To the Editor,
The clinical presentation and course of patients previously treated with immunomodulatory therapeutics, with coronavirus disease 2019 (COVID‐19) remain unclear. Rituximab is an anti‐CD20 monoclonal antibody used to treat B‐cell lymphoid malignancies and autoimmune diseases. There is little data on COVID‐19 patients previously treated with rituximab. 1 , 2 Here, we present a protracted severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection with an atypical course in two patients previously treated with rituximab for autoimmune disease.
The first patient was a 65‐year‐old man treated with rituximab for 2 years for neuromyelitis optica spectrum disorder. He reported flu‐like symptoms for 10 days in June 2020. SARS‐CoV‐2 infection was confirmed by polymerase chain reaction (PCR) on a nasopharyngeal swab. Seven days after symptom resolution, he experienced fever, asthenia, weight loss, arthromyalgia, and diarrhea, requiring hospitalization on Day 32. He had elevated C‐reactive protein at 155 mg/L, no B‐cell and hypogammaglobinemia at 4.2 g/L. The chest computed tomography (CT) on 32 days after symptom onset showed bilateral ground‐glass opacities (10%‒25%), with condensation and air bronchogram (Figure 1A). SARS‐CoV‐2 PCR was negative on the nasopharyngeal swab (Table 1). Despite broad‐spectrum antibiotic therapy, the patient deteriorated with persistent fever and increased oxygen requirements. Bronchoalveolar lavage (BAL) on Day 39, revealed a high SARS‐CoV‐2 viral load (Table 1). There was no virological, bacteriological, fungal, or parasitological co‐infection. Intravenous methylprednisolone at 1 mg/kg daily was started. SARS‐CoV‐2 PCR was positive in serum on Day 39. On Day 42, the patient was transferred to the intensive care unit due to respiratory distress. The chest CT showed a significant increase of lung involvement to more than 50% of the parenchyma (Figure 1A). The patient received mechanical ventilation for 17 days and corticosteroid therapy for 27 days, improving gradually. He returned home on Day 81. His immunoglobulin A (IgA) anti‐SARS‐CoV‐2 level peaked after more than forty days of evolution, but his immunoglobulin G (IgG) level remained doubtful on Day 68 (Table 1).
Figure 1.
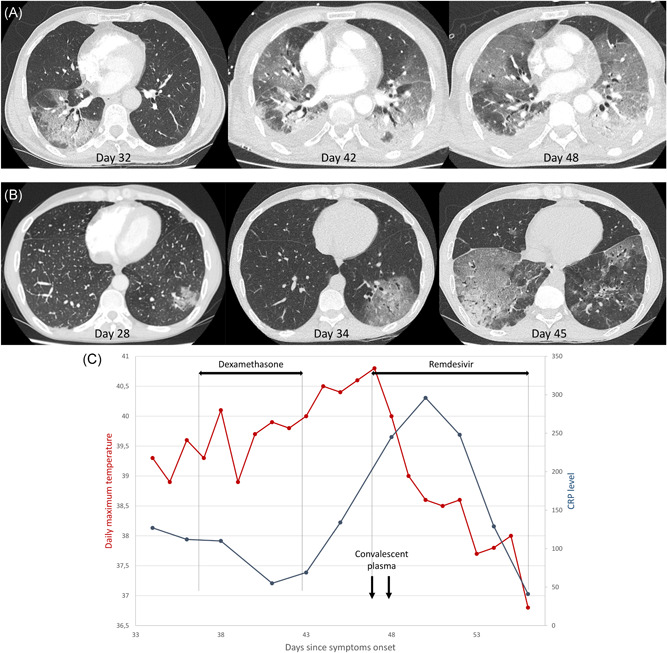
(A) The change in the chest computed tomography (CT) of the first patient, a 65‐year‐old man treated with rituximab for neuromyelitis optica spectrum disorder. (B) The change in the chest CT of the second patient, a 46‐year‐old man treated with rituximab for rheumatoid arthritis. (C) The change in the daily maximal temperature and C‐reactive protein level of the second patient over time according to the therapeutics initiated
Table 1.
