Abstract
Background
Since early 2020, convalescent plasma has been widely used for treating coronavirus disease 2019 (COVID‐19). There is limited information regarding donor tolerability of convalescent plasma donation. In this study, we evaluated the short‐term donor tolerability of convalescent plasma donation.
Methods
A prospective study of 309 convalescent plasma donation related adverse events were conducted at Wuhan Blood Center of China, from February 12 to April 1, 2020. Additionally, up to 28‐day post‐donation follow‐ups were performed on the donors.
Results
Sixteen (5.2%) adverse events were reported in 309 donations. All of these were mild vasovagal without loss of consciousness. The frequency of adverse reactions was higher in donors with a per donation volume of >8 mL/kg body weight or ≥ 600 mL, <100 mm Hg in pre‐donation systolic blood pressure, or less than 28 days from the onset of COVID‐19 symptoms. There was no correlation to donation history, weight, sex, ABO blood type, pre‐donation diastolic blood pressure, pulse, or hemoglobin.
Conclusion
The donation of convalescent plasma is generally safe. Mitigation of risk factors associated with adverse events can further enhance donor tolerability of convalescent plasma donation.
Keywords: adverse donation reaction, convalescent plasma, coronavirus disease 2019
1. INTRODUCTION
The current outbreak of coronavirus disease 2019 (COVID‐19) has become a major public health challenge around the world. Convalescent plasma has been considered as a potential therapeutic option for patients with severe COVID‐19 disease. 1 , 2 Selection of optimal convalescent plasma donors thus far has been mostly focused on identifying donors with high levels of neutralizing antibodies or equivalent and donors' returning to asymptomatic stages; however, it is not known if convalescent plasma donation is well tolerated by donors. Even though thousands of convalescent plasma collections have been performed, there is no up‐to‐date report on relevant donor tolerability.
Plasma donation is generally considered to be safe, 3 but adverse reactions of varying severity may occasionally occur. Based on the international society of blood transfusion (ISBT) definitions, donation reactions may include complications with local symptoms such as hematoma and complications with generalized symptoms such as vasovagal reactions, allergic reactions, apheresis procedure‐related complications, and others. 4 Reactions can be classified as immediate or delayed based on the interval between procedure end time and the time of reaction, with reactions occurring outside the transfusion site generally considered as delayed. 5 Donor reaction can have the following severities: mild, which includes sweating, pallor, dizziness, headache, and malaise, without loss of consciousness; moderate, which includes vomiting, loss of consciousness, or hypotension; severe, which includes convulsions or loss of consciousness, and requires hospitalization or surgery; rarely life‐threatening, which requires immediate medical intervention to prevent death; and death. 6 , 7
In this study we investigated the frequency, type, and severity of adverse events in convalescent plasma donation and identified risk factors associated with the events.
2. METHODS
2.1. Donor selection criteria
This is a prospective, single‐center study of all adverse reactions related to convalescent plasma donations performed between February 12 and April 1, 2020, at the Wuhan Blood Center. All donors gave written, informed consent. The selection criteria were the following: (a) aged 18‐55 years; (b) eligible for plasma donation: time interval no less than 14 days and donation volume no more than 600 mL 8 ; (c) diagnosed with COVID‐19. (d) had no COVID‐19 symptoms for more than 2 weeks before convalescent plasma donation. 9
2.2. Donation process
Convalescent plasma donations were performed via plasmapheresis, which is similar to other standard plasmapheresis equipment used globally. (DigiPIa80, Sichuan Nangeer Biotechnology Co., Ltd, Chengdu, China; XCF 3000, Sichuan Nangeer Biotechnology Co., Ltd, Chengdu, China). The volume of plasma collection was determined based on the donor's weight, range from 200 to 600 mL. Replacement fluids were 0.9% saline (NS). For donors weighing less than 60 kg, the volume of the replacement fluid was 1/2 of the plasma collected volume. Anticoagulant was performed with 4% citrate. Plasma collection procedure is a one‐arm procedure. The donor was asked to rest at the collection site for at least 15 minutes post‐donation and provided beverages and snacks. Donors were monitored for adverse reactions and allowed to leave the blood center only if they felt well or free of symptoms from adverse reactions. The donor and donation records captured donor demographics and donation characteristics, including donation history, age, gender, weight, ABO blood type, pre‐donation systolic blood pressure (BP) and diastolic BP, pulse, Hemoglobin (Hb) level, and apheresis collection records including volume of the collection. Time from COVID‐19 symptom onset to plasma donation was also recorded.
