Abstract
In this study, we aimed to investigate the changes of lymphocyte subsets (CD3+, CD4+, CD8+) and inflammatory factors (interleukin‐6 [IL‐6], hypersensitive C‐reactive protein [HS‐CRP], and procalcitonin [PCT]) of alveolar lavage fluid in patients with severe corona virus‐2019 (COVID‐19) pneumonia and their clinical impact on the assessment of disease severity and prognosis. Twenty‐four patients with severe COVID‐19 pneumonia were admitted to the intensive care unit (ICU) of the Ezhou Central Hospital from February 1 to March 22, 2020. According to the 28‐day prognosis, they were assigned to a death group and a survival group. On the 3rd day of ICU admission, peripheral blood and alveolar lavage fluid were collected for examination of lymphocyte subsets and inflammatory factors by flow cytometry and immunoturbidimetry, respectively. The CD3+, CD4+, and CD8+ cell counts in alveolar lavage fluid and serum were significantly higher in the survival group than those of the death group (p < .05). The levels of IL‐6, HS‐CRP, and PCT in the alveolar lavage fluid and serum of the death group were statistically higher than those of the survival group (p < .05); The CD3+, CD4+ cell count, and IL‐6 level were negatively correlated with Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II scores, respectively (p < .05). The CD4+ cell and SOFA score have a regression relationship for the prognosis of COVID‐19 severe patients. The CD3+, CD4+, CD8+ cells, and IL‐6 levels are valuable in determining the prognosis of severe COVID‐19 pneumonia and are strongly correlated with the severity of the disease; the CD4+ cell is an independent risk factor affecting the prognosis of COVID‐19 pneumonia.
Keywords: alveolar lavage fluid, cellular immunity, COVID‐19, inflammatory factors
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2) is a non‐segmented single‐stranded RNA virus with an envelope, with a diameter of about 50–200 nm, 1 it is the seventh member of the coronavirus family that can infect humans. In December 2019, an outbreak of corona virus‐2019 (COVID‐19) began in Wuhan, China, spreading to 206 countries or regions around the world, gradually becoming a public health emergency of global concern. Until now, WHO reports more than 85 million confirmed cases, with over 1.8 million deaths. China has reported more than 80,000 confirmed cases and more than 3000 deaths, with a mortality rate of 4.0% 2 , 3 , 4 , 5 ; existing research results indicate that SARS‐CoV‐2 has angiotensin‐converting enzyme 2 (ACE2) receptor binding domain, which can enter host cells through ACE2 6 and cause lung injury by invading cells with ACE2 as the receptor, and in addition to directly causing damage to lung tissue, the cytokine storm it triggers further aggravates the inflammatory response and can even develop into a systemic inflammatory response accompanied by shock, diffuse intravascular coagulation, and multiple organ failure (MOF). 2 , 7 , 8 Furthermore, a currently published retrospective study of COVID‐19 cases shows that cytokine storm (cytokine release syndrome, CRS) is associated with high mortality of the disease 9 and the progressive increase of inflammatory factors such as C reactive protein (CRP) and interleukin‐6 (IL‐6) in peripheral blood can be used as early warning indicators, suggesting that CRP and IL‐6 are of great significance to the severity of COVID‐19 disease. 10 The level of T lymphocyte subsets has been found to provide certain guidance for the clinical treatment and prognosis of patients with viral pneumonia, 2 and T lymphocyte subsets participate in immune regulation and play an important role in the body's immune system against viral infections. Among them, CD4+ and CD8+ cells are important components of the T lymphocyte subsets that maintain the body in a stable state of immunity and thus can resist the invasion of external pathogens. 11
Previous studies have only described the general epidemiological characteristics, clinical manifestations, and outcomes of COVID‐19 pneumonia. However, evidence regarding the cellular immunity and inflammatory factor assays of alveolar lavage fluid in patients who suffer from severe COVID‐19 pneumonia remains unknown. In this study, by examining the changes of cellular immunity and inflammatory factors in the alveolar lavage fluid and serum of patients with severe COVID‐19 pneumonia, we attempt to understand the impact of immune function and inflammatory response on the progression and prognosis of disease to provide a theoretical basis for the immune status and systemic inflammatory response of patients with severe COVID‐19 pneumonia.
