Abstract
Experts have forewarned about the coronavirus disease 2019 (COVID‐19) pandemic environment fomenting the rising incidence of alcohol use disorder (AUD) and alcohol‐associated liver disease (ALD). We performed a cross‐sectional study of ALD at our liver transplantation (LT) center, located in the initial U.S. epicenter, New York City. Centered around the “stay at home” order date in New York state, March 22, 2020, we defined three time periods: “pre‐COVID” (January 1, 2020‐March 21, 2020), “COVID‐quarantine” (March 22, 2020‐April 22, 2020), and “declining‐COVID” (April 23, 2020‐August 25, 2020). We found a 62% increase in interhospital patient transfers for ALD from pre‐COVID (20 of 93, 21%) to the declining‐COVID period (43 of 127, 34%). Our inpatient liver census with ALD also increased: 38% pre‐COVID, 45% COVID‐quarantine, and 49% declining‐COVID. Among 30 patients with severe alcoholic hepatitis (AH) not responding to medical therapy since March 22, 2020, 9 underwent early LT for AH (16% of the total number of early LT during our 8‐year program). Three of 9 early‐LT recipients reported specific COVID‐related stressors. All 25 previous LT recipients with established abstinence pre‐COVID maintained abstinence at follow‐up visits during the declining‐COVID period. Of the 6 recipients with sustained alcohol use within 6 months before March 22, 2020, half regained abstinence during the declining‐COVID period. Our findings help confirm the predictions of rising AUD and ALD as an immediate consequence of the COVID‐19 pandemic. This aftershock particularly affected ethnically diverse patients with ALD with high inpatient mortality, reflecting the disproportionate impact of COVID‐19 on underserved and minority populations. Alcohol relapse did not occur in long‐term early LT for AH recipients during the time of COVID‐19. This lends further support to AH being a viable indication for LT, with recipients able to demonstrate ongoing resilience in the face of this unprecedented universal stressor.
Abbreviations
- AH
alcoholic hepatitis
- ALD
alcohol‐associated liver disease
- AUD
alcohol use disorder
- COVID‐19
coronavirus disease 2019
- LT
liver transplantation
The coronavirus disease 2019 (COVID‐19) pandemic has been a uniquely universal stressor with widespread health fears, social isolation, and severe economic anxiety. Even during peak periods of lockdown, liquors stores remained open as essential businesses, online sales of alcohol increased 262% from 2019,( 1 ) and there has been a spike in self‐reported alcohol use.( 2 ) Medical experts have forewarned about the pandemic environment fomenting the rising incidence of alcohol use disorder (AUD) and alcohol‐associated liver disease (ALD).( 3 , 4 )
To provide an early account of this anticipated aftershock from COVID‐19, we performed a cross‐sectional study of ALD at our liver transplantation (LT) center, located in the initial U.S. epicenter, New York City.
Methods
Centered around the governor’s “stay‐at‐home” order date in New York State, March 22, 2020, we defined three time periods: “pre‐COVID” (January 1, 2020‐March 21, 2020), “COVID‐quarantine” (March 22, 2020‐April 22, 2020), and “declining‐COVID” (April 23, 2020‐August 25, 2020). The month period from March 22 to April 22 was chosen to allow the COVID‐related stressors to accumulate. While imprecise, we surmised that there would be some lag time for patients to adopt unhealthy coping strategies, leading to changes in alcohol use pattern.
We performed chart reviews of three separate cohorts of patients: (1) all patients transferred to our institution with a primary diagnosis of ALD at time of transfer (confirmed by chart review) during 2020 both before and since the COVID pandemic; (2) all patients who underwent early LT for severe alcoholic hepatitis (AH) since the onset of the pandemic; and (3) alcohol use since the pandemic started in prior early‐LT recipients for AH (a prospectively maintained cohort).
A diagnosis of AH was defined using National Institutes of Health Alumni Association criteria for probable or definite AH,( 5 ) based on chart reviews of clinical notes and biopsies where available. ALD was defined as alcohol‐associated cirrhosis or AH, and based on chart review of laboratory results, imaging studies, and clinical notes. Patients were included if the treating team felt that ALD was the most likely diagnosis.