2.3. Follow up
All convalescent plasma donors in this study received a telephone interview from a qualified member of the blood center staff 1 week after their donation. The main contents of the survey include: 1. Did you feel any discomfort during or immediately after blood donation or in the rest area? 2. Did you have sweating, pallor, dizziness, headache, malaise, or loss of consciousness, and so forth? 3. Did you experience any discomfort at the phlebotomy site? 4. Did you have any bleeding (delayed bleeding), bruise, hematoma, pain, pain along the vein (thrombophlebitis), or redness/itching (allergy), and so forth? 5. When and where it happened? 6. Did these uncomfortable symptoms improve after rest? In addition, all donors were instructed to contact the blood center if they had any adverse reactions, experienced problems, or had any concerns about their health up to one‐month (28 days) post‐donation.
2.4. Characterization of adverse reaction
Characterization of adverse reactions was based on the standard for surveillance of complications related to blood donation by ISBT. 4
2.5. Statistical analysis
To determine the significance of differences in the rates of adverse reactions, a Chi‐square test or a Fisher exact test (the expected frequencies <5%) was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, California). Data are shown as the mean ± SD. Significance was defined as a P‐value of less than .05.
3. RESULTS
3.1. Donor demographics and adverse reactions
In this study, there were a total of 309 plasma donation events provided by 309 donors, including 186 males and 123 females. They consisted of 181 (58.6%) first‐time donors and 128 (41.4%) repeat donors. The mean (SD) age of donors was 38 ± 8 years, with a range of 20‐55 years. The mean (SD) weight was 69 ± 13 kg, with a range of 45 to 117 kg. The mean (SD) pre‐donation systolic BP, diastolic BP, and pulse of donors were 122 ± 12 mm Hg, 80 ± 8 mm Hg, and 87 ± 10 bpm, respectively. Days from COVID‐19 symptom onset to donation ranged from 17 to 85 days, with 96.8% donating more than 28 days after disease onset. (Table 1).
TABLE 1.
Donor demographics and characteristics
| Total donations a N = 309 (%) | Donations without adverse reactions N = 293 (%) | Adverse reactions N = 16 (%) | |
|---|---|---|---|
| Plasma donation history | |||
| First time | 181 (58.6%) | 171 (58.4%) | 10 (62.5%) |
| Repeat | 128 (41.4%) | 122 (41.6%) | 6 (37.5%) |
| Sex | |||
| Male | 186 (60.2%) | 176 (60.1%) | 10 (62.5%) |
| Female | 123 (39.8%) | 117 (39.9%) | 6 (37.5%) |
| Age group, years | |||
| Mean ± SD | 38 ± 8 | 38 ± 8 | 40 ± 9 |
| Range | 20–55 | 20–55 | 21‐52 |
