Abstract
The epidemiology and mycology of invasive candidiasis in the ICU is well‐described in certain types of critically ill patients but not in others. One population that has been scarcely studied is non‐neutropenic patients admitted specifically to medical ICUs. Even less is known about the broader category of medical ICU patients without active oncological disease. This group constitutes a very large share of the patients requiring critical care across the globe, especially in the era of the SARS‐CoV‐2 pandemic. We analysed medical ICU candidaemia episodes that occurred in non‐oncological patients in our tertiary academic centre in the United States from May 2014 to October 2020 to determine the incidence and species distribution of the associated isolates. We then separately considered non‐COVID‐19 and COVID‐19 cases and compared their characteristics. In the non‐COVID‐19 group, there were 38 cases for an incidence of 1.1% and rate of 11/1000 admissions. In the COVID‐19 group, there were 12 cases for an incidence of 5.1% and rate of 51/1000 admissions. In the entire sample, as well as separately in the non‐COVID‐19 and COVID‐19 groups,Candida albicans accounted for a minority of isolates. Compared to non‐COVID‐19 patients with candidaemia, COVID‐19 patients had lower ICU admission SOFA score but longer ICU length of stay and central venous catheter dwell time at candidaemia detection. This study provides valuable insight into the incidence and species distribution of candidaemia cases occurring in non‐oncological critically ill patients and identifies informative differences between non‐COVID‐19 and COVID‐19 patients.
Keywords: Candidaemia, coronavirus, COVID‐19, invasive candidiasis, medical intensive care unit, mycology
1. INTRODUCTION
Invasive mycosis caused by Candida species, most commonly detected clinically as candidaemia, is a major form of nosocomial infection particularly prevalent in intensive care units (ICUs). Historically, invasive candidiasis (IC) has been associated most closely with two categories of ICU cases: Oncological patients with neutropenia and surgical patients with abdominal pathology. While the importance of Candida as a pathogen in non‐neutropenic medical ICU patients has been increasingly recognised, studies restricted to this fundamental ICU population remain few. Among the features of IC rarely studied in this specific population is the relative distribution of albicans versus non‐albicans species among contemporary isolates, an issue of importance because some studies in undifferentiated ICU populations have suggested a shift from the traditional majority of the former to a majority of the latter. 1 , 2 , 3 The ongoing pandemic of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), which has created a global explosion in the number of critically ill patients with respiratory failure, has contributed an additional question: Has the pandemic changed the incidence, patient characteristics, and speciation of IC cases in medical ICUs? We performed the present analysis of our medical ICU non‐oncological candidaemia cases in order to describe the epidemiology and mycology of IC in this population as well as to compare those with and without coronavirus disease 2019 (COVID).
2. PATIENTS AND METHODS
All patients older than 18 years of age belonging to the medical ICU service at Westchester Medical Center (WMC), a tertiary university hospital in Valhalla, NY, between 1 May 2014 and 31 October 2020 whose blood cultures drawn at any time following admission to the ICU yielded Candida species were potentially eligible for inclusion in this study. These cases were identified through a query of the pathogen database maintained by the WMC Department of Microbiology. In WMC, identification and speciation of fungal isolates are performed using the Matrix Assisted Laser Desorption Ionization‐Time of Flight (MALDI‐TOF) Biotyper CA system (BRUKER Corp.). Patients with severe neutropenia (absolute neutrophil count < 500 cells/µl) at the time of index ICU admission and also active recipients of chemotherapy for an oncological diagnosis at the time of the index hospitalisation were excluded. Patients eligible for analysis after the application of these inclusion and exclusion criteria were then screened for positivity for SARS‐CoV‐2 by polymerase chain reaction testing of a nasopharyngeal swab specimen. Those who were found to be positive for SARS‐CoV‐2 formed the COVID group, within the study; all others comprised the non‐COVID group. Demographic, clinical and mycological characteristics of the study patients were extracted retrospectively from the institutional electronic medical record. The date of the first positive fungal blood culture was used for calculation of all durations. Receipt of antibacterial therapy was counted only if the last dose had been administered within 30 days prior to the acquisition of the blood culture. Patients were considered corticosteroid recipients if they had been exposed to these agents at any dose within 3 days of the blood culture. Central venous catheter (CVC) dwell time in days was calculated from the date of insertion (day 0) to the date of the blood culture. If the insertion date was not available (eg, for patients transferred from outlying hospitals), the date of medical ICU entry was taken as day 1 of CVC dwell. In case of the presence of more than one CVC at the time of positive blood culture, the CVC with the longest dwell time was the one counted. Patients designated as recipients of new renal replacement therapy (RRT) were those started on RRT during the index hospitalisation and prior to the date of the positive blood culture. Patients designated as having end‐stage renal disease (ESRD) were recipients of ambulatory RRT prior to hospitalisation. The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The institutional review board of New York Medical College approved this study (protocol #14091).