Alcohol use was captured by reviewing all patient visits to any provider, where alcohol use was asked about or where alcohol biomarkers were checked. Sustained alcohol use was defined as alcohol consumption of four or more drinks in a day or at least one drink for 4 or more days in succession after LT. A “slip” was defined as any brief alcohol consumption episode followed by resumed abstinence.( 6 ) We defined abstinence as no alcohol use over the preceding 6 months.
COVID‐related stressors were defined as a stressor that could be directly attributed to the pandemic. We captured this retrospectively by reviewing all social worker notes, searching for mention of the pandemic resulting in a change in patient’s mental health or stress levels.
During the pandemic, a negative COVID nasopharyngeal swab polymerase chain reaction test was required before inpatient transfer, but no other changes to our interhospital transfer practice. During the “COVID‐quarantine” period, less‐urgent transfers were discouraged to our dedicated liver medicine service, due to hospital rationing.
Results
Hospitalizations for ALD in the Time of COVID‐19
There was a 62% increase in interhospital patient transfers for ALD from pre‐COVID (20 of 93, 21%) to the declining‐COVID period (43 of 127, 34%) (Supporting Fig. S1). Transfers to our center peaked in the period from May 22 to June 22, about 2 months after the stay‐at‐home order, when 13 of 28 transfers (46%) had a primary diagnosis of ALD. Geographically, most ALD transfers were from the New York City metropolitan area: Long Island (33%), followed by Brooklyn (17%), the Bronx (17%) and 10% each from Queens, Manhattan, and New Jersey.
While our liver service remained “open,” a sharp decline was seen in the median total liver census, from 44 inpatients pre‐COVID to 9 during COVID‐quarantine and rising to 23 in the declining‐COVID period. This was likely due to the marked decline of interhospital transfers during COVID‐quarantine (three ALD transfers) and the avoidance of health care facilities by patients. Overall, there was a rising proportion of patients with ALD out of the total liver census during the three time periods: 30 of 86 (35%) pre‐COVID, 23 of 47 (49%) COVID‐quarantine, and 33 of 79 (42%) declining‐COVID (Fig. 1).
FIG. 1.
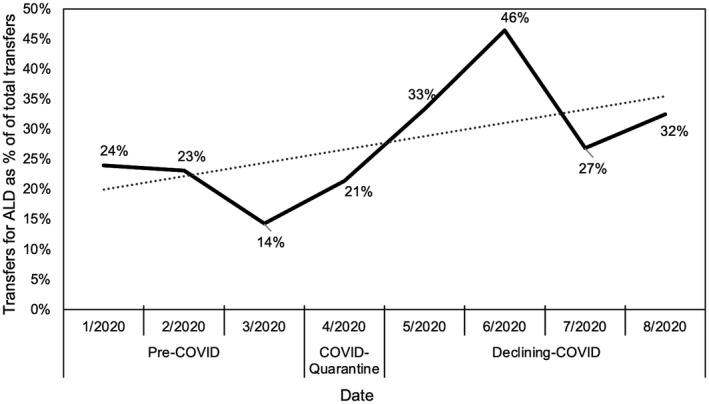
Transfers for ALD as a percentage of total transfers in 2020.
Nearly all (92%) of the 39 patients with AH transferred during the declining‐COVID period were new referrals (not previously seen at our center). Patients admitted with AH (49 in total) during this period were more likely to be male (P ≤ 0.001), less likely to be Caucasian (P = 0.01), and more likely to be of Hispanic ethnicity (P = 0.001), and trended toward being sicker based on discriminant function (P = 0.09) than those in the pre‐COVID period (Table 1). The overall inpatient mortality rate for patients with AH was 31% (21 of 67), and similarly high within each of the three time periods.
TABLE 1.