| ≤24 | 15 (4.9%) | 13 (4.4%) | 2 (12.5%) |
| 25‐29 | 26 (8.4%) | 25 (8.5%) | 1 (6.3%) |
| 30‐34 | 66 (21.4%) | 63 (21.5%) | 3 (18.8%) |
| 35‐39 | 84 (27.2%) | 82 (28.0%) | 2 (12.5%) |
| 40‐44 | 42 (13.6%) | 40 (13.7%) | 2 (12.5%) |
| 45‐49 | 49 (15.8%) | 46 (15.7%) | 3 (18.7%) |
| ≥50 | 27 (8.7%) | 24 (8.2%) | 3 (18.7%) |
| Weight (Kg) | |||
| Mean ± SD | 69 ± 13 | 69 ± 13 | 72 ± 15 |
| Range | 45–117 | 45–117 | 49‐113 |
| 45‐59 | 80 (25.9%) | 77 (26.3%) | 3 (18.8%) |
| 60‐74 | 129 (41.7%) | 123 (42.0%) | 6 (37.5%) |
| 75‐89 | 80 (25.9%) | 75 (25.6%) | 5 (31.2%) |
| ≥90 | 20 (6.5%) | 18 (6.1%) | 2 (12.5%) |
| Pre‐donation systolic BP (mmHg) | |||
| Mean ± SD | 122 ± 12 | 123 ± 12 | 120 ± 13 |
| Range | 84‐140 | 90‐140 | 84–140 |
| <100 | 18 (5.5%) | 15 (5.1%) | 3 (18.7%) |
| 100‐130 | 200 (64.7%) | 189 (64.5%) | 11 (68.8%) |
| 130‐140 | 91 (29.8%) | 89 (30.4%) | 2 (12.5%) |
| Pre‐donation diastolic BP (mm Hg) | |||
| Mean ± SD | 80 ± 8 | 80 ± 8 | 80 ± 6 |
| Range | 60‐90 | 60‐90 | 68‐90 |
| <70 | 42 (13.6%) | 39 (13.3%) | 3 (18.7%) |
| 70‐85 | 161 (52.1%) | 152 (51.9%) | 9 (56.3%) |
| 85‐90 | 106 (34.3%) | 102 (34.8%) | 4 (25%) |
| Pre‐donation pulse (bpm) | |||
| Mean ± SD | 87 ± 10 | 87 ± 10 | 89 ± 10 |
| Range | 62‐107 | 62‐107 | 66‐100 |
| <65 | 3 (1.0%) | 3 (1.0%) | 0 (0%) |
| 65‐90 | 190 (61.5%) | 183 (62.5%) | 7 (43.8%) |
| >90 | 116 (37.5%) | 107 (36.5%) | 9 (56.2%) |
| Hemoglobin, Hb (g/L) | |||
| Mean ± SD | 142 ± 12 | 142 ± 13 | 141 ± 11 |
| Range | 115‐175 | 115–175 | 125‐161 |
| <120 | 3 (1.0%) | 3 (1.0%) | 0 (0%) |
| 120‐134 | 102 (33.0%) | 95 (32.4%) | 7 (43.8%) |
| 135‐150 | 118 (38.2%) | 113 (38.6%) | 5 (31.2%) |
| >150 | 86 (27.8%) | 82 (28.0%) | 4 (25%) |
| Days of disease onset to donation | |||
| Mean ± SD | 52 ± 12b | 51 ± 12b | 42 ± 10 |
| Range | 17‐85 | 17‐93 | 23‐56 |
| <28 | 10 (3.2%) | 7 (2.4%) | 3 (18.8%) |
| 28‐42 | 66 (21.4%) | 61 (20.8%) | 5 (31.2%) |
| 43‐56 | 138 (44.7%) | 130 (44.4%) | 8 (50%) |
| 57‐70 | 84 (27.2%) | 84 (28.7%) | 0 (0%) |
| ≥71 | 9 (2.9%) | 9 (3.1%) | 0 (0%) |
| Volume of per‐donation (mL) | |||
| 200 | 61(19.7%) | 61 (20.8%) | 0 (0%) |
| 300 | 42 (13.6%) | 40 (13.7%) | 2 (12.5%) |
| 400 | 202 (65.4%) | 191 (65.2%) | 11 (68.8%) |
| 600 | 4 (1.3%) | 1 (0.3%) | 3 (18.7%) |
| ABO Blood type | |||
| Type A | 121 (39.2%) | 117 (40.0%) | 4 (25.0%) |
| Type AB | 28 (9.0%) | 27 (9.2%) | 1 (6.3%) |
| Type B | 84 (27.2%) | 76 (25.9%) | 8 (50.0%) |
| Type O | 76 (24.6%) | 73 (24.9%) | 3 (18.7%) |
| Symptoms of adverse reactions | |||
| Discomfort, weakness, dizziness, sweating, or nausea | ‐ | ‐ | 14 (87.5%) |
| More than one symptom | ‐ | ‐ | 2 (12.5%) |
All donors made only one plasma donation events.
