Abstract
Background
The general medical impacts of coronavirus (COVID‐19) are increasingly appreciated. However, its impact on neurocognitive, psychiatric health and quality of life (QoL) in survivors after the acute phase is poorly understood. We aimed to evaluate neurocognitive function, psychiatric symptoms and QoL in COVID‐19 survivors shortly after hospital discharge.
Methods
This was a cross‐sectional analysis of a prospective study of hospitalized COVID‐19 survivors followed up for 2 months after discharge. A battery of standardized instruments evaluating neurocognitive function, psychiatric morbidity and QoL (mental and physical components) was administered by telephone.
Results
Of the 229 screened patients, 179 were included in the final analysis. Amongst survivors, the prevalence of moderately impaired immediate verbal memory and learning was 38%, delayed verbal memory (11.8%), verbal fluency (34.6%) and working memory (executive function) (6.1%), respectively. Moreover, 58.7% of patients had neurocognitive impairment in at least one function. Rates of positive screening for anxiety, depression and post‐traumatic stress disorder were 29.6%, 26.8% and 25.1%, respectively. In addition, 39.1% of the patients had psychiatric morbidity. Low QoL for physical and mental components was detected in 44.1% and 39.1% of patients respectively. Delirium and psychiatric morbidity were associated with neurocognitive impairment, and female gender was related with psychiatric morbidity.
Conclusion
Hospitalized COVID‐19 survivors showed a considerable prevalence of neurocognitive impairment, psychiatric morbidity and poor QoL in the short term. It is uncertain if these impacts persist over the long term.
Keywords: COVID‐19, sequelae, neurocognitive, psychiatric morbidity, quality of life
Abbreviations
- ANT
animal naming test
- ARDS
acute respiratory distress syndrome
- COVID‐19
coronavirus disease 2019
- COWAT
controlled oral word association test
- CPAP
continuous positive airway pressure
- DTS
Davidson Trauma Scale
- GAD‐7
Generalized Anxiety Disorder 7
- ICU
intensive care unit
- MCS
mental component summary
- MERS‐CoV
Middle East respiratory syndrome CoV
- PHQ‐2
Patient Health Questionnaire 2
- PTSD
post‐traumatic stress disorder
- QoL
quality of life
- PCS
physical component summary
- PSI
Pneumonia Severity Index
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SCIP
subtest screen for cognitive impairment in psychiatry
- SF‐12
short‐form health survey 12 item
- SpO2/FiO2
blood oxygen saturation/fraction of inspired oxygen
- WAIS‐III
Wechsler Adult Intelligence Scale, Third Edition
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was identified in December 2019 [1]. As of 18 December, SARS‐CoV‐2 caused more than 71.5 million confirmed cases of coronavirus disease and over 1.6 million deaths worldwide [2]. Efforts by the international community are focusing on decreasing the virus transmission as well as reducing its mortality. Despite the considerable mortality rate, the vast majority of patients survive coronavirus disease 2019 (COVID‐19); however, potential systemic sequelae are poorly understood and may be an additional global public health issue [3].
Pulmonary fibrosis, cardiovascular damage and neurological impairment are amongst the potential sequelae of COVID‐19 in survivors. It is known that many human coronaviruses display neurotropism, such as HCoV‐OC43, SARS‐CoV‐1 and the Middle East respiratory syndrome CoV (MERS‐CoV) [4]. Psychiatric and neurological presentations have been found in the acute and postillness stage in other severe coronavirus infections [5]. Nevertheless, most of the studies were of either low or medium quality and focused on the acute phase of infection [5].
During hospitalization, more than a half of COVID‐19 patients have nonspecific neurological symptoms, and their persistence after the acute phase is currently under study [6, 7, 8]. The most frequently observed symptoms are headache, dizziness, fatigue, anorexia, ageusia and anosmia along with respiratory symptoms [9]. Of note, neurocognitive deficits in the postacute stage of COVID‐19 and other coronavirus infections are insufficiently described [10]. Only preliminary data support the neurological and neuropsychiatric impact in COVID‐19 patients [11]. In a national surveillance study with voluntary case notification, Varatharaj et al reported that 31% of patients had an altered mental status including neuropsychiatric and neurocognitive symptoms in the acute phase [12]. To the authors’ knowledge, there are no previous studies in COVID‐19 simultaneously evaluating neurocognitive function, psychiatric symptoms (including depression, anxiety and post‐traumatic stress disorder [PTSD]) and quality of life (QoL) following the acute phase in survivors. To date, most of the studies focus on the mental health of the general population although it has been scarcely studied in COVID‐19 survivors [13].
We hypothesized that COVID‐19 survivors may present with persistent impaired cognition, psychiatric symptoms, and poorer QoL. Severity at admission, hypoxaemia, systemic inflammation, and respiratory support during hospitalization may be associated with neuropsychiatric and QoL impairment in COVID‐19. We thus conducted a cross‐sectional cohort study in COVID‐19 survivors posthospitalization to assess the neuropsychiatric and QoL consequences 2 months after hospital discharge.