2. MATERIALS AND METHODS
2.1. Research object
A total of 24 severe COVID‐19 pneumonia patients in the intensive care unit (ICU) of the Ezhou Central Hospital (Ezhou, Hubei) from February 1, 2020, to March 22, 2020, aged 40–79 years, mean age (64.7 ± 13.42) years old, including 17 males and 7 females. COVID‐19 pneumonia with primary disease: four cases of chronic obstructive pulmonary disease (COPD), two cases of heart disease (coronary heart disease, cardiac arrhythmia and chronic heart failure), two cases of type II diabetes, and two cases of hypertension.
2.2. Diagnostic and exclusion criteria
Diagnostic criteria: All patients are eligible for the intermediate‐severe type of the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (7th Version)” 10 : which is defined as the occurrence of respiratory failure and the need for mechanical ventilation (MV), the presence of shock, and the combination of other organs failures requiring ICU monitoring treatment.
Exclusion criteria: (1) patients with autoimmune diseases, immunodeficiency diseases, hypersensitivity diseases; (2) patients with malignant tumors and radiochemotherapy; (3) patients who were taking hormones and immunomodulators within 3 months; (4) pregnant women; (5) negative result for SARS‐CoV‐2 in throat swabs by reverse‐transcription polymerase chain reaction (RT‐PCR) test. According to the testing guidelines issued by the National Health Commission of China, 10 a specific PCR test for two targets (ORF1ab and N) of the SARS‐CoV‐2 in one sample is required to improve specificity, and if both Ct values are less than 37, the patient is reported positive and the infection is confirmed. The nucleic acid detection kit is produced by Shenzhen BGI, China, and the PCR instrument provided by Thermo Fisher Scientific.
2.3. Grouping of research subjects
According to the 28‐day prognosis, patients with severe COVID‐19 pneumonia were assigned to a survival group and a death group.
Survival group: the clinical symptoms of patients and relevant laboratory indicators have improved, and the survival time exceeded 28 days.
Death group: the clinical symptoms have deteriorated, and the relevant laboratory examination indicators did not improve or even worsened, and the patient died within 28 days of hospitalization.
Ten patients were in the survival group, including seven males and three females, aged 40–76 years, with an average age of (61.30 ± 13.40) years. 14 patients were involved in the death group, including 10 males and 4 females, aged 45‐80 years, with an average age of (65.80 ± 12.40) years.
2.4. Treatments
All patients were treated in accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (7th edition) 5 : invasive MV, antiviral, anti‐infection, fluid resuscitation, organ protection, maintenance of water‐electrolyte acid–base balance, nutrition support, hormones, Chinese herbal medicine, and other supportive treatments.
2.5. Testing indicators
All patients' age, gender, and existing primary disease, clinical vital signs (breathing, pulse, blood pressure, body temperature, and GCS scores after transferring to ICU are recorded. Within 24 h of admission to ICU, 5 ml of peripheral venous blood was collected for routine blood tests, electrolytes, liver function, kidney function, and blood gas analysis, and so on. Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) score are calculated. On the 3rd day of admission to the ICU, peripheral blood and alveolar lavage fluid were collected and sent to detect lymphocyte subsets (CD3+, CD4+, CD8+ cells) and inflammatory mediators (IL‐6, hypersensitive CRP [HS‐CRP], procalcitonin [PCT]). The alveolar lavage operation technique is in accordance with the Expert Consensus of the Chinese Medical Association Respiratory Branch Regarding Pulmonary Infectious Disease Bronchial Alveolar Lavage Pathogen Detection and Specimen Collection (2017 Edition). 12
2.6. Laboratory testing methods
Lymphocyte subsets and inflammatory mediator specimens were tested at the clinical laboratory center of the Ezhou Central Hospital (Ezhou, Hubei). Lymphocyte subgroups were detected using flow cytometry. Inflammatory factors such as IL‐6, PCT, and HS‐CRP were measured by immunoturbidimetry.
2.7. Evaluation indicators
Comparing the survival group and death group with alveolar lavage fluid and peripheral blood on CD3+, CD4+, CD8+, IL‐6, HS‐CRP, and PCT.