2.1. Statistical analysis
All categorical variables are expressed as frequencies and percentages. Comparisons of categorical variables were performed using Fisher's Exact Test. Continuous variables are expressed as median (IQR) unless otherwise specified, in which case they are expressed as mean ± SD. The Mann‐Whitney U Test was used for statistical inference on non‐normally distributed data while the two‐tailed Student's T test was used for statistical inference on data not expected to violate normality. P‐value of < 0.05 was taken to be statistically significant. Microsoft Excel 2016 supplemented by a calculator application publically available at www.statskingdom.com was used for the statistical analysis.
3. RESULTS
During the 78‐month span of the study period, there were 3,568 non‐COVID adults and 236 COVID adults admitted to the medical ICU service at WMC. After the application of our exclusion criteria, a total of 50 unique patients with candidaemia were identified for an overall incidence of 1.3% and a corresponding incidence rate of 13/1000 admissions. There were 38 candidaemic patients in the non‐COVID group and 12 in the COVID group. These numbers translated into an incidence of 1.1% in the non‐COVID group and 5.1% in the COVID group. The corresponding incidence rates were 11/1000 admissions in the non‐COVID group and 51/1000 admissions in the COVID group. The demographic and clinical characteristics of the study population are summarised in Table 1, which also compares the non‐COVID and COVID groups. There were no statistically significant differences between the two groups with respect to demographic features, frequency of any of the tabulated risk factors for IC or pre‐candidaemia corticosteroid exposure. There was a statistically significant difference in the sequential organ failure assessment (SOFA) score on admission to the ICU, which was higher in the non‐COVID group (10, IQR 8‐12 vs. 5, IQR 4‐9; P =.006) indicating a greater burden of organ dysfunction in those patients. On the other hand, the ICU length of stay prior to development of candidaemia was significantly longer in the COVID group (19 days, IQR 11‐28 vs. 5 days, IQR 2‐9; P =.001) as was the CVC dwell time (12.5 days, IQR 7‐19 vs. 5.5 days, IQR 2‐9; P =.022). There was no significant difference in crude hospital mortality between the two groups. The Candida species distribution in the overall population as well as separately in the non‐COVID and COVID groups is presented in Table 2 and depicted graphically in Figure 1. Out of a total of 52 Candida isolates, a minority (15/52, 29%) were Candida albicans. Candida albicans likewise constituted a minority of isolates in both the non‐COVID (11/39, 28%) and COVID (4/13, 31%) groups. The distribution of albicans vs. non‐albicans species was not significantly different between the two groups (P = 1.00). The single most common species in the non‐COVID group was Candida glabrata (12/39, 31%) and in the COVID group it was Candida albicans (4/13, 31%). None of the patients had received antifungal therapy in the ICU prior to blood culture positivity.
TABLE 1.