Materials and methods
Design and participants
We conducted a cross‐sectional analysis of an ongoing prospective cohort study in a large tertiary care hospital in Valencia, Spain. All hospitalized patients with COVID‐19 were diagnosed with reverse transcription‐polymerase chain reaction (RT‐PCR) of nasopharyngeal swab or sputum samples. All screened patients required hospitalization between 8 March and 25 April 2020 in the Department of Pneumology and/or the intensive care unit (ICU). Survivors were referred to the COVID‐19 outpatient clinic in the Pneumology Department for clinical control where they were invited to participate in the study and they signed the informed consent to participate in the study. Exclusion criteria included patients aged ≥ 85 or < 18 years, non‐Spanish speaking subjects, nursing‐home residents, pre‐existing dementia, pre‐existing or cognitive decline under evaluation, previous brain injury with cognitive sequelae, current alcohol/substance use disorder (except for nicotine) and previously lifetime history of major psychiatric disorders. The Biomedical Research Ethics Committee of La Fe University and Polytechnic Hospital reviewed and approved the study (2020‐280‐1). The hospital discharge dates were between 23 March to 7 July, and outpatient clinic visits were from 18 May to 24 July. All recruited patients were contacted by telephone 2 (±1) months from the date of hospital discharge.
Neuropsychiatric and quality of life assessment
A battery of standardized instruments evaluating neurocognitive function, psychiatric morbidity and QoL was administered by telephone (Table S1). The neuropsychiatric evaluation included immediate verbal memory and learning, delayed verbal memory, verbal fluency and working memory (executive function) for the neurocognitive domain; anxiety, depression and PTSD were evaluated for the psychiatric domain. The telephone battery was designed by two psychiatrists experienced in neurocognition [14]. An independent researcher, blind to clinical data and the study hypothesis, conducted pilot interviews under monitoring of her supervisors before enrolment. The duration of the interview was approximately 20 minutes. Three telephone contact attempts were made with the patients at different times before desisting. The data obtained from the interview were attached to the database with clinical data collected by an independent researcher. Additional information on interview is detailed in the Supplemental file.
Neurocognitive assessment tests included the following: (i) the verbal learning – immediate, and (ii) delayed memory subtests from the Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), (iii) animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT) for semantic verbal fluency (iv) and the subtest Digit Span backward from the Wechsler Adult Intelligence Scale, Third Edition (WAIS‐III) for working memory (executive function). Psychiatric morbidity was assessed with screening questionnaires, including the following: (i) Generalized Anxiety Disorder 7 (GAD‐7) for anxiety, (ii) Patient Health Questionnaire 2 (PHQ‐2) for depression (iii) and 17‐item Davidson Trauma Scale (DTS) for PTSD. Finally, for QoL assessment, the Short‐Form Health Survey 12 item (SF‐12, First version) was administered. This QoL test provides two independent scores, a physical component summary (PCS) and a mental component summary score (MCS). No control group was included due to the descriptive nature of the study. Moreover, all used tests have been validated in the Spanish general population according to age, sex and educational level. Additional information on neuropsychiatric and QoL battery, including cut‐off scores, is detailed in the Supplemental file.
Clinical data
Relevant variables such as demographics, comorbidities, chronic treatments, laboratory parameters, and radiographic data were recorded. Initial severity was measured with Pneumonia Severity Index (PSI), a classical prognostic score for community‐acquired pneumonia, given the absence of COVID‐19‐specific prognostic scores when the study was designed. Blood oxygen saturation/fraction of inspired oxygen (SpO2/FiO2) at admission, respiratory support (need for supplementary oxygen, high‐flow nasal cannula oxygen therapy, continuous positive airway pressure [CPAP], noninvasive mechanical ventilation and/or invasive mechanical ventilation) and laboratory data during hospitalization were recorded. Clinical outcomes included length of hospital stay, ICU admission, venous thromboembolic event or delirium. Delirium, defined as an acute, reversible state of confusion, was recorded.
Outcomes
Neurocognitive domain impairment was predefined as moderate/severe impairment of any of the four neuropsychological tests. In the same way, psychiatric morbidity was predefined as positive screening in any of the three questionnaires assessing psychiatric morbidity. This definition was used in previous studies of acute lung injury and neurocognitive evaluation [15]. The reference values and cut‐off points for the QoL questionnaire and for each variable in the neurocognitive and psychiatric domains are detailed in the Supplemental file.
Statistical analysis
Data were summarized as N (%) or median [1st quartile, 3rd quartile], as appropriate. Correlations were estimated using Spearman’s rank correlation. An exploratory analysis with predefined risk factors included known risk factors in acute respiratory distress syndrome (ARDS) such as delirium, use of corticosteroids, mechanical ventilation, length of ICU stay, metabolic alterations (renal and hepatic) along with those previously described in the hypothesis (severity at admission measured by PSI, hypoxaemia, systemic inflammation and respiratory support during hospitalization) [16, 17]. Multivariable Bayesian logistic regressions with weakly regularizing priors, N(0,1), were used to evaluate the association of risk factors with neurocognitive impairment and psychiatric morbidity in COVID‐19 survivors. Association between psychiatric morbidity and neurocognitive impairment was assessed with chi‐square test. Statistical significance was considered if P < 0.05. Statistical analyses were performed using R (version 4.0.2).