Pearson correlation analysis of pulmonary alveolar lavage fluid and peripheral blood on CD3+, CD4+, CD8+, IL‐6, HS‐CRP, and PCT with SOFA and APACHE II score, respectively.
Application of binary logistic regression was adopted to analyze the prognostic factors affecting severe COVID‐19 patients.
2.8. Statistical methods
SPSS23.0 (SPSS Inc.) was used for statistical analysis. The measurement data are expressed as mean ± SD. The homogeneity of variance is analyzed by one‐way ANOVA, LSD‐t‐Test, and t‐test, uneven variance using the Kruskal‐Wallis test. The adoption rate and composition ratio (%) of count data are described. Pearson correlation analysis and binary logistic regression analysis were used. Diagnostic efficacy was analyzed by plotting the receiver operating curve (ROC). p < .05 indicates a statistical difference.
3. RESULTS
3.1. Comparison of gender, age, and primary disease in the survival group and death group
There was no statistically significant difference between the survival group and death group in gender and age (p > .05), and there was no significant difference in the primary disease between the survival group and death group (p > .05) (Table 1).
Table 1.
Gender, age and primary disease in the survival group and death group ( ± S)
| Gender | Primary diseases | ||||||
|---|---|---|---|---|---|---|---|
| Group | No. of cases | (male/female) | Age | COPD | Heart disease | Hypertension | Diabetes |
| Survival group | 10 | 7/3 | 61.3 ± 13.4 | 2 | 0 | 1 | 1 |
| Death group | 14 | 10/4 | 65.8 ± 12.4 | 2 | 2 | 1 | 1 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
3.2. Comparison of APACHE II and SOFA scores in survival group and death group
The SOFA and APACHE are two scoring systems that determine the prognosis of patients by measuring the degree of impairment of major organ function and are mainly used in ICU units. The higher the score, the higher the risk of death. In the study, the scores of SOFA and APACHE II were higher in the death group compared to the survival group (p < .05) (Table 2).
Table 2.
Comparison of SOFA score and APACHE II score between survival group and death group ( ± S)
| Group | Number of cases | SOFA Score | APACHE II Score |
|---|---|---|---|
| Survival group | 10 | 3.22±2.06 | 14.85 ± 4.46 |
| Death Group | 14 | 8.86±2.84a | 24.08±3.78a |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
p<0.05 comparison between two groups.
3.3. Comparison of lymphocyte subsets and inflammatory factors in alveolar lavage fluid and serum between survival group and death group
It is well known that lymphocyte subsets are closely associated with cellular immunity in humans. In terms of general trends, whether in serum or in alveolar lavage fluid, the lymphocyte subsets of the death group are greatly decreased compared to the survival group. on the other hand, there was a significant increase in the related inflammatory factors in the death group (Figure 1). Specifically, the cell counts of CD3+, CD4+, and CD8+ in alveolar lavage fluid and serum in the survival group were significantly higher compared to the death group and were significantly lower in the alveolar lavage fluid of the death group than those in the serum (p <.05); the levels of IL‐6, HS‐CRP, and PCT of the alveolar lavage fluid and serum in the survival group were lower compared to the death group, in addition, these inflammatory factors were significantly higher in the alveolar lavage fluid of the death group than those in the serum group (p < .05) (Table 3).
Figure 1.
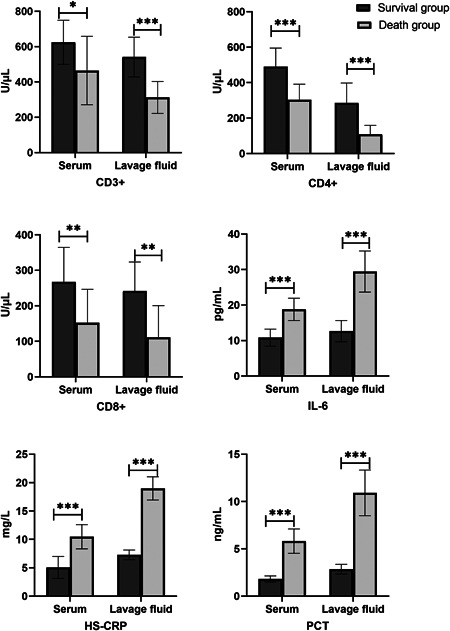
Lymphocyte subsets (CD3+, CD4+, CD8+ cells) and inflammatory factors (IL‐6, HS‐CRP, PCT) in alveolar lavage fluid and serum between survival group and death group on COVID‐19 patients. Data are expressed as mean ± SD and were compared with the t test. COVID‐19, coronavirus disease 2019; HS‐CRP, hypersensitive c‐reactive protein; IL‐6, interleukin‐6; PCT, procalcitonin. *p < .05, **p < .01,***p < .001
Table 3.