Two data missing.
A total of 16 adverse events (5.2%) were reported in 309 donations, occurring in roughly one out of every 20 donations, and all were reported during follow‐up 1 week later (Table 1). This reaction rate is higher than that of routine plasmapheresis donation at the Wuhan Blood Center (5.2% vs <0.1%, P < .01). All reactions were delayed in timing and mild in severity, with self‐reported improvement after resting. Vasovagal reactions, such as discomfort, weakness, dizziness, sweating, and nausea, were the most common adverse reactions. Fourteen (87.5%) donors had one symptom, and two (12.5%) donors had two or three symptoms (Table 1). Among the 16 donors with adverse reactions, 10 (62.5%) were first‐time donors and 6 (37.5%) repeated donors, with no statistical significance.
3.2. Risk factors related to adverse reactions
We assessed the correlation of the occurrence of adverse reactions to donor or donation factors including age, gender, weight, donation history, pre‐donation systolic BP and diastolic BP, pulse and Hb level, apheresis collection volume, and time interval from COVID‐19 symptom onset to donation. We found that three of eight donors had adverse reactions with a per donation volume of >8 mL/kg body weight, the reaction rate was significantly higher (37.5% vs 5.9%, P < .05) (Figure 1A) and per donation volume of ≥600 mL (75% vs 5.4%, P < .001) (Figure 1B). The number of donors collected with a volume of ≥600 mL was only 4.
FIGURE 1.
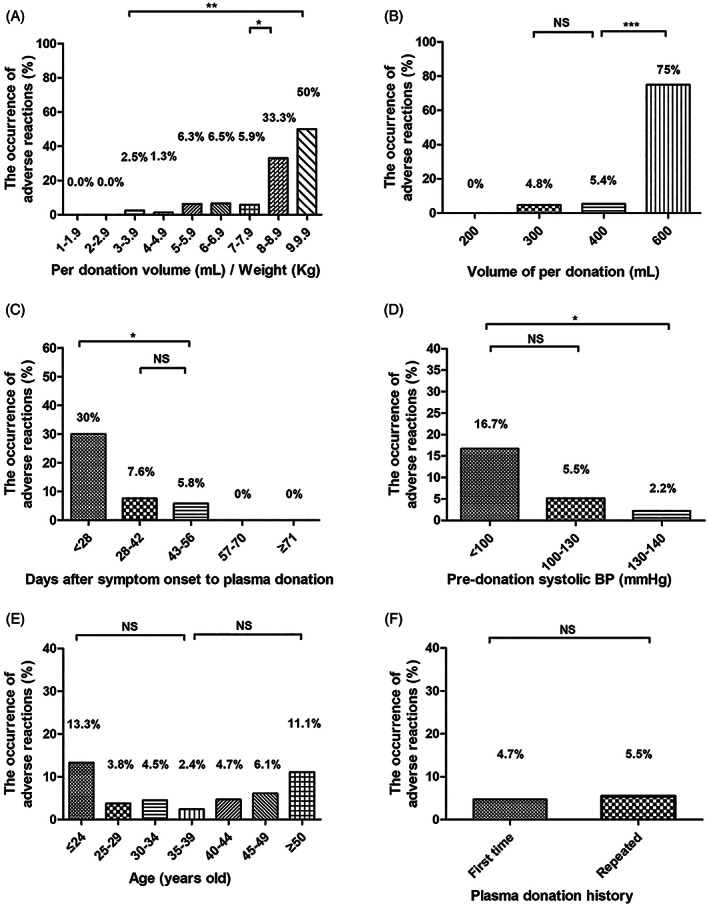
Factors related to adverse reactions. A, The occurrence of adverse reactions based on per donation volume/weight. B, The occurrence of adverse reactions based on per donation volume of 200, 300, 400, and 600 mL. C, The occurrence of adverse reactions based on the time interval from COVID‐19 symptom onset to plasma donation: less than 28 days, 28 to 42 days, 43 to 56 days, 57 to 70 days, and 71 or more days. D, The occurrence of adverse reactions of donors based on pre‐donation systolic blood pressure (BP): <100, 100 to 130, and 130 to 140 mm Hg. E, The occurrence of adverse reactions of donors based on age: ≤29, 30 to 39, 40 to 49, and ≥ 50. F, The occurrence of adverse reactions in first‐time donors and repeated donors. The differences between the groups were analyzed using a Chi‐square test or a Fisher exact test. ***P < .001; **P < .01; *P < .05; NS, nonsignificant
Days from COVID‐19 symptom onset to donation for donors with adverse reactions were significantly less than with donors without reactions (42 ± 10 vs 51 ± 12, P < .01). The rates were higher for donors who donated within 28 days from the onset of symptoms than for those who donated later than 28 days (30% vs 7.6%, P < .05) (Figure 1C). Donors with pre‐donation systolic BP below 100 mm Hg had higher rates of reactions than those with systolic BP between 100 and 140 mm Hg (16.7% vs 4.5%, P < .05) (Figure 1D). The systolic BP range of convalescent plasma donors in this study was as low as 84 mm Hg, while 90 mm Hg is the minimum value required by AABB/ FDA regulations. Notably, of the 15 donors in the ≤24 year age group, 2 (13.3%) had a reaction; of the 267 in the 25 to 49 year age group, 11 (4.1%) had a reaction; and of the 27 in the ≥50 year age group, 3 (11.1%) had a reaction, although the differences were not statistically significant (P = .1) (Figure 1E). Regarding the history of blood donation, the adverse reaction rates were 5.5% for first‐time donors and 4.7% for repeat plasma donors, with no statistically significant differences (P = .7) (Figure 1F). Additionally, the occurrence of adverse reactions had no correlation with the weight, sex, ABO blood type, pre‐donation diastolic BP, pulse, or hemoglobin. (Figure ure S1).