Results
Recruitment and patient baseline characteristics
A total of 229 patients discharged after COVID‐19 hospitalization were followed in the outpatient clinic of the Pneumology Department. Of those, 197 were contacted by telephone, and 179 completed the test battery (Fig. 1). The final data analysis was performed in this cohort. Patients screened but not included in the cohort (N = 50) showed similar characteristics to those included (Table S2).
Fig. 1.
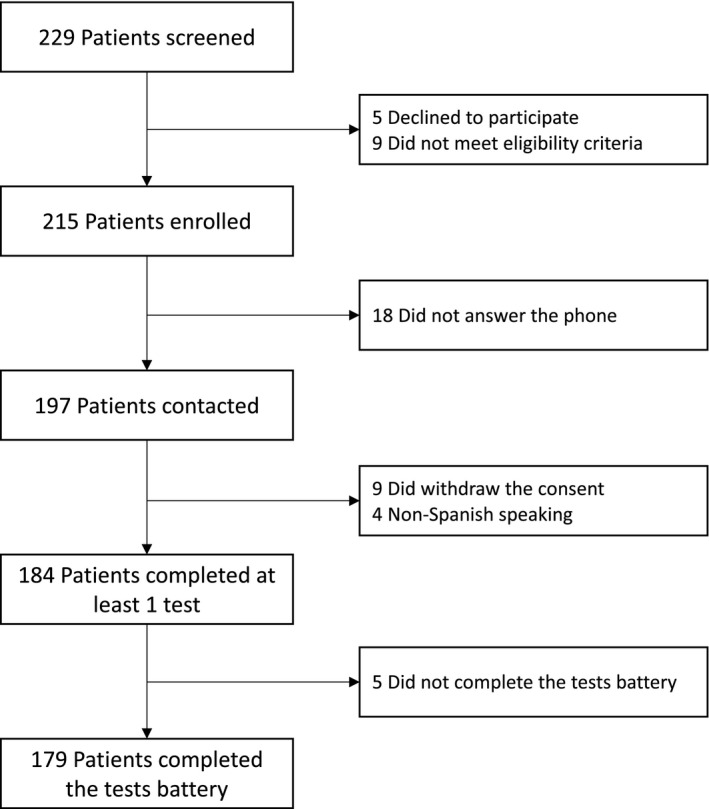
Flow chart.
Table 1 describes the main baseline characteristics of the study sample. Participants’ age ranged from 22 to 81 years, and 74 (41.3%) were female. Of the 179 survivors studied, 99 (55.3%) had at least one comorbidity. Only 1.1% of the patients did not complete any formal education and 31.3%, 35.2%, 24.6% and 7.8% of the patients completed primary, secondary, university and postgraduate education, respectively. The patients had a median of 7 [6, 10] days from the onset of symptoms to when they were hospitalized. Initial severity measured by PSI score, analytical parameters, respiratory support, treatments and clinical outcomes during hospitalization also appear in Table 1. No patient had any cerebrovascular accident during the study period.
Table 1.
Characteristics of the patients at baseline, severity, treatment received, analytical parameters, level of respiratory support and outcomes
| Total (N = 179) | |
|---|---|
| Demographics | |
| Age, yr, median [1st, 3rd quartile] | 57 [49,67] |
| Distribution, no. (%) | |
| <50, yr | 52 (29.1) |
| 50 to 69, yr | 94 (52.5) |
| ≥70, yr | 33 (18.4) |
| Male sex, no. (%) | 105 (58.7) |
| Level of education, yr, median [1st, 3rd quartile] | 11 [8, 16] |
| Smoking, no. (%) | |
| Never | 125 (69.8) |
| Former | 44 (24.6) |
| Current | 10 (5.6) |
| Coexisting conditions, no. (%) | |
| Any | 99 (55.3) |
| Hypertension | 58 (32.4) |
| Diabetes | 29 (16.2) |
| Dyslipidaemia | 52 (29.1) |
| Chronic heart disease | 10 (5.6) |
| Chronic renal disease a | 3 (1.7) |
| Chronic liver disease | 3 (1.7) |
| Cancer | 3 (1.7) |
| Chronic respiratory disease | 21 (11.7) |
| Previous medication use, no. (%) | |
| Antiplatelets | 6 (3.4) |
| Statins | 37 (20.7) |
| ACE inhibitor | 14 (7.8) |
| Angiotensin II‐receptor antagonist | 28 (15.6) |
| SpO2/FiO2 at admission, median [1st, 3rd quartile] | 452.4 [442.9, 461.9] |
| Radiological data at admission | |
| Lung infiltrates, no. (%) | 177 (98.9) |
| Bilateral infiltrates, no. (%) | 120 (67) |
| Severity | |
| PSI score, median [1st, 3rd quartile] | 57 [45,71] |
| Distribution, no. (%) | |
| I–III | 169 (94.4) |
| IV–V | 10 (5.6) |
| Analytical parameters | |
| Peak LDH, UI/L, median [1st, 3rd quartile] | 317 [258,436] |
| Peak C‐reactive protein, mg/L, median [1st, 3rd quartile] | 97.2 [45.3, 174.3] |
| Nadir lymphocyte count, cells/mL, median [1st, 3rd quartile] | 920 [640,1260] |
| Peak D‐dimer, ng/mL, median [1st, 3rd quartile] | 962 [498,2102] |
| Treatment, no. (%) | |
| Hydroxychloroquine, no. (%) | 168 (93.9) |