Comparison of immune and inflammatory factors in serum and alveolar lavage fluid in survival and death groups
| Item | Survival group (n = 10) | Death group (n = 14) | |||
|---|---|---|---|---|---|
| CD3+ (/μl) | |||||
| Serum | 624.70 ± 124.76 | 464.83±193.91a | |||
| Lavage fluid | 541.43 ± 112.39 | 312.45±90.05b | |||
| CD4+ (/μl) | |||||
| Serum | 490.50 ± 104.10 | 303.23±88.01a | |||
| Lavage fluid | 285.53 ± 112.39 | 108.13±50.82b | |||
| CD8+ (/μl) | |||||
| Serum | 267.57 ± 97.01 | 152.23±94.27a | |||
| Lavage fluid | 241.63 ± 81.97 | 111.44±89.15b | |||
| IL‐6 (pg/ml) | |||||
| Serum | 10.82 ± 2.41 | 18.79±3.14a | |||
| Lavage fluid | 12.66 ± 2.98 | 29.44±5.80b | |||
| HS‐CRP (mg/L) | |||||
| Serum | 5.06 ± 1.93 | 10.48±2.13a | |||
| Lavage fluid | 7.28 ± 0.85 | 18.99±2.05b | |||
| PCT (ng/ml) | |||||
| Serum | 1.81 ± 0.33 | 5.82±1.29a | |||
| Lavage fluid | 2.86 ± 0.51 | 10.92±2.42b | |||
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; HS‐CRP, hypersensitive C‐reactive protein; IL‐6, interleukin‐6; PCT, procalcitonin; SOFA, sequential organ failure assessment.
p < .05 comparison of serum between two groups.
p < .05 comparison of alveolar lavage fluid between two groups.
3.4. Correlation analysis of immune and inflammatory factor indexes with SOFA score and APACHE II score in patients with severe COVID‐19 pneumonia
The levels of CD3+, CD4+ cells, and IL‐6 in alveolar lavage fluid of patients with severe COVID‐19 pneumonia were negatively correlated with the SOFA score and APACHE II score, respectively (p < .05). In addition, the levels of CD8+ cells, PCT, and HS‐CRP were not correlated with SOFA score and APACHE II score, respectively (p > .05) (Table 4).
Table 4.
Correlation analysis of immune and inflammatory factor indexes with SOFA score and APACHE II score in critical COVID‐19 pneumonia
| r | ||
|---|---|---|
| Item (n = 60) | SOFA | APACHE II |
| CD3+ | −0.516a | −0.559a |
| CD4+ | −0.549a | −0.648a |
| CD8+ | −0.161 | −0.070 |
| IL‐6 | 0.252a | 0.041a |
| HS‐CRP | 0.130 | −0.039 |
| PCT | 0.035 | 0.077 |
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; HS‐CRP, hypersensitive C‐reactive protein; IL‐6, interleukin‐6; SOFA, sequential organ failure assessment.
p < .05.
3.5. Logistic analysis of factors affecting the prognosis of severe COVID‐19 pneumonia
Taking the 28‐day death and survival of the patient as the dependent variables, it may be related to the prognosis of severe COVID‐19 pneumonia, and the statistically significant indicators (CD3+, CD4+ cells, APACHE II score, SOFA score) are independent variables, the results of logistic regression analysis indicated that the SOFA score, CD4+ had a regressive relationship with the prognosis of sepsis patients (Table 5), suggesting that the levels of CD4+ and SOFA score are independent risk factors affecting the prognosis of patients with severe COVID‐19 pneumonia.
Table 5.