4. DISCUSSION
To encourage convalescent plasma donation and ensure adequate convalescent plasma inventory, it is important to evaluate donor tolerability of convalescent plasma donation. There have been few studies observing the rates of adverse reactions related to convalescent plasma donation. 10 While we confirmed the overall safety of convalescent plasma donation in this study, the occurrence of adverse reactions in our study was higher than that of routine plasmapheresis donation based on the information from the Wuhan Blood Center (5.2% vs <0.1%). 11 A report from Serious Hazards of Transfusion (SHOT) showed that the rates of serious adverse events after donations in 2018 was 0.23 per 10 000 donations in the United Kingdom. 12 The rate of adverse events from plasmapheresis donations was 2.3% in Australia 13 and 0.16% in Italy. 14 In this study, the rate of adverse reactions, all delayed, was 5.2% (16/309). All events were determined to be definite mild vasovagal without loss of consciousness. A recent Italian retrospective study of 494 CCP donors over a period of ~3.5 months had an adverse reaction rate of 2.6%, all of which were mild and did not require therapeutic intervention. The results showed that convalescent plasma donation was safe, which was similar to the results of this study. 10 In addition, many studies confirm that delayed adverse reactions, consisting mainly of vasovagal reactions and hematoma, usually occurred when a donor was just leaving the donation center or later. 15 , 16 There is a strong link between the occurrence of these adverse reactions and subsequent discontinuation of donations. Donors and those who are made aware of the event often are unwilling to donate again. 12 , 17
Vasovagal reactions were the most common adverse events; severe complications were not reported in our study. Most donors experienced presyncope reactions, such as dizziness and fatigue, which may be triggered by various physical (eg, standing up after donation) and psychological stimuli (eg, stress or pain), resulting in decreased arterial blood pressure and cerebral perfusion. 18 High per donation volume and low pre‐donation systolic BP are more likely to be associated with insufficient circulating blood volume. 19 Some studies also indicated that donors with low estimated blood volume were at a higher potential risk of adverse reactions. 20 Regarding the time intervals from COVID‐19 symptom onset to blood donation, it is reasonable to speculate that convalescent plasma donors who donated earlier may not have fully recovered in their physical conditions. In recent years, several studies have demonstrated that first‐time blood donors have higher rates of adverse reactions than repeat donors; female sex, and younger or older age were also noted as risk factors. 21 , 22 However, no statistically significant differences were observed in this study, and it is not known whether this phenomenon is related to COVID‐19. It should be noted that the inclusion criteria of this study ranged from 18 to 55 years, so we do not know the incidence of adverse reactions in older patients.