| Azithromycin, no. (%) | 166 (92.7) |
| Lopinavir/Ritonavir, no. (%) | 76 (42.5) |
| Interferon β, no. (%) | 26 (14.5) |
| Tocilizumab, no. (%) | 43 (24) |
| Baricitinib, no. (%) | 18 (10.1) |
| Corticosteroids, no. (%) | 65 (36.3) |
| Respiratory support, no. (%) b | |
| Room air, no. (%) | 89 (49.7) |
| O2 nasal cannula, no. (%) | 20 (11.2) |
| O2 venturi mask, no. (%) | 38 (21.2) |
| HFNC/CPAP/NIV, no. (%) | 8 (4.5) |
| MV, no. (%) | 23 (12.8) |
| Median length of MV, days [1st, 3rd quartile] | 13 [10.5, 25.5] |
| ECMO, no. (%) | 1 (0.6) |
| Outcomes and complications c | |
| Median length of hospital stay, days [1st, 3rd quartile] | 12 [9,18] |
| ICU admission, no. (%) d | 34 (19) |
| Median length of ICU stay, days [1st, 3rd quartile] | 18.5 [11,26] |
| Delirium, no. (%) | 8 (4.5) |
| Cerebrovascular event, no. (%) | 0 (0) |
| VTE, no. (%) | 17 (9.5) |
| Acute kidney injury, no. (%) e | 9 (5) |
| Acute liver injury, no (%) f | 59 (33) |
| Shock, no. (%) | 2 (1.1) |
Data are summarized as n (%) and medians [1st, 3rd quartile], as appropriate. ACE denotes angiotensin‐converting enzyme, ECMO denotes extracorporeal membrane oxygenation, HFNC/CPAP/NIV denotes high‐flow nasal cannula/continuous positive airway pressure/noninvasive ventilation, ICU denotes intensive care unit, LDH denotes lactate dehydrogenase, MV denotes mechanical ventilation, SpO2/FiO2 denotes peripheral blood oxygen saturation/fraction of inspired oxygen, VTE denotes venous thromboembolic event.
Stage ≥ 2.
Maximum respiratory support needed during hospital stay.
Complications were considered until the date of the interview administration.
Need for ICU admission at any time during hospitalization.
≥ 2‐fold increase in baseline serum creatinine or ≥ 50% decrease in baseline glomerular filtration rate.
Elevation of alanine transaminase (ALT) or aspartate transaminase (AST) enzymes > 2 × the upper limit of normal.
Short‐term neurocognitive function and psychiatric morbidity assessment
For neurocognitive assessment, immediate verbal memory/learning, delayed verbal memory, semantic verbal fluency and working memory were evaluated. From the full sample, 38% of patients presented moderate impairment and 11.2% severe impairment in immediate verbal memory. In relation to delayed memory, 11.8% of survivors had moderate impairment and 2.8% had severe impairment. In semantic verbal fluency, 34.6% of patients had moderate deficits and 8.4% severe deficits. Lastly, working memory was moderately impaired in 6.1% and severely impaired in 1.1% of survivors. The percentiles or scalar score distribution for each neurocognitive function test is shown in Fig. S1. Finally, 105 (58.7%) patients met criteria for moderate neurocognitive impairment and 33 (18.4%) for severe neurocognitive impairment, as previously defined. Figure 2A details data regarding patients with affected neurocognitive tests.
Fig. 2.
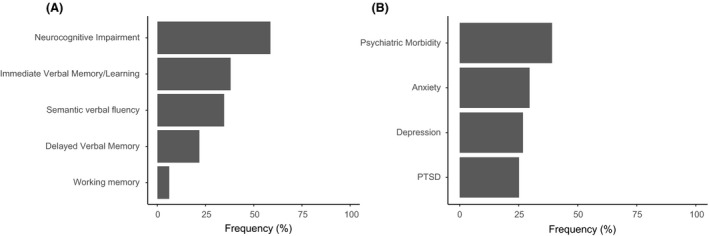
(A) Neurocognitive impairment and (B) psychiatric morbidity prevalence. PTSD denotes post‐traumatic stress disorder.