Logistic regression analysis of prognostic factors in patients with severe COVID‐19 pneumonia
| Variable | B | Wald | Sig. | Exp(B) |
|---|---|---|---|---|
| SOFA | 0.320 | 3.860 | 0.049 | 1.377 |
| CD4+ | −0.009 | 3.930 | 0.047 | 0.991 |
| Constant | −1.160 | 1.767 | 0.431 | 0.314 |
3.6. Plotting the receiver operating curve (ROC)
The area under the CD4+ curve is 0.777 (77.7%) with a sensitivity of 90.5% and the specificity of 53.8%. The area under the SOFA score curve is 0.775 (77.5%), the sensitivity is 85.7%, and the specificity is 59.0% (Table 6, Figure 2). The area under the CD4+ curve is 77.7%, while the area under the SOFA score curve is 77.5%, suggesting that the CD4+ have a certain clinical value in determining the prognosis of patients with severe COVID‐19 pneumonia instead of the SOFA.
Table 6.
ROC analysis of factors affecting the prognosis of severe COVID‐19 pneumonia
| Variable | AUC | p value | Sensitivity | Specificity |
|---|---|---|---|---|
| SOFA | 0.775 | .000 | 85.7 | 59.0 |
| CD4+ | 0.777 | .000 | 90.5 | 53.8 |
Abbreviations: COVID‐19, coronavirus disease 2019; ROC, receiver operating curve; SOFA, Sequential Organ Failure Assessment.
Figure 2.
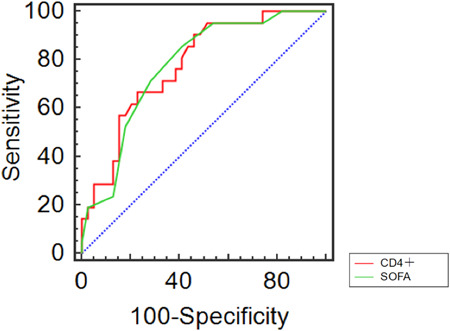
ROC curve of prognostic factors in patients with severe COVID‐19 pneumonia.COVID‐19, coronavirus disease 2019; ROC, receiver operating curve; SOFA, Sequential Organ Failure Assessment
4. DISCUSSION
Pneumonia caused by COVID‐19 has strong infectivity and a high mortality rate, threatening the lives of people all over the world. 5 The SARS‐CoV outbreak in 2003 can not only infect lung tissue but also other tissues and organs that express ACE2 protein, unfortunately, the SARS‐CoV‐2 in 2019 has also been confirmed to have such characteristics. 13 , 14 , 15 Gurzu et al. 16 have reported that lung injury caused by SARS‐CoV‐like infection leads to pathological changes in multiple organs throughout the body, such as liver centrilobular fatty change, splenomegaly, proliferative glomerulonephritis, which may cause multiple organ dysfunction syndrome (MODS) eventually.
SARS‐CoV‐2 infection mainly affects the respiratory system with typical clinical symptoms of fever, dry cough, fatigue, dyspnea, hypoxemia, acute respiratory distress syndrome (ARDS), and multiple organ insufficiency. 16 Recent reports have suggested that SARS‐CoV‐2 infection can also affect the cardiovascular system, causing acute myocardial injury with rapid progression and poor prognosis in severe cases. 2 , 8 , 9 Based on previous studies related to SARS‐CoV and the Middle East Respiratory Syndrome Coronavirus (MERS‐CoV), it is currently hypothesized that acute myocardial injury in patients with COVID‐19 is associated with direct viral invasion of myocardial cells, severe hypoxemia‐related myocardial oxygen supply‐demand imbalance, and excessive inflammatory response‐mediated myocardial injury. The myocardium is a highly oxygen‐dependent tissue, and in addition to coronary blood flow, blood oxygen content plays an equally important role in maintaining normal myocardial metabolism. Several studies have further demonstrated that severe and persistent hypoxemia can lead to the development of acute myocardial injury through various mechanisms, such as vascular endothelial cell dysfunction and apoptosis, 17 , 18 , 19 mitochondrial functional impairment, 20 , 21 , 22 and vasospasm. 23
