwise healthy individuals are consistent with the low numbers of active cases then prevalent in the four participating states. In the state of Victoria, the study period preceded then overlapped with the early stages of the ‘second wave’. Despite this, there were no detections in more than 3000 cases screened, of which 863 were recruited from Victoria. Notably, the two of the three participating hospitals in NSW are the referral centres for the communities most affected during the ‘first wave’ of infections in Australia. These results provide critical reassurance that when few cases of SARS‐CoV‐2 are detected in a community with high rates of testing, the likelihood of an asymptomatic patient with SARS CoV‐2 presenting for elective surgery is very low.
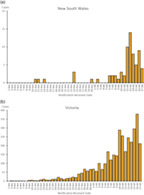
In Australia during the study period, community transmission was detectable at various points in the study regions (Fig. 2a,b); however, South Australia and Western Australia had virtually no detectable cases of community transmission. While the restrictions on community movement and interactions were variable during the study period, there were no ‘lockdown’‐style severe movement restrictions in place in any study region. The first case was recruited on 2 June when 485 cases were known in Australia, and the last case on 17 July, when 2548 active cases were known (National Incident Room, personal communication), but 90% had been recruited by 13 July, when 2730 cases were known. Our serology results suggest that as few as five patients were likely to have had prior infection.
Fig 2.
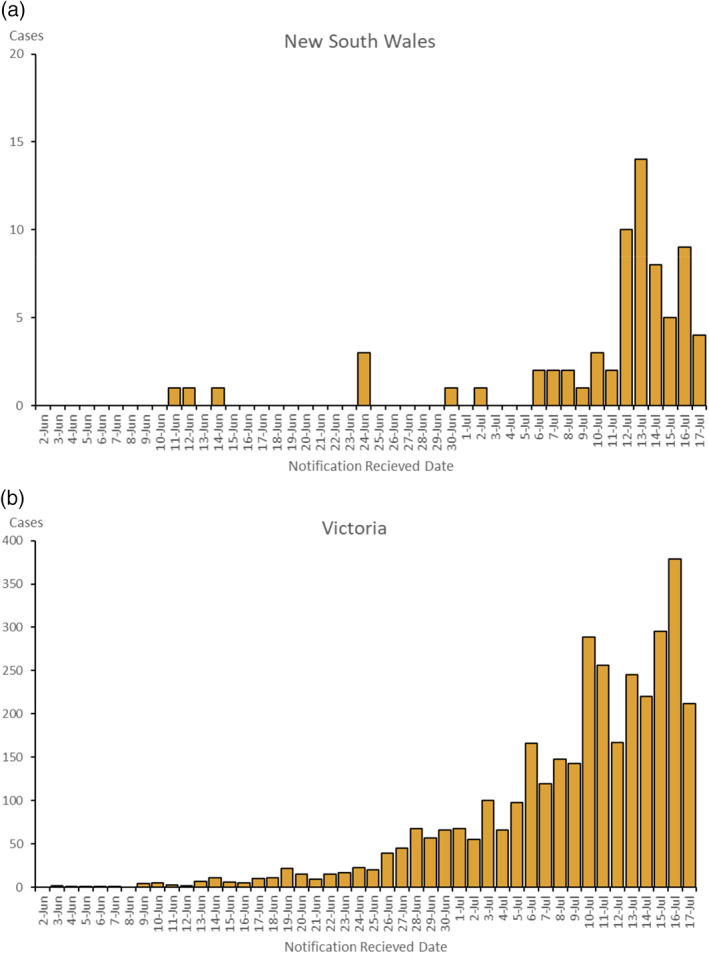
(a) New South Wales and (b) Victoria – Locally acquired cases by notification received date from 2 June to 17 July 2020.
Our study did not examine patients undergoing emergency surgery, nor those who had been inpatients for significant time. We are not aware of any SARS‐CoV2 infections in healthcare workers directly caring for elective surgery patients during this study. Hospitals have become locations of SARS CoV‐2 transmission, where infected and unwell patients pose risks to other patients and health workers. Rigorous infection control practices including the use of particulate filter respirators for suspected or confirmed COVID‐positive patients undergoing surgery is required. 13 While the results of this study do not support the use of particulate filter respirators for all anaesthetic inductions and aerosol generating operations when community prevalence is low, it is possible that if community prevalence was considerably higher the risk of transmission by asymptomatic patients undergoing surgery may be higher, and PPE guidance may require modification. Further data is being collected throughout the Victorian resurgence to test whether or not this is the case.
During the study, 15 patients had epidemiological risk factors including two who had contact with confirmed SARS CoV‐2 cases in the 2 weeks prior to their elective procedure. This indicates that even in a study environment screening tools have their limitations and this needs to be factored into policy decisions. Ideal management would have been to postpone those cases until greater than 2 weeks post exposure, or to test prior to surgery. We would recommend a similar approach for patients returning from overseas travel, however at the time of writing all returned travellers to Australia are undertaking 14 days mandatory quarantine.