Demographic and clinical characteristics of the total study population as well as its non‐COVID and COVID subgroups
| Parameter | Total N = 50 | Non‐COVID N = 38 | COVID N = 12 | P‐value |
|---|---|---|---|---|
| Age (mean ± SD) | 58 ± 16 | 56 ± 15 | 62 ± 16 | 0.30 |
| Male (%) | 37 (74) | 28 (74) | 9 (75) | 0.67 |
| DM (%) | 4 (8) | 3 (8) | 1 (8) | 1.00 |
| CLD a (%) | 11 (22) | 10 (26) | 1 (8) | 0.26 |
| ESRD (%) | 3 (6) | 3 (8) | 0 (0) | 0.54 |
| SOT b (%) | 3 (6) | 3 (8) | 0 (0) | 1.00 |
| Therapeutic IS c (%) | 3 (6) | 3 (8) | 0 (0) | 1.00 |
| SOFA Score d | 9 (6‐12) | 10 (8‐12) | 5 (4‐9) | 0.006 |
| MV (%) | 44 (88) | 33 (87) | 11 (92) | 1.00 |
| ECMO (%) | 2 (4) | 0 (0) | 2 (17) | 0.054 |
| CVC (%) | 48 (96) | 36 (95) | 12 (100) | 1.00 |
| Days CVC Dwell | 6.5 (4‐12) | 5.5 (2‐9) | 12.5 (7‐19) | 0.022 |
| New RRT (%) | 32 (68) d | 22 (63) d | 10 (83) | 0.29 |
| TPN (%) | 7 (12) | 5 (13) | 2 (16) | 1.00 |
| CS Therapy (%) | 22 (45) d | 16 (43) d | 6 (50) d | 0.74 |
| ABX Therapy (%) | 48 (96) | 36 (95) | 12 (100) | 1.00 |
| ICU Days to Positive Culture | 7 (2.25‐18.5) | 5 (2‐9) | 19 (11‐28) | 0.001 |
| Mortality (%) | 32 (64) | 23 (61) | 9 (75) | 0.50 |
Abbreviation: ABX, antibacterial agents; CLD, chronic liver disease; CS, corticosteroids; CVC, central venous catheter; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; ESRD, end‐stage renal disease; ICU, intensive care unit; IS, immunosuppression; MV, mechanical ventilation; RRT, renal replacement therapy; SOFA, sequential organ failure assessment; SOT, solid organ transplant; TPN, total parenteral nutrition.
Child‐Pugh class B or C cirrhosis
Includes 2 patients with liver transplantation and 1 patient with kidney transplantation
Includes 3 patients receiving tacrolimus
Calculated at time of ICU entry
Based on N = 47 that excludes patients with ESRD
Based on N = 35 that excludes patients with ESRD
Based on N = 49 due to a patient with missing data
Based on N = 37 due to a patient with missing data
Six patients in the COVID group and none in the non‐COVID group received corticosteroids at pulse dose (ie, methylprednisolone 1gm)
TABLE 2.
Candida species distribution in the overall study population and separately according to COVID status
| Candida Species | Non‐COVID | COVID | Total |
|---|---|---|---|
| C. albicans | 11 | 4 | 15 |
| C. glabrata | 12 | 2 | 14 |
| C. parapsilosis | 7 | 3 | 10 |
| C. tropicalis | 5 | 2 | 7 |
| C. dubliniensis | 3 | 1 | 4 |
| C. krusei | 1 | 0 | 1 |
| Other non‐C.albicans | 0 | 1 | 1 |
| 39 a | 13 b | 52 |
One patient in the non‐COVID group had two types of non‐albicans Candida isolated
One patient in the COVID group had both albicans and an unidentified non‐albicans Candida species isolated
FIGURE 1.
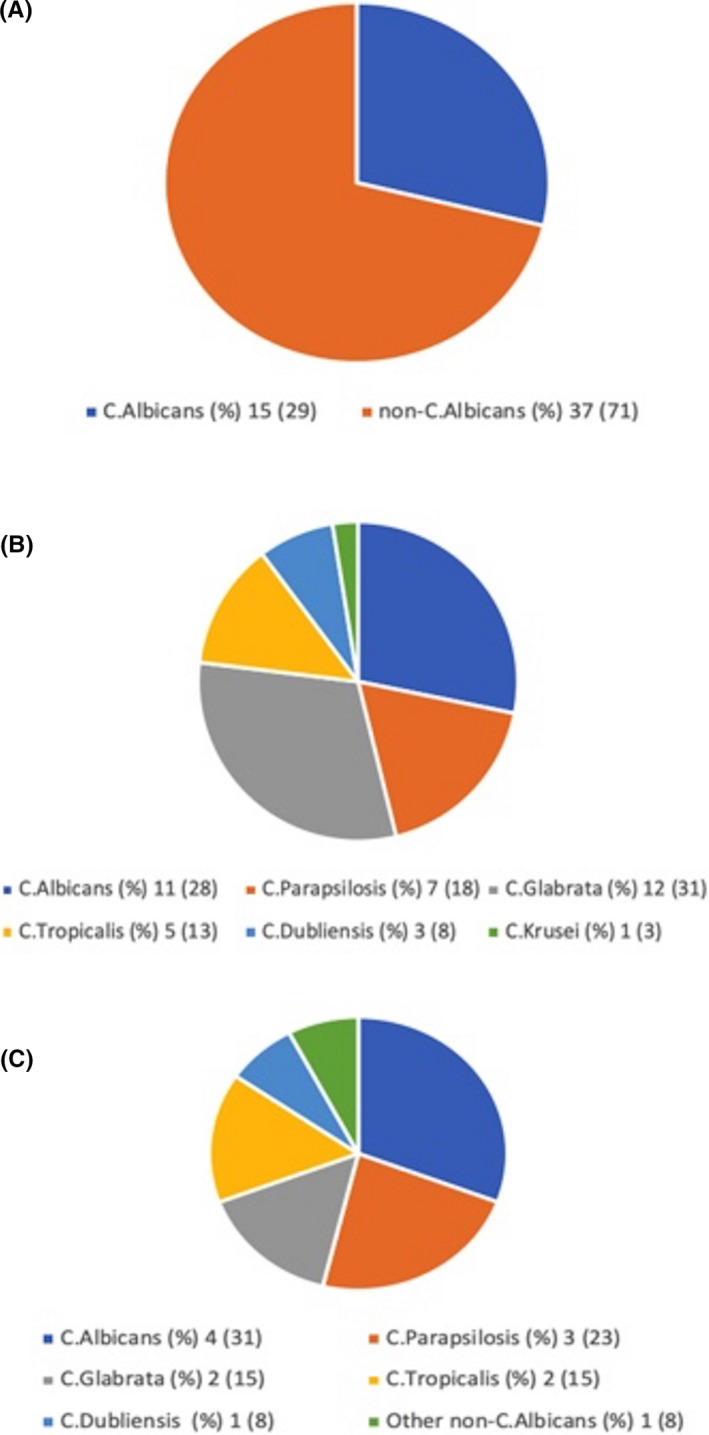
Graphical depiction of (A) the C.albicans vs. non‐C.albicans Candida distribution in the entire study population, (B) the Candida species distribution in the non‐COVID group, and (C) the Candida species distribution in the COVID group
4. DISCUSSION
Our study contributes several important observations to the limited published information on the epidemiology and mycology of IC as detected by candidaemia in non‐oncological medical ICU patients, a specific critically ill population not heretofore explored separately in this regard to our knowledge. First, it provides an estimate of the incidence rate (11/1000 medical ICU admissions) of candidaemia among such patients in a United States tertiary referral centre in the absence of COVID. In general, there has been a paucity of data available from the United States on the incidence of candidaemia in non‐surgical, non‐neutropenic ICU patients. Existing multicentre collaborative studies performed outside the United States do report candidaemia incidence rates restricted to either medical or non‐neutropenic ICU patients but not both. For example, a mixed medical‐surgical ICU study from France reported a mean incidence rate of 5.3/1000 admissions across the 12 participating medical ICUs, but close to 20% of the overall study population was neutropenic. 