The occurrence of urinary complications in COVID‐19 patients may be related to organ ischemia and hypoxia, high ACE2 expression, inflammatory factors, age, drugs, and other factors. Firstly, it is well known that the kidney is an organ with high blood flow and high perfusion, which is more sensitive to changes in oxygen supply and tension, and is therefore susceptible to hypoxic injury when the organism is hypoxic and ischemic. Patients with severe COVID‐19 are usually treated by MV in a hypoxic condition. Firstly, MV can reduce the blood return to the heart and alter the hemodynamics of the heart, which in turn leads to a decrease in glomerular filtration rate and subsequent renal injury. 24 Secondly, due to the high expression of ACE2 in renal tubules, 25 SARS‐CoV‐2 may bind to ACE2 in the kidney and cause renal injury. A study found that SARS‐CoV‐2 has a higher affinity to ACE2 protein than SARS‐CoV, 26 which might be the reason why SARS‐CoV‐2 is more likely to cause kidney injury. In addition, a few studies 27 , 28 have indicated that some inflammatory factors can exacerbate kidney injuries, such as tumor necrosis factor and IL‐6. Moreover, Li et al. 29 have reported that cytokine storm may be an important mechanism of viral sepsis and MODS in patients with COVID‐19, including acute kidney injury. Furthermore, some autopsy findings have revealed that extensive endothelial cell damage in the heart, kidney, small intestine and liver in COVID‐19, 30 triggering excessive thrombin production, inhibiting fibrinolysis, and activating the complement pathway, causing a thrombotic inflammatory response, ultimately leading to microthrombus deposition and microvascular dysfunction, which may promote further evolution of acute tubular necrosis into cortical necrosis, resulting in irreversible renal failure. 16 , 31
Obviously, SARS‐CoV‐2 can affect the cellular immunity of patients. T lymphocytes are involved in the cellular immune function of the body and can be divided into two subgroups, CD4+ and CD8+ T lymphocytes, which have killing and auxiliary killing functions, respectively. One clinical study has demonstrated that most patients with SARS‐CoV‐2 infection have decreased lymphocytes, 32 in addition, another study has shown that a significant decrease in the total number of lymphocytes indicates that coronavirus consumes many immune cells and suppresses the immune function of human cells, and the damage of T lymphocytes may be an important factor leading to the deterioration of the patient's condition. 33 There are differences in T lymphocyte subsets between critical and noncritical groups, but CD4+ T lymphocytes were more significantly decreased than CD8+ T lymphocytes in the critical group. 34 During the SARS outbreak in 2003, investigators also paid more attention to the T lymphocyte subsets. In the lung interstitium of SARS patients, CD8+ T cells account for approximately 80% of total infiltrating inflammatory cells and play an important role in clearing SARS‐CoV from infected cells and inducing immune damage. 35 The depletion of CD4+ T cells is associated with recruitment of lymphocytes in the lung, neutralizing antibodies and decreased cytokine production, resulting in strong immune response and interstitial pneumonia, which delays the clearance of SARS‐CoV from the lung. 36 The combination of virus replication in the lower respiratory tract and abnormal immune response may have an impact on the severity of the disease, similar to what has been demonstrated in SARS and MERS. 33 Chiu, W et al. 37 have found that the level of T lymphocytes is strongly associated with the prognosis of patients with severe influenza‐A virus infection and that patients with low CD4+ T cells have a higher risk of death within 28 days after admission. In inclusion of more than 100 SARS‐CoV‐2 infection cases with obesity, diabetes, and cardio‐cerebrovascular diseases also have continuously decreased in lymphocytes and increased ferritin and cytokines, such as IL‐6, which may cause the disease rapidly developed into ARDS and prolonged disease course. 32 As a cytokine, IL‐6 has a wide range of functions and diverse biological efficacies. It can be produced by a variety of cells, such as vascular endothelial cells, monocytes and macrophages, and helper T cells. A study showed that IL‐6 is an important mediator synthesized during acute inflammation and an important cytokine involved in the inflammatory response, playing a role in the induction of acute‐phase proteins synthesis in hepatocytes and pro‐inflammatory effects on a variety of cells. 