The patients were also assessed for psychiatric morbidity. Of the 179 patients, a positive screening for anxiety was present in 29.6%, depression in 26.8% and PTSD in 25.1% (Fig. 2B). As previously defined, 39.1% of the patients screened positive for psychiatric morbidity. The score distribution for the anxiety, depression and PTSD questionnaires is shown in Fig. S2. Patients with psychiatric morbidity presented a significantly higher prevalence of neurocognitive impairment compared with those without psychiatric symptoms (P < 0.001) (Table S3).
Clinical associations for neurocognitive impairment and psychiatric morbidity
The Table S3 shows clinical data regarding neurocognitive impairment and psychiatric morbidity. Logistic regression models were performed to identify potential predictors for neurocognitive impairment and a positive screening for psychiatric morbidity (Table 2). All the factors stated in the hypothesis and the previously described clinical data were included in the logistic regression models. The variables that reached the final models are shown in Table 2. A psychological trauma event prior to hospitalization due to COVID‐19 was assessed as detailed in the Supplemental file. Trauma events were only identified in 17 patients. Of these, 3 had any PTSD symptoms in the past, but no patient reported long‐lasting symptoms related to previous trauma at the time of assessment. For this reason, past history of traumatic event was not included in the models. Finally, delirium during the hospitalization and a positive screening for psychiatric morbidity increased the odds of neurocognitive impairment approximately fivefold. On the other hand, female gender was related to a 2.5‐fold increase in the odds of psychiatric morbidity.
Table 2.
Multivariable analysis for neurocognitive impairment and psychiatric morbidity prediction
| Variables | OR | 95% credibility interval |
|---|---|---|
| Neurocognitive impairment model | ||
| Any comorbidity | 1.61 | 0.85–3.07 |
| SpO2/FiO2 | 1.01 | 1.0–1.01 |
| MV | 0.53 | 0.18–1.51 |
| Delirium | 4.05 | 1.03–16.4 |
| Acute kidney injury | 2.52 | 0.71–10.05 |
| Positive screening for stress‐related symptoms | 3.97 | 2.06–7.80 |
| Psychiatric morbidity model | ||
| Female gender | 2.50 | 1.31–4.85 |
| Neurocognitive impairment | 4.56 | 2.27–9.47 |
MV denotes mechanical ventilation, OR denotes odds ratio, SpO2/FiO2 denotes peripheral blood oxygen saturation/fraction of inspired oxygen. Bold text denotes P < 0.05.
Quality of life and functionality assessment
The PCS and MCS scores for QoL appear in Fig. 3. The mean PCS was 42.5 (± 11.2), and the mean MCS was 45.5 (± 11.5). Briefly, 44.1% and a 39.1% of this cohort presented low QoL for PCS and MCS, respectively. Only 1.1% and 9.5% presented high QoL for PCS and MCS, respectively. PCS and MCS scores did not significantly (P > 0.05) correlate with each other (rho 0.030) and were also not correlated with age (rho −0.143 and rho 0.096) and years of education (rho 0.126 and rho 0.073). The Table S5 describes the quality of life scores in the physical and mental components of neurocognitive and psychiatric domains. Patients with neurocognitive impairment or a positive screening for psychiatric morbidity showed lower scores for both PCS and MCS.
Fig. 3.
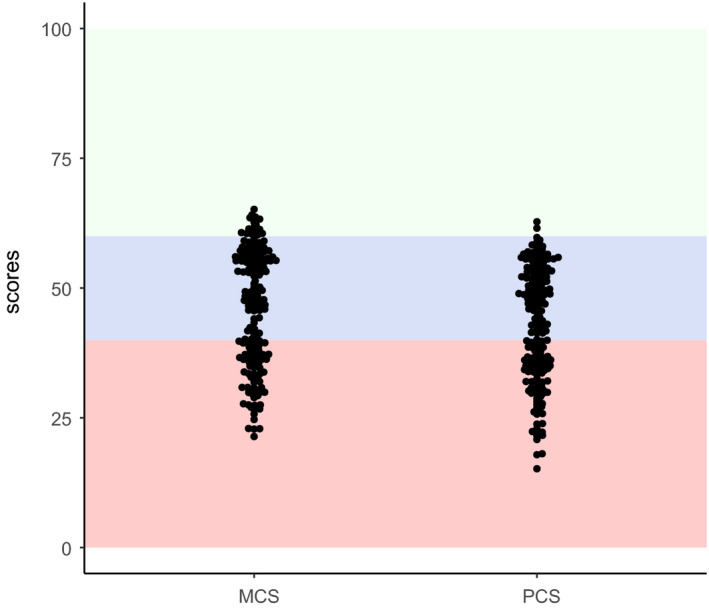
Quality of life. Scores for mental component summary (MCS) and physical component summary (PSC). Green, blue and red shaded areas denote high, normal and low quality of life, respectively.
Discussion
To our knowledge, this is the first study to simultaneously evaluate the persistence of COVID‐19 attributable symptoms, neurocognitive function, psychiatric symptoms, and QoL in mild‐to‐severe COVID‐19 survivors requiring hospitalization after the acute phase. The main findings of the study are briefly summarized as follows: 58.7% presented at least moderate neurocognitive decline, 39.1% had psychiatric morbidity and approximately 40% had poor QoL. The secondary findings were as follows: (i) delirium during hospitalization and psychiatric comorbidity were associated with neurocognitive impairment; (ii) and female gender was related to psychiatric morbidity in the short‐term.