4 Conversely, an Australian study restricted to non‐neutropenic ICU patients reported a mean incidence rate of 2.06/1000 admissions, but approximately 68% of the population had undergone recent surgery. 5 Contrary to precedent, we elected to limit our study population to not just those without neutropenia but also to those without actively treated malignancy. This was done to investigate a population that is largely unexplored in IC research yet one that constitutes the core of the pathology treated in general medical ICUs across the globe. Another reason for this decision was that even those oncological patients who are not neutropenic at time of adjudication of their status for study purposes may have been neutropenic in the recent past and may possess unique complicating factors as a result of their active malignancy and treatment thereof such as long‐term intravascular catheters, prophylactic antibiotic administration, and lower threshold for empirical antifungal therapy among others. The third rationale was facilitation of the comparison between non‐COVID and COVID ICU patients with candidaemia since the former contingent was considered a priori likely to be relatively enriched in patients with active malignancy and chemotherapy receipt, as has already been demonstrated in a small Italian series in which—contrary to the non‐COVID group—the COVID group with candidaemia contained zero patients with active malignancy or recent chemotherapy. 6
Another utility of our study results is the ability to compare and contrast the Candida species distribution in a representative medical ICU population in the United States with that of other parts of the world. To begin with, there is a paucity of data on albicans vs. non‐albicans Candida distribution in medical ICU patients in the United States in general. We found that during an approximately 6‐year contemporary period in our institution's medical ICU, non‐albicans Candida collectively constituted the majority of isolates in candidaemic patients, considering non‐COVID and COVID cases together. A 2009 multi‐continent study of ICU patients from Australia, Europe and South America showed that non‐albicans Candida represented the majority of isolates only in South America. 7 Since then, more contemporary ICU studies have reaffirmed an albicans majority in Europe 8 , 9 and Australia, 5 but not in Asia 1 and not in South America. 2 , 3 All of the aforementioned studies included mixed ICU populations with varying neutropenic contingents, making direct correlation with our results challenging. When the non‐COVID and COVID groups are considered separately, non‐albicans Candida made up the majority of isolates in each of the two groups. Of note, the predominant single species in the non‐COVID group of our study was Candida glabrata, whereas in the COVID group it was Candida albicans. Data are beginning to emerge from around the globe describing the speciation of candidaemia in COVID patients, with major such reports summarised in Table 3. 6 , 19 As in our study, a non‐albicans majority has been observed among COVID candidaemia isolates in another tertiary academic centre in the United States, 18 whereas an albicans majority was recorded across three Brazilian tertiary hospitals 17 , 19 as well as in the United Kingdom 16 and Italy. 6 Unlike our study of exclusively ICU patients, not all of these comparator studies of COVID candidaemia were limited to patients cared for in an ICU setting.