The PCR detection and serology of SARS‐CoV‐2 after symptoms onset
| Patient | Days from symptom onset | PCR result (viral copies/reaction) a | Sampling site | Serology result (realized on serum samples) b |
|---|---|---|---|---|
| 1 | 8 | Positive (unknown) | NPS | |
| 32 | Negative | NPS | ||
| 35 | Negative | NPS | ||
| 39 | Positive (590,000) | BAL | IgG 1.22 (doubtful) | |
| Positive (54) | Serum | IgA 0.89 (doubtful) | ||
| 42 | Positive (478,000) | ETA | ||
| Negative | NPS | |||
| 48 | Positive (9,700) | BAL | ||
| Positive (60,000) | ETA | |||
| Negative | NPS | |||
| 55 | Positive (11,950,000) | ETA | ||
| Positive (62) | NPS | |||
| 67 | Positive (low) | NPS | ||
| 68 | Negative | Serum | IgG 0.74 (doubtful) | |
| IgA 8.24 (positive) | ||||
| 73 | Negative | NPS | ||
| 77 | Negative | NPS | ||
| 2 | 1 | Positivec | NPS | |
| 34 | Negative | NPS | ||
| 35 | Positive (24,630,000) | BAL | IgG 0.61 (doubtful) | |
| Negative | Serum | IgA 5.34 (positive) | ||
| 41 | Negative | NPS | ||
| 43 | / | IgG 1.16 (doubtful) | ||
| IgA 2.97 (positive) | ||||
| 45 | Positive (97) | Plasma | ||
| 54 | Negative | Serum |
Abbreviations: BAL, bronchoalveolar lavage; ETA, endotracheal aspirate; IgA, immunoglobulin A; IgG, immunoglobulin G; NPS, nasopharyngeal swab; PCR, polymerase chain reaction; RT‐PCR, reverse transcription PCR; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
SARS‐CoV‐2 was identified via RT‐PCR according to the current guidelines (Institut Pasteur and the WHO technical guidance). The assay targets two regions of the viral RNA‐dependent RNA polymerase (RdRp) gene and has the threshold limit of detection of 10 copies per reaction.
SARS‐CoV‐2 serological diagnostic was performed according manufacturer instructions:
– IgA were detected using the Euroimmun assays (negative: ratio <0.8; doubtful: 0.8‒1.1; positive: >1.1).
– IgG were detected using the Abbott architect assay (negative: ratio <0.49; doubtful: 0.49‒1.4; positive: >1.4).
Viral load was not available for this sample; the threshold cycle value was 27.
The second patient was a 46‐year‐old man treated with rituximab for 2 years for severe seropositive rheumatoid arthritis. He was diagnosed with SARS‐CoV‐2 infection in August 2020. Due to persistent fever over 39°C with significant weight loss, the patient was hospitalized 34 days after symptom onset, with elevated C‐reactive protein at 107 mg/L, absence of B‐cell but normal gammaglobulin level at 7.1 g/L. The chest CT showed an increase of ground‐glass opacities, to 10%‒25% of the parenchyma (Figure 1B). On day 34, SARS‐CoV‐2 PCR was negative on the nasopharyngeal swab, but BAL revealed a high viral load (Table 1). Bacterial culture on BAL showed only 102 colony‐forming unit/ml Pseudomonas aeruginosa in culture. Intravenous dexamethasone at 6 mg/day and 2 g of ceftazidime every 8 h were initiated (Figure 1C). After 7 days, the patient still presented fever over 40°C and started to require supplemental oxygen. SARS‐CoV‐2 PCR was positive on plasma on Day 45. The chest CT revealed new ground‐glass opacities reaching 25%–50% of the parenchyma (Figure 1B). Remdesivir 200 mg was started on Day 47, followed by 100 mg/day for 9 days; also, two infusions of two units of convalescent plasma were performed. The patient's fever immediately decreased (Figure 1C). He was discharged on Day 56. On day 84, the patient remained afebrile. The IgA level was positive on Day 35, but the IgG level remained doubtful on Day 43 (Table 1). Five months later, serology was still negative.