38 In the early stage of infection, an appropriate increase in IL‐6 can help maintain the internal homeostasis of the body, defend against various pathogens and help control the progression of inflammation. However, its large release can induce excessive inflammatory response and damage to tissues and organs, as well as induce a series of pathological changes in the microcirculation and vascular endothelium, 39 , 40 which may be related to the occurrence of acute myocardial injury in COVID‐19 patients. Nevertheless, a recent study from Romania has shown that the level of IL‐6 in serum is not necessarily related to inflammation at the tissue level in the early stage of infection, suggesting that it may not be an appropriate indicator for diagnosing and predicting the onset of mild inflammation. 41
In this study, we found that in patients with severe COVID‐19 pneumonia, CD3+, CD4+, CD8+ cell counts of the alveolar lavage fluid and serum in the survival group were significantly higher than those in the death group. In the survival group, the IL‐6, HS‐CRP, and PCT of the level alveolar lavage fluid and serum were lower than those in the death group, the difference was statistically significant (p < .05); suggesting that the T lymphocyte count and inflammatory factors are positively correlated with the severity of the disease. The study also found that the levels of CD3+, CD4+, CD8+ in alveolar lavage fluid of the death group were significantly lower than those in the serum, while the levels of IL‐6, PCT, and HS‐CRP, were higher than those in the serum, indicating that viral infection leads to abnormal oxygen and carbon dioxide exchange in the alveoli, as well as secondary inflammation‐mediated immune response disorder, which results in rapid disease progression. The correlation analysis of this study found that the levels of CD3+, CD4+, and IL‐6 were significantly negatively correlated with SOFA and APACHE II score, suggesting that with the multiple organ dysfunction, the decrease of CD3+ and CD4+ and the increase of IL‐6 are related to the severity of COVID‐19 pneumonia.
To accurately assess the criticality of a patient's condition, ICU physicians usually need to collect a variety of clinical laboratory data to calculate SOFA scores, including oxygenation index, platelet count, bilirubin, blood pressure, Glasgow score, creatinine, and urine output, which is a relatively time‐consuming process. By performing a binary logistic analysis in this study, the CD4+ cells and SOFA score were considered as independent risk factors affecting the prognosis of patients with severe COVID‐19 pneumonia. Furthermore, the area under the curve of CD4+ was 77.7% through the ROC curve, suggesting that the level of CD4+ cell has a certain clinical value in determining the prognosis of patients with COVID‐19 pneumonia and its diagnostic efficacy is not different from SOFA with the area of 77.5% under the curve.
However, there are a few limitations of this study: first, this is a single‐center study, so the sample size is not large enough and there may be biases in the results. Second, this study lacks the correlations between these inflammatory factors and lymphocytes in serum and tissues. In this case, autopsies would be useful. Finally, we were unable to further investigate the long‐term prognosis of patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Liang Liao performed the sample collections, medical care and attention, and statistics. Guohui Yang offered guidance for this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethics committee of the Ezhou Central Hospital approved the study. As the disease was highly contagious, written informed consent was abandoned.