Our study covers patients with different ranges of severity. Most of the available literature on neuropsychiatric consequences focused on critically ill patients with pneumonia, sepsis or ARDS who require intubation or admission to the ICU and there are no cohorts with similar severity to compare our results [17, 18, 19, 20, 21, 22]. The few studies in noncritically ill patients are focused on elderly patients. Moreover, previous studies have shown a declined neurocognitive function at hospital discharge in up to 100% of patients and up to 30% 1 year later [23, 24, 25]. However, currently there are no data on neurocognitive function in the postacute phase of COVID‐19.
In the acute phase, several studies reported neurological problems such as stroke, encephalitis or delirium [6, 26]. In a small study of COVID‐19, one third of ICU survivors were found to have a dysexecutive syndrome [27]. In this regard, follow‐up studies in other coronavirus outbreaks in the postacute stage may be informative. In a recent review of 40 such studies after resolution of acute infection, Rogers et al [5] reported substantial rates of memory impairment (44%). Our findings show a considerable prevalence for neurocognitive impairment (58.7%), immediate verbal memory/learning (38%), delayed verbal memory (11.8%), semantic verbal fluency (34.6%) and executive function (6.1%) in a mild‐to‐severe cohort. Previous studies have found similar neurocognitive outcomes in critically ill cohorts [19]. In our study, delirium and having concurrent psychiatric morbidity were linked to neurocognitive impairment. Delirium is a recognized risk factor for cognitive decline and reveals acute neurological damage that can persist over time [16, 17, 21]. Furthermore, psychiatric disorders with coexisting psychiatric morbidity such as PTSD and depression are associated with neurocognitive impairment [28]. Screening for delirium and psychiatric morbidity may be useful to identify vulnerable subjects. No other convincing associations were found in the analysis, such as severity, levels of systemic inflammation, thrombosis‐related biomarkers, metabolic disorders, respiratory support or oxygenation. There was also no association with age, although it should be noted that the neurocognitive tests were age‐adjusted. Additional factors or mechanisms not explored in this study may further explain neurocognitive decline, such as potential neuroinvasion of SARS‐CoV‐2, endothelial injury, blood–brain barrier disruption or neuroinflammation [29].
In ICU survivors, similar rates of psychiatric morbidity have been identified 3 and 12 months after discharge compared with our much less severe cohort [30, 31]. In other coronavirus infections, substantial rates of persistent depressed mood and anxiety have been reported [5]. Recently, a study of COVID‐19 survivors analysed the presence of PTSD, depression, anxiety, obsessive–compulsive and insomnia symptomatology [32]. The authors reported very similar frequencies to those found in this study and showed that the female gender along with previous psychiatric diagnoses, but not systemic inflammation, was associated with a higher prevalence of psychiatric symptoms. Indeed, women are two to three times more at risk to develop stress‐related disorders [33]. Recently, a new study with electronic health records supports the idea of a possible causal relationship between COVID‐19 and the risk of psychiatric sequelae [34]. However, neurocognition and clinical data such as comorbidities, severity or clinical outcomes (ICU admission, mechanical ventilation, delirium) were not characterized in those studies.
Few studies about QoL in COVID‐19 exist. In a recent report, worsened QoL was observed in 44.1% of patients discharged as measured with the EuroQoL visual analogue scale, defined as a 10‐point different between the COVID‐19 and postacute stage visit [7]. Our findings show that patients with neurocognitive impairment or psychiatric symptoms presented a poorer physical and mental quality of life. Both clinical problems have been associated with diminished quality of life in patients with psychiatric disorders and other diseases [35]. Neurocognitive remediation and treatment of neuropsychiatric morbidity may improve QoL [36]. In the context of the current pandemic [37], online‐delivered treatments such as cognitive behavioural therapy could be useful options in this regard [38].
Our study has several limitations: (1) this is a single‐centre study and the application of our findings to other populations should be extrapolated with caution, requiring external validation. (2) The tests were administered by telephone. The psychiatric screening for symptoms of anxiety, depression and PTSD used self‐reported measures after administering validated questionnaires. A face‐to‐face approach to the neurocognitive function and psychiatric morbidity by means of a structured clinical interview and objective measures, such as neuroimaging, may provide more precise information [39]. (3) Although a dropout rate of 21.8% is not likely to bias the present results, dropouts might correspond to those patients with higher disabilities. If that were the case, the present findings would even underestimate the true prevalence of the outcomes. (4) The findings do not allow us to discern whether the outcomes were due to the direct action of the virus on the central nervous system or whether they were the cognitive sequelae of the acute stress reaction caused by a life‐threatening illness. Additional studies focused on neuroinflammation and other biological pathways are needed.