Rituximab predisposes patients to a higher risk of infection, especially viral, although it was prescribed with other immunosuppressive agents in most reported cases of infection. 3 , 4
Here, we described protracted forms of SARS‐CoV‐2 infection with persistent fever and late respiratory worsening in two patients treated only with rituximab for autoimmune diseases. In SARS‐CoV‐2 infection, over 90% of immunocompetent patients develop immunoglobulin M and/or IgG within the first 14 days. 5 , 6 , 7 Interestingly, IgG anti‐SARS‐CoV‐2 were never positive in our two patients.
We hypothesized an incomplete clearance of SARS‐CoV‐2 due to an impaired or delayed humoral response. Previously, seven patients treated with anti‐CD20 agents with COVID‐19 showed inconsistent seroconversion but a favorable outcome. 1
Our second observation does not allow us to conclude the effectiveness of convalescent plasma, especially since the patient received remdesivir simultaneously. Nevertheless, the patient finally improved after more than 45 days of fever and persistent positive viral loads.
The use of convalescent plasma against SARS‐CoV‐2 could be effective in patients treated with anti‐CD20 antibodies.8, 9 Recently, Libster et al. showed that early administration of high‐titer convalescent plasma reduced the progression of COVID‐19.10 This treatment may be efficacious in patients with depleted B‐cells and protracted COVID‐19,8 justifying controlled trials in this population.
CONFLICT OF INTERESTS
Yves Hansmann reports personal fees from Pfizer, MSD, and Astellas, outside the submitted work. François Danion declares personal fees from Gilead, outside the submitted work. The other authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Concept and design: Yvon Ruch, Victor Gerber, and Louis Boehn. Management of patients: Yvon Ruch, Victor Gerber, Louis Boehn, Charlotte Kaeuffer, Axel Ursenbach, Estelle Rougier, Yves Hansmann, Nicolas Lefebvre, and François Danion. Collection of clinical data: all authors. Virological analyses: Aurélie Velay and Morgane Solis. Writing original draft: Victor Gerber, Yvon Ruch, and Louis Boehn. Writing review and editing: all authors.
Contributor Information
Victor Gerber, Email: victor_gerber@hotmail.fr.
Yvon Ruch, Email: yvon.ruch@chru-strasbourg.fr.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV‐negative patients: a report of 57 cases from the research on adverse drug events and reports project. Blood. 2009;113(20):4834‐4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldberg SL, Pecora AL, Alter RS, et al. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high‐dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood. 2002;99(4):1486‐1488. [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID‐19. J Autoimmun. 2020;114:102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID‐19. Clin Infect Dis Avr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B‐cell depleted patients with protracted COVID‐19 disease. Blood. 2020;136:2290‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinot M, Jary A, Fafi‐Kremer S, et al. Remdesivir failure with SARS‐CoV‐2 RNA‐dependent RNA‐polymerase mutation in a B‐cell immunodeficient patient with protracted COVID‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Libster R, Perez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe COVID‐19 in older adults. N Engl J Med. 2020. 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meca‐Lallana V, Aguirre C, Beatrizdel Río, Cardeñoso L, Alarcon T, Vivancos J. COVID‐19 in 7 multiple sclerosis patients in treatment with ANTI‐CD20 therapies. Mult Scler Relat Disord. 2020;44:102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tepasse PR, Hafezi W, Lutz M, et al. Persisting SARS‐CoV‐2 viremia after rituximab therapy: two cases with fatal outcome and a review of literature. Br J Haematol. 2020;190:185‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fafi‐Kremer S, Bruel T, Madec Y, et al. Serologic responses to SARS‐CoV‐2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020;59:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