TABLE 3.
Comparison of the current study with available studies reporting the incidence of candidaemia and/or speciation of Candida isolates in COVID positive patients
| Study | Country | N | ICU Only Y/N | MV % | Incidence (%) | Incidence Rate a | Incidence Density | Isolates | C. albicans % |
|---|---|---|---|---|---|---|---|---|---|
| Cataldo b 10 | Italy | 5 | Y | NR | 8.8 | NR | NR | 6 | 33 |
| Giacobbe b 11 | Italy | 3 | Y | NR | 3.8 | NR | NR | 3 | 33 |
| Bonazzetti b 12 | Italy | 3 | Y | NR | 3.4 | NR | NR | 3 | 100 |
| Antinori 13 | Italy | 3 | N | NR | NR | NR | NR | 3 | 33 |
| Al‐Hatmi 14 | Oman | 4 | Y | 100 | NR | NR | NR | 5 | 60 |
| Chowdhary 15 | India | 15 | Y | 53 | 2.5 | NR | NR | 15 | 20 c |
| White d 16 | UK | 5 | Y | 91 | 3.7 | NR | NR | 6 | 83 |
| Mastrangelo 6 | Italy | 21 | N | NR | NR | NR | 82 aa | 21 | 67 |
| Riche 17 | Brazil | 11 | N | NR | NR | NR | 10‐12 bb , cc | 11 | 73 |
| Bishburg 18 | USA | 8 | Y | NR | 8.9 | NR | NR | 8 | 25 |
| Nucci 19 | Brazil | 9 | N | 100 | 1.5 | 15 | NR | 9 | 56 |
| Current | USA | 12 | Y | 92 | 5.1 | 51 | NR | 13 | 31 |
Abbreviations: ICU, intensive care unit; MV, mechanical ventilation; NR, not reported.
Expressed per 1000 admissions
Studies of both bacterial and fungal bloodstream infection
Sixty‐seven percent of the isolates in this study were Candida auris
Study of invasive mould and yeast infections
Expressed per 10,000 patient‐days of follow up
Expressed per 1000 patient‐days
Two different hospitals were studied
The third major contribution of this study is the comparison between candidaemic ICU patients without COVID and those with COVID in our institution. We detected a nearly 5‐fold greater incidence of ICU candidaemia among COVID patients as compared to non‐COVID patients. Such an increase is consistent with other early reports of comparative incidence available at the time of this writing (Table 3). Mastrangelo et al found a nearly six‐fold greater incidence density of candidaemia expressed per 10,000 patient‐days of follow up in the ICU subset of their COVID patients as compared to historical non‐COVID controls. Reporting candidaemia incidence density per 1000 patient‐days, Riche et al found a greater than 8‐fold increase in their ICUs. Nucci et al expressed their findings as an incidence rate per 1000 admissions, making their results the most directly comparable to ours. They found a 3‐fold difference in the incidence rate of candidaemia favouring COVID patients over non‐COVID patients admitted during the same time period: 15/1000 admissions versus 5/1000 admissions, respectively. It is once again important to recall that the non‐COVID groups in these studies contain variable percentages of surgical and oncological cases, patient types missing from our non‐COVID population as per the study design.