Study strengths not previously mentioned include the methodologically rigorous assessment of the outcomes. In previous studies, cognitive deficits were based on self‐report [5]. Therefore, no study has used neuropsychological tests to objectively establish cognitive deficits. All the selected tests were previously validated in the Spanish general population and widely used in other diseases. These tests included cut‐off points for cognitive impairment or psychiatric screening. Moreover, confounding variables such as age and educational level were controlled in the case of cognitive performance. The study was performed in a well‐characterized cohort and the high percentage of eligible patients who completed the assessment adds internal validation. Finally, very elderly patients and those with previous cognitive impairment or major psychiatric disorders were excluded. Further research on these more vulnerable patients is needed in the postacute phase and in the long term.
Conclusions
In summary, our findings reveal a considerable prevalence of neurocognitive impairment, psychiatric comorbidity and poor QoL in COVID‐19 survivors after the acute phase, even in noncritically ill patients. These data have important clinical consequences and hence implications for health policies aimed to mitigate a wave of mental ill health following the current pandemic. Public health prevention, early detection, neurocognitive remediation and treatment of psychiatric symptoms should be prioritized in COVID‐19 survivors which may ultimately lead to an improvement in QoL and daily functioning.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Hospital Universitario y Politécnico La Fe (2020‐280‐1).
Consent for publication
All authors have accepted the publication of the manuscript.
Conflict of interests
Dr. M. Berk reports grants from National Institute of Health, grants from Cooperative Research Centre, grants from Autism Foundation, grants from Cancer Council of Victoria, grants from Stanley Medical Research Foundation, grants from Medical Benefits Fund, grants from National Health and Medical Research Council, grants from Medical Research Futures Fund, grants from Beyond Blue, grants from Rotary Health, grants from A2 milk company, grants from Meat and Livestock Board, grants from Woolworths, grants from Avant and the Harry Windsor Foundation, nonfinancial support from Astra Zeneca, nonfinancial support from Lundbeck, nonfinancial support from Merck, other from Pfizer, other from Allergan, other from Astra Zeneca, other from BioAdvantex, other from Bionomics, other from Collaborative Medicinal Development, other from Servier, outside the submitted work.
Funding Information
Raúl Méndez is the recipient of a Río Hortega grant supported by the Instituto de Salud Carlos III (ISCIII [CM19/00182]). Paula González‐Jiménez is the recipient of a postresident research grant supported by the Health Research Institute La Fe (2019‐053‐1). Michael Berk is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072).
Role of the Funding Source
There was no specific funding for this study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author Contribution
Raúl Méndez: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (lead). Vicent Balanzá‐Martínez: Conceptualization (equal); Methodology (lead); Writing‐original draft (equal); Writing‐review & editing (equal). Sussy Carolina Luperdi: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Itziar Estrada: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Ana Latorre: Data curation (equal); Writing‐review & editing (equal). Paula Gonzalez‐Jimenez: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Laura Feced: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Leyre Bouzas: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Katheryn Yépez: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Ana Ferrando: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). David Hervás: Formal analysis (equal); Methodology (equal); Writing‐review & editing (equal). Enrique Zaldívar: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Soledad Reyes: Data curation (equal); Investigation (equal); Writing‐review & editing (equal). Michael Berk: Data curation (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Rosario Menendez: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing‐review & editing (equal).
Supporting information
Figure S1. Percentiles distribution for Immediate Verbal Memory/Learning (A) and Delayed Verbal Memory (B). Scalar scores distribution for Semantic Verbal Fluency (C) and Working Memory (D). Density corresponds to relative frequency/bin width. The dashed line is located at 1 standard deviation above the mean of the general population.
Figure S2. Scores distribution in the psychiatric morbidity screening questionnaires.
Acknowledgements
We are indebted to all patients and colleagues for their cooperation and assistance in this study, always in our memory those who are no longer amongst us due to this pandemic.
Méndez R, Balanzá‐Martínez V, Luperdi SC, Estrada I, Latorre A, González‐Jiménez P, Feced L, Bouzas L, Yépez K, Ferrando A, Hervás D, Zaldívar E, Reyes S, Berk M, Menéndez R (La Fe University and Polytechnic Hospital, Valencia; Health Research Institute La Fe, Valencia; University of Barcelona, Barcelona, University of Valencia, CIBERSAM, Valencia; La Fe University and Polytechnic Hospital, Valencia; University of Valencia, Valencia; Health Research Institute La Fe, Valencia, Spain; Barwon Health, Deakin University, Geelong; The University of Melbourne, Melbourne, Victoria, Australia; Center for Biomedical Research Network in Respiratory Diseases (CIBERES), Madrid, Spain). Short‐term neuropsychiatric outcomes and quality of life in COVID‐19 survivors. J Intern Med 2021; 290: 621–631.
R. Méndez, V. Balanzá‐Martínez and S. C. Luperdi contributed equally.
Data Availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . COVID‐19 situation update worldwide.