5. CONCLUSIONS
Our study confirms the general donor tolerability of convalescent plasma donation. To enhance donation safety, we recommend evaluating the impact of donation volume, pre‐donation systolic blood pressure, and intervals from COVID‐19 symptom onset to blood donation in risk mitigation.
6. LIMITATIONS
This study has several limitations. First, the reporting of adverse reactions was based partly on subjective donor feeling and interpretation, so there is a risk of under‐reporting or over‐reporting. Second, the sample size was small. Third, our experience may be related to local practice with plasma donation and donor monitoring. Our data should be validated by large sample size and different blood collection centers.
AUTHOR CONTRIBUTIONS
Rui He was responsible for data analysis and manuscript‐writing, Hui Lin was responsible for recruiting, tracking donors and collecting information.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to disclose.
Supporting information
Figure S1 Reaction rates based on weight, sex, ABO blood type, pre‐donation diastolic BP, pulse, and hemoglobin.
He R, Lin H, Xie S, et al. Donor tolerability of convalescent plasma donation. J Clin Apher. 2021;36:429–436. 10.1002/jca.21882
Funding information the CAMS Innovation Fund for Medical Sciences (CIFMS), Grant/Award Numbers: 2016‐I2M‐3‐024, 2017‐I2M‐1‐009, 2020‐I2M‐CoV19‐006
Contributor Information
Yanyun Wu, Email: yxw1366@med.miami.edu.
Zhong Liu, Email: liuz@ibt.pumc.edu.cn.
DATA AVAILABILITY STATEMENT
Data Availability Statement The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Roback JD, Guarner J. Convalescent plasma to treat COVID‐19: possibilities and challenges. JAMA. 2020;323(16):1561‐1562. [DOI] [PubMed] [Google Scholar]
- 2. Food and Drug Administration (FDA) . Recommendations for Investigational COVID‐19 Convalescent Plasma. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma Accessed August 10, 2020.
- 3. Pathak C, Pujani M, Pahuja S, Jain M. Adverse reactions in whole blood donors: an Indian scenario. Blood Transfus. 2011;9(1):46‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International society of blood transfusion working party on haemovigilance in collaboration with the international haemovigilance network and the AABB donor haemovigilance working group. Standard for Surveillance of Complications Related to Blood Donation. http://aabb.org/research/hemovigilance/Documents/Donor-Standard-Definitions.pdf Accessed June 26, 2020.
- 5. Advancing transfusion and cellular therapies worldwide . AABB Donor hemovigilance report 2012‐2014. http://www.aabb.org/research/hemovigilance/Documents/2012-2014-AABB-Donor-Hemovigilance-Report.pdf Accessed August 10, 2020.