We found no significant differences between the non‐COVID and COVID groups with respect to demographics or traditional risk factors for IC, including corticosteroid exposure. This latter finding was somewhat surprising given the widespread use of corticosteroids specifically for the treatment of SARS‐CoV‐2 lung disease, although Mastrangelo et al also encountered a similar lack of significant difference in this parameter. However, of the COVID patients, six had received at least one dose of pulse corticosteroids (ie, methylprednisolone 1gm), raising a question about possibly greater intensity of corticosteroid exposure in the COVID patients, which has been proposed as an explanation for the dramatic spike in candidaemia incidence in their institution by Riche et al The lower SOFA score on ICU admission of the non‐COVID group likely reflects the often isolated respiratory failure that characterises critical illness due to COVID, at least at ICU entry. Notably, COVID patients developed candidaemia significantly later in their ICU course than did non‐COVID patients. A trend towards longer hospital stays preceding onset of candidaemia in COVID cases was likewise observed by Mastrangelo et al and by Nucci et al, though in the latter study only when comparing contemporaneous non‐COVID and COVID admissions. Besides the present study, to our knowledge, these are the only other studies to have made this comparison. This pattern suggests that in critically ill COVID patients, candidaemia may be a feature of prolonged ICU stay to a greater degree than it is for their non‐COVID counterparts. We also compared CVC dwell time, and found it to be significantly longer in the COVID group. This was an expected finding given the naturally higher threshold for CVC manipulations at the height of the pandemic from a staff exposure perspective. Taken together, the longer ICU stay and CVC dwell time prior to detection of IC that differentiate our COVID group from the non‐COVID group suggest that the increased candidaemia incidence rates emerging in reports such as ours may stem from the prolonged stays and extended CVC days that appear to characterise the ICU course of these patients. The precise impact of length of ICU stay, CVC dwell time, and intensity of corticosteroid exposure on the development of candidaemia in critically ill patients with COVID remains in need of further elucidation as these patients may differ from traditional medical ICU patients with respect to these parameters. The high crude hospital mortality rate of non‐COVID candidaemic patients (61%) and ones with COVID (75%) is comparable with reports from both within 6 , 17 , 19 and outside 20 , 21 , 22 the pandemic era.
Our study suffers from a number of limitations, primary among them its retrospective nature and the interrelated problems of single‐centre design and relatively small sample size. We made the unconventional decision to exclude not only neutropenic patients, which is typical but also active oncological patients, the rationale for this decision having been discussed earlier. The justification for the latter exclusion is debatable. Additionally, we compared COVID patients whose admissions were limited to a 5‐month‐period in 2020 to non‐COVID patients some of whose admissions dated to as far back as 2014. As a result, evolution in ICU management approaches over the span of the study may have influenced the comparison. Finally, we reported corticosteroid exposure as a binary variable rather than calculating the cumulative corticosteroid dosing, which may have masked the impact of intensity of corticosteroid therapy.
This single‐centre retrospective study from an academic tertiary referral centre in the United States provides an estimate of the incidence of IC as measured by candidaemia in a population of non‐neutropenic, non‐oncological medical ICU patients. Furthermore, this study adds the United States to the shortlist of countries whose ICUs have reported non‐albicans Candida together comprising the majority of candidaemia isolates. Finally, we add to the small but growing body of literature comparing non‐COVID ICU candidaemia patients to those with COVID; we observed a lower ICU admission SOFA score in the COVID group, indicating a tendency of these patients to enter the ICU with dysfunction of fewer organs; we also found a greater ICU length of stay prior to the development of candidaemia in the COVID group, suggesting that it is a more delayed phenomenon in the course of critical illness in these patients. The longer CVC dwell duration at time of candidaemia in the COVID group of our study merits consideration as one of the reasons for the recent increase in the incidence of this infection in the ICU brought about by the impact of the SARS‐CoV‐2 pandemic on vascular catheter management.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION
Precious Macauley: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing‐original draft (equal). Oleg Epelbaum: Conceptualization (equal); Methodology (supporting); Supervision (equal); Writing‐review & editing (equal).
ACKNOWLEDGEMENT
None.