Liao L, Yang G‐H. Clinical significance of cellular immunity function and inflammatory factors assays in alveolar lavage fluid for severe COVID‐19 pneumonia. J Med Virol. 2021;93:2979‐2987. 10.1002/jmv.26827
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457‐460. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heymann DL, Shindo N, Bedford J, et al. COVID‐19: what is next for public health? Lancet. 2020;395(10224):542‐545. 10.1016/S0140-6736(20)30374-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019‐nCoV) patients. Can J Anesth. 2020;67(5):568‐576. 10.1007/s12630-020-01591-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Gayle AA, Wilder‐Smith A, Rocklov J. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2):taaa021. 10.1093/jtm/taaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33(4):869‐889. 10.1016/j.idc.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei P‐F. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J. 2020;133(9):1087‐1095. 10.1097/cm9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braun J, Loyal L, Frentsch M, et al. SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature. 2020;587(7833):270‐274. 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 12. Wang E, Zhu M, Bu H, Chen J, Sun B. Consensus of Chinese experts on detection of related drive genes in target therapy of non‐small cell lung cancer. Zhonghua Bing Li Xue Za Zhi, 45(2):73‐77. [DOI] [PubMed] [Google Scholar]
- 13. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622‐630. 10.1002/path.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurzu S, Satala CB, Melit LE, et al. COVID‐19 like findings in a fatal case of idiopathic desquamative interstitial pneumonia associated with IgA glomerulonephritis in a 13‐month‐old child. Front Pediatr. 2020;8:586666. 10.3389/fped.2020.586666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54(6):1425‐1430. [PubMed] [Google Scholar]
- 18. Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280(4):C719‐C741. 10.1152/ajpcell.2001.280.4.C719 [DOI] [PubMed] [Google Scholar]
- 19. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF‐kappaB. J Appl Physiol. 2008;105(4):1333‐1341. 10.1152/japplphysiol.90470.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Y, Liu Y, Dorn GW, 2nd . Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109(12):1327‐1331. 10.1161/CIRCRESAHA.111.258723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294(2):C460‐C466. 10.1152/ajpcell.00211.2007 [DOI] [PubMed] [Google Scholar]
- 22. Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. 10.1038/cddis.2011.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nunez C, Victor VM, Marti M, D'Ocon P. Role of endothelial nitric oxide in pulmonary and systemic arteries during hypoxia. Nitric Oxide. 2014;37:17‐27. 10.1016/j.niox.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 24. Abreu KLS, Silva Junior GB, Muniz TD, et al. Acute kidney injury in critically ill patients with lung disease: kidney‐lung crosstalk. Rev Bras Ter Intensiva. 2013;25(2):130‐136. 10.5935/0103-507X.20130024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang YM, Zhang H. Genetic roadmap for kidney involvement of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. Clin J Am Soc Nephrol. 2020;15(7):1044‐1046. 10.2215/CJN.04370420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID‐19 outbreak: a study based on single‐cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114‐1116. 10.1007/s00134-020-06026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McAdoo SP, Pusey CD. Is there a role for TNFalpha blockade in ANCA‐associated vasculitis and glomerulonephritis? Nephrol Dial Transplant. 2017;32(suppl_1):i80‐i88. 10.1093/ndt/gfw361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durlacher‐Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh‐Many T. Interleukin‐6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315‐325. 10.1016/j.kint.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 29. Li H, Liu L, Zhang D, et al. SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517‐1520. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bikdeli B, Madhavan MV, Gupta A, et al. Pharmacological Agents Targeting Thromboinflammation in COVID‐19: review and Implications for Future Research. Thromb Haemost. 2020;120(7):1004‐1024. 10.1055/s-0040-1713152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu WJ, Zhao M, Liu K, et al. T‐cell immunity of SARS‐CoV: implications for vaccine development against MERS‐CoV. Antiviral Res. 2017;137:82‐92. 10.1016/j.antiviral.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maloir Q, Ghysen K, von Frenckell C, Louis R, Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev Med Liege. 2018;73(7‐8):370‐375. [PubMed] [Google Scholar]
- 36. Le Bert N, Tan AT, Kunasegaran K, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020. 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 37. Chiu WJ, Kuo ML, Chen LC, et al. Evaluation of clinical and immunological effects of inactivated influenza vaccine in children with asthma. Pediatr Allergy Immunol. 2003;14(6):429‐436. 10.1111/j.1399-3038.2003.00058.x [DOI] [PubMed] [Google Scholar]
- 38. Finlay BB, McFadden G. Anti‐immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767‐82. 10.1016/j.cell.2006.01.034 [DOI] [PubMed] [Google Scholar]
- 39. Cheekatla SS, Tripathi D, Venkatasubramanian S, et al. NK‐CD11c+ cell crosstalk in diabetes enhances IL‐6‐mediated inflammation during Mycobacterium tuberculosis infection. PLOS Pathog. 2016;12(10):e1005972. 10.1371/journal.ppat.1005972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heldt S, Eigl S, Prattes J, et al. Levels of interleukin (IL)‐6 and IL‐8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses. 2017;60(12):818‐825. 10.1111/myc.12679 [DOI] [PubMed] [Google Scholar]
- 41. Badea IM, Azamfirei R, Grigorescu BL, et al. The role of interleukin‐6 as an early predictor of sepsis in a murine sepsis model. Rom J Morphol Embryol. 2019;60(1):69‐75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