- 6. Riga A, Sapey T, Bacanu M, Py JY, Dehaut F. Blood donor serious adverse reactions (SAR) 2010‐2014 EFS Châteauroux, France. Transfus Clin Biol. 2015;22(2):62‐65. [DOI] [PubMed] [Google Scholar]
- 7. Land KJ, Townsend M, Goldman M, Whitaker BI, Perez GE, Wiersum‐Osselton JC. International validation of harmonized definitions for complications of blood donations. Transfusion. 2018;58(11):2589‐2595. [DOI] [PubMed] [Google Scholar]
- 8. National Health Commission of the People's Republic of China . Standards for Apheresis Plasma Collection Stations. Accessed January 13, 2021. http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=18684
- 9. Li L, Yang R, Wang J, et al. Feasibility of a pilot program for COVID‐19 convalescent plasma collection in Wuhan, China. Transfusion. 2020;60(8):1773‐1777. 10.1111/trf.15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Fante C, Franchini M, Baldanti F, et al. A retrospective study assessing the characteristics of COVID‐19 convalescent plasma donors and donations. Transfusion. 2020; 10.1111/trf.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Ru Y. Analysis of the adverse reaction of blood donation and the necessity of establishing Haemovigilance system. Chin J Blood Trans. 2016;29(9):968‐970. [Google Scholar]
- 12. The serious hazards of transfusion (SHOT) , 2018. SHOT Annual Report and Summary. https://www.shotuk.org/wp-content/uploads/myimages/5.-Donor-Haemovigilance-2.pdf. Accessed June 30, 2020.
- 13. Thijsen A, Masser B, Gemelli CN, Davison TE. Trends in return behavior after an adverse event in Australian whole blood and plasma donors. Transfusion. 2019;59(10):3157‐3163. [DOI] [PubMed] [Google Scholar]
- 14. Crocco I, Franchini M, Garozzo G, et al. Adverse reactions in blood and apheresis donors: experience from two Italian transfusion centres. Blood Transfus. 2009;7(1):35‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Py JY, Durieux S, Barnoux M, Sapey T. Delayed adverse reactions to blood donation: from haemovigilance data to specific studies. Transfus Clin Biol. 2016;23(4):233‐239. [DOI] [PubMed] [Google Scholar]
- 16. Esplendori GF. Adverse reactions to whole blood donation, basic human needs and nursing diagnoses: a reflection. Rev Esc Enferm USP. 2018;51:3284‐3291. [DOI] [PubMed] [Google Scholar]
- 17. Custer B, Rios JA, Schlumpf K, et al. Adverse reactions and other factors that impact subsequent blood donation visits. Transfusion. 2012;52(1):118‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilchrist PT, McGovern GE, Bekkouche N, et al. The vasovagal response during confrontation with blood‐injury‐injection stimuli: the role of perceived control. J Anxiety Disord. 2015;31:43‐48. [DOI] [PubMed] [Google Scholar]
- 19. Grindon AJ. Adverse reactions to whole blood donation and plasmapheresis. Crit Rev Clin Lab Sci. 1982;17(1):51‐75. [DOI] [PubMed] [Google Scholar]
- 20. Kamel H, Tomasulo P, Bravo M, et al. Blood donors and blood collection: delayed adverse reactions to blood donation. Transfusion. 2010;50:556‐565. [DOI] [PubMed] [Google Scholar]
- 21. Eder AF, Hillyer CD, Dy BA, Notari EP, Benjamin RJ. Adverse reactions to allogeneic whole blood donation by 16 and 17 year olds. JAMA. 2008;299:2279‐2286. [DOI] [PubMed] [Google Scholar]
- 22. Inaba S, Takanashi M, Matsuzaki K, et al. Analysis of a questionnaire on adverse reactions to blood donation in Japan. Transfus Apher Sci. 2013;48(1):21‐34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Reaction rates based on weight, sex, ABO blood type, pre‐donation diastolic BP, pulse, and hemoglobin.
Data Availability Statement
Data Availability Statement The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