Macauley P, Epelbaum O. Epidemiology and mycology of candidaemia in non‐oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non‐COVID‐19 and COVID‐19 cases. Mycoses. 2021;64:634–640. 10.1111/myc.13258
REFERENCES
- 1. Chakrabarti A, Sood P, Rudramurthy SM, et al. Incidence, characteristics and outcome of ICU‐acquired candidemia in India. Intensive Care Med. 2015;41:285‐295. [DOI] [PubMed] [Google Scholar]
- 2. Motoa G, Muñoz JS, Oñate J, Pallares CJ, Hernández C, Villegas MV. Epidemiology of Candida isolates from Intensive Care Units in Colombia from 2010 to 2013. Rev Iberoam Micol. 2017;34:17‐22. [DOI] [PubMed] [Google Scholar]
- 3. da Silva RB, Neves RP, Hinrichsen SL, de Lima‐Neto RG. Candidemia in a public hospital in Northeastern Brazil: epidemiological features and risk factors in critically ill patients. Rev Iberoam Micol. 2019;36:181‐185. [DOI] [PubMed] [Google Scholar]
- 4. Bougnoux ME, Kac G, Aegerter P, d'Enfert C, Fagon JY, CandiRea Study Group . Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med. 2008;34:292‐299. [DOI] [PubMed] [Google Scholar]
- 5. Marriott DJE, Playford EG, Chen S, et al. Determinants of mortality in non‐neutropenic ICU patients with candidaemia. Crit Care. 2009;13:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mastrangelo A, Germinario BN, Ferrante M, et al. Candidemia in COVID‐19 patients: incidence and characteristics in a prospective cohort compared to historical non‐COVID‐19 controls. [published online ahead of print, 2020 Oct 30].Clin Infect Dis. 2020;ciaa1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holley A, Dulhunty J, Blot S, et al. Temporal trends, risk factors and outcomes in albicans and non‐albicans candidaemia: an international epidemiological study in four multidisciplinary intensive care units. Int J Antimicrob Agents. 2009;33:554.e1‐554.e7. [DOI] [PubMed] [Google Scholar]
- 8. Shahin J, Allen EJ, Patel K, et al. Predicting invasive fungal disease due to Candida species in non‐neutropenic, critically ill, adult patients in United Kingdom critical care units. BMC Infect Dis. 2016;16:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single‐center retrospective 10‐year analysis. Ann Intensive Care. 2020;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cataldo MA, Tetaj N, Selleri M, et al. Incidence of bacterial and fungal bloodstream infections in COVID‐19 patients in intensive care: an alarming "collateral effect.". J Glob Antimicrob Resist. 2020;23:290‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID‐19. Eur J Clin Invest. 2020;50:e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonazzetti C, Morena V, Giacomelli A, et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49:e31‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID‐19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19:102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Hatmi AMS, Mohsin J, Al‐Huraizi A, Khamis F. COVID‐19 associated invasive candidiasis. J Infect. 2021;82(2):e45‐e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug‐resistant candida auris infections in critically ill coronavirus disease patients, India, April‐July 2020. Emerg Infect Dis. 2020;26:2694‐2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White PL, Dhillon R, Cordey A, et al. A National strategy to diagnose COVID‐19 associated invasive fungal disease in the ICU.Clin Infect Dis. 2020;ciaa1298. [published online ahead of print, 2020 Aug 29]. [Google Scholar]
- 17. Riche CVW, Cassol R, Pasqualotto AC. Is the frequency of candidemia increasing in covid‐19 patients receiving corticosteroids? J Fungi (Basel). 2020;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bishburg E, Okoh A, Nagarakanti SR, Lindner M, Migliore C, Patel P. Fungemia in covid ‐19 ICU patients, a single medical center experience. J Med Virol. 2020. 10.1002/jmv.26633. [published online ahead of print, 2020 Oct 27]. [DOI] [PubMed] [Google Scholar]
- 19. Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID‐19 pandemic.Mycoses. 2021;64(2):152‐156. 10.1111/myc.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leroy O, Gangneux JP, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med. 2009;37:1612‐1618. [DOI] [PubMed] [Google Scholar]
- 21. Lortholary O, Renaudat C, Sitbon K, et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014;40:1303‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colombo AL, Guimarães T, Sukienik T, et al. Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9‐year period. Intensive Care Med. 2014;40:1489‐1498.[published correction appears in Intensive Care Med. 2014 Dec; 40(12):1974] [DOI] [PMC free article] [PubMed] [Google Scholar]