Nonetheless, the findings of this study do not lend support to a policy of routine preoperative screening for SARS CoV‐2 in asymptomatic elective surgery patients in a low prevalence setting, among whom the likelihood of detecting an active or infectious case under the most conservative of assumptions is no more than 1 in 710 and likely lower than 1 in 918. Doing so may cause significant patient inconvenience and be poor use of limited testing and human resources, leading to delays in testing for other affected individuals in the community. Similarly, while personnel with close patient contact must use appropriate PPE, this study has demonstrated that the risk to operating theatre staff of contracting SARS CoV‐2 in the workplace is small. Where community prevalence is low the use of standard surgical PPE is proportionate to the low risk that we have found of exposure to SARS‐CoV‐2 in operating suite environments during elective surgery for asymptomatic patients.
Author Contributions
Nicholas Coatsworth: Conceptualization; funding acquisition; investigation; methodology; project administration; writing‐original draft; writing‐review and editing. Paul Myles: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; writing‐original draft; writing‐review and editing. Graham Mann: Conceptualization; formal analysis; funding acquisition; investigation; methodology; writing‐original draft; writing‐review and editing. Ian Cockburn: Formal analysis; methodology; supervision; validation; writing‐original draft; writing‐review and editing. Andrew Forbes: Formal analysis; investigation; methodology; writing‐original draft; writing‐review and editing. Elizabeth Gardiner: Data curation; formal analysis; investigation; methodology; writing‐original draft; writing‐review and editing. Gary Lum: Data curation; formal analysis; project administration; resources; writing‐review and editing. Allen Cheng: Conceptualization; formal analysis; methodology; writing‐review and editing. Russell Gruen: Conceptualization; funding acquisition; methodology; project administration; writing‐original draft; writing‐review and editing.
Conflicts of interest
None declared.
Supporting information
Appendix S1. SARS‐CoV‐2 testing in elective surgery collaborators.
Acknowledgements
The study was supported by grants from the Australian Department of Health and Ageing and the Medibank Private Foundation, which had no direct role in the recruitment of patients, processing of tests, nor the analysis of their results. NC and GL, who are employees of the Department, contributed to study design, interpretation of results, and writing and approval of the manuscript. The authors acknowledge the contributions of all participants, the Australian and New Zealand College of Anaesthetists Clinical Trials Network, study sites, local investigators, surgical and anaesthetic teams, the study coordinators Sophie Wallace and Lucy Morris, the efforts in viral RNA testing at all participating laboratories, and the implementation of high‐throughput serologic testing at ANU by Sarah Hicks, Kai Pohl, Hayley McNamara, Samina Nazir, Sidra Ali, Amee George, Kate Parsons, Jinshu He and Philip Wu.
N. Coatsworth MB,BS MIntPH; P. S. Myles MBBS DSc; G. J. Mann PhD; I. A. Cockburn PhD; A. B. Forbes PhD; E. E. Gardiner PhD; G. Lum MBBS; A. C. Cheng PhD; R. L. Gruen PhD.
References
- 1. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein A. Australia keeps a lid on COVID‐19 – for now. New Sci. 2020; 426: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anon . Australian Health Sector Emergency Response Plan for Novel Coronavirus (COVID‐19). Publication Number 12723. Canberra: Australian Government Department of Health. [Cited 15 Sep 2020.] Available from URL: https://www.health.gov.au/resources/publications/australian-health-sector-emergency-response-plan-for-novel-coronavirus-covid-19
- 5. COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 396: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myles PS, Maswime S. Mitigating the risks of surgery during the COVID‐19 pandemic. Lancet 2020; 396: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anon . Coronavirus (COVID‐19) in Australia – Pandemic Health Intelligence Plan. Canberra: Australian Government Department of Health. [Cited 15 Sep 2020.] Available from URL: https://www.health.gov.au/sites/default/files/documents/2020/05/coronavirus-covid-19-in-australia-pandemic-health-intelligence-plan_1.pdf
- 8. Amanat F, Stadlbauer D, Strohmeier S et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat. Med. 2020; 26: 1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hicks S, Pohl K, Neeman T et al. A dual antigen ELISA allows the assessment SARS‐CoV‐2 seroprevalence in a low transmission setting. J. Infect. Dis. 2021; 223: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burki TK. Testing for COVID‐19. Lancet Respir. Med. 2020; 8: e63–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS‐CoV‐2 infection – challenges and implications. N. Engl. J. Med. 2020; 383: e38. [DOI] [PubMed] [Google Scholar]
- 12. Mohammadi A, Esmaeilzadeh E, Li Y, Bosch RJ, Li JZ. SARS‐CoV‐2 detection in different respiratory sites: a systematic review and meta‐analysis. EBioMedicine 2020; 59: 102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Infection Control Expert Group . The Use of Face Masks and Respirators in the Context of COVID‐19. Canberra: Australian Government Department of Health. [Cited 7 Sep 2020.] Available from URL: https://www.health.gov.au/resources/publications/the‐use‐of‐face‐masks‐and‐respirators‐in‐the‐context‐of‐covid‐19
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. SARS‐CoV‐2 testing in elective surgery collaborators.