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA ‐ J Am Med Assoc. 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. JAMA Neurol. 2020;77:1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. The Lancet Psychiatry. 2020;7:611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology. 2020;95:e1060–70. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carfì A, Bernabei R, Landi F. Group for the GAC‐19 P‐ACS. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19:767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hao F, Tam W, Hu X, Tan W, Jiang L, Jiang X et al. A quantitative and qualitative study on the neuropsychiatric sequelae of acutely ill COVID‐19 inpatients in isolation facilities. Transl Psychiatry. 2020;10:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiat. 2020;7:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tran BX, Ha GH, Nguyen LH, Vu GT, Hoang MT, Le HT et al. Studies of Novel Coronavirus Disease 19 (COVID‐19) pandemic: a global analysis of literature. Int J Environ Res Public Health. 2020;17:4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luperdi SC, Tabarés‐Seisdedos R, Livianos L, Vieta E, Cuesta MJ, Balanzá‐Martínez V. Neurocognitive endophenotypes in schizophrenia and bipolar disorder: a systematic review of longitudinal family studies. Schizophr Res. 2019;210:21–9. [DOI] [PubMed] [Google Scholar]
- 15. Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL et al. The adult respiratory distress syndrome cognitive outcomes study: long‐term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long‐term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson Jl, Pun BT et al. Long‐term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herridge MS, Cheung AM, Tansey CM, Matte‐Martyn A, Diaz‐Granados N, Al‐Saidi F et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF. Two‐year cognitive, emotional, and quality‐of‐life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–7. [DOI] [PubMed] [Google Scholar]
- 20. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long‐term Cognitive Impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis‐associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honarmand K, Lalli RS, Priestap F, Chen JL, McIntyre CW, Owen AM et al. Natural history of cognitive impairment in critical illness survivors. A systematic review. Am J Respir Crit Care Med. 2020;202:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Girard TD, Self WH, Edwards KM, Grijalva CG, Zhu Y, Williams DJ et al. Long‐term cognitive impairment after hospitalization for community‐acquired pneumonia: a prospective cohort study. J Gen Intern Med. 2018;33:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown CH 4th, Sharrett AR, Coresh J, Schneider ALC, Alonso A, Knopman DS et al. Association of hospitalization with long‐term cognitive and brain MRI changes in the ARIC cohort. Neurology. 2015;84:1443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in COVID‐19 patients: a retrospective multicenter study. Neurology. 2020;95:e1868–82. 10.1212/WNL.0000000000010112 [DOI] [PubMed] [Google Scholar]
- 27. Helms J, Kremer S, Merdji H, Clere‐Jehl R, Schenck M, Kummerlen C et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382:2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NiS et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–68. [DOI] [PubMed] [Google Scholar]
- 29. Kanberg N, Ashton NJ, Andersson L‐M, Yilmaz A, Lindh M, Nilsson S et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID‐19. Neurology. 2020;95:e1754–9. 10.1212/WNL.0000000000010111 [DOI] [PubMed] [Google Scholar]
- 30. Wolters AE, Peelen LM, Welling MC, Kok L, de Lange DW, Cremer OL et al. Long‐term mental health problems after delirium in the ICU. Crit Care Med. 2016;44:1808–13. [DOI] [PubMed] [Google Scholar]
- 31. Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and post traumatic stress disorder after critical illness: a UK‐wide prospective cohort study. Crit Care. 2018;22:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gennaro Mazza M, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I et al. Anxiety and depression in COVID‐19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020. 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anon . WHO | Depression and Other Common Mental Disorders. WHO. 2017. [Google Scholar]
- 34. Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID‐19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID‐19 cases in the USA. Lancet Psychiat. 2021;8:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wittchen HU, Carter RM, Pfister H, Montgomery SA, Kessler RC. Disabilities and quality of life in pure and comorbid generalized anxiety disorder and major depression in a national survey. Int Clin Psychopharmacol. 2000;15:319–28. [DOI] [PubMed] [Google Scholar]
- 36. Hofmann SG, Wu JQ, Boettcher H, Sturm J. Effect of pharmacotherapy for anxiety disorders on quality of life: a meta‐analysis. Qual Life Res. 2014;23:1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balanzá‐Martínez V, Atienza‐Carbonell B, Kapczinski F, De Boni RB. Lifestyle behaviours during the COVID‐19 ‐ time to connect. Acta Psychiatr Scand. 2020;141:399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soh HL, Ho RC, Ho CS, Tam WW. Efficacy of digital cognitive behavioural therapy for insomnia: a meta‐analysis of randomised controlled trials. Sleep Med. 2020;75:315–25. [DOI] [PubMed] [Google Scholar]
- 39. Ho CSH, Lim LJH, Lim AQ, Chan NHC, Tan RS, Lee SH et al. Diagnostic and predictive applications of functional near‐infrared spectroscopy for major depressive disorder: a systematic review. Front Psychiatry. 2020;11:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percentiles distribution for Immediate Verbal Memory/Learning (A) and Delayed Verbal Memory (B). Scalar scores distribution for Semantic Verbal Fluency (C) and Working Memory (D). Density corresponds to relative frequency/bin width. The dashed line is located at 1 standard deviation above the mean of the general population.
Figure S2. Scores distribution in the psychiatric morbidity screening questionnaires.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