Characteristics of Patients With AH in the Time of COVID‐19
| Characteristic | Pre‐COVID | Declining‐COVID | P Value |
|---|---|---|---|
| Number of patients, n | 18 | 49 | |
| Median age in years (IQR) | 49 (37.5‐57.5) | 47 (39.5‐53) | 0.65 |
| Sex, female | 9 (50%) | 18 (37%) | <0.001 |
| Ethnicity | |||
| Caucasian | 15 (83%) | 24 (49%) | 0.01 |
| Hispanic | 0 | 13 (27%) | 0.01 |
| Asian‐Pacific Islander | 0 | 4 (8%) | 0.21 |
| African‐American | 2 (11%) | 6 (12%) | 0.90 |
| Unknown | 1 (6%) | 2 (4%) | |
| Drinks/day before admission, median (IQR) | 11 (5‐21.5) | 11.5 (6‐19) | 0.86 |
| Years of heavy drinking, median (IQR) | 9 (3.5‐18.5) | 10 (5‐20) | 0.46 |
| Discriminant function, median (IQR) | 62.5 (49‐87) | 80 (55‐97) | 0.09 |
| MELD‐Na on admission, median (IQR) | 32.5 (29‐35) | 34 (29‐40) | 0.24 |
| Creatinine in mg/dL, median (IQR) | 1.45 (0.6‐2.5) | 1.79 (0.91‐4.2) | 0.25 |
| Length of stay, median days (IQR) | 25 (14‐33) | 20 (10.5‐36) | 0.98 |
| Inpatient mortality rate | 7 (39%) | 14 (29%) | 0.42 |
Abbreviations: IQR, interquartile range; MELD‐Na, Model for End‐Stage Liver Disease–Sodium.
Early LT for AH in the Time of COVID‐19
Among 30 patients with severe AH not responding to medical therapy evaluated from March 22, 2020, 28 arrived through interhospital transfer, with 22 from a floor bed and 6 from an ICU. A total of 9 patients underwent early LT (<6 months of abstinence) for AH, as previously described.( 6 ) These 9 LTs performed over 5 months following the stay‐at‐home order (March 22, 2020‐August 25, 2020) accounted for 16% of the total number of early LT in the history of our 8‐year program. This demonstrated an accelerated rate of early LT from an average of 0.5 LT/month pre‐COVID to nearly 2 LTs/month.( 6 ) Two of these patients were transplanted during the COVID‐quarantine period. None required mechanical ventilation pre‐LT, and 5 out of 9 (56%) were on renal replacement therapy at the time of LT. Despite this accelerated tempo of early LT, our selection of candidates with favorable psychosocial profiles and life‐threatening ALD adhered to evidence‐based expert consensus (Supporting Table S1).( 7 ) Three of the 9 early‐LT recipients reported specific COVID‐related stressors: “colleagues [in funeral home] dying from COVID,” “drinking more due to the pandemic,” and “stress of job as police officer and frustration with inadequate personal protective equipment.”
Alcohol Use in Recipients of Early LT for AH in the Time of COVID‐19
To determine the impact of COVID‐19 stress on early LT for AH recipients, we examined their follow‐up visits and laboratory data in the declining‐COVID period. After excluding deaths and recent LT recipients (< 6 months following LT), 42 early LT for AH recipients were analyzed. Somewhat remarkably, all 25 recipients with established abstinence pre‐COVID (median 1,034 days following LT [interquartile range 485‐1670]) maintained abstinence at a follow‐visit during the declining‐COVID period based on patient interviews and negative alcohol biomarkers (urine ethyl glucuronide and serum phosphatidylethanol), drawn routinely at each visit.( 6 ) Telehealth was used in 34% of visits, with 46% having corresponding laboratory testing. Of the 6 recipients with sustained alcohol use within 6 months before March 22, 2020, half continued to drink, but the other half regained abstinence during the declining‐COVID period, as confirmed by alcohol biomarkers. Eleven recipients did not have a follow‐up visit during this latter period.
Discussion
This early cross‐sectional report from the initial U.S. epicenter of COVID‐19 demonstrates a significant aftershock of ALD that may reverberate throughout the country in different stages based on regional COVID‐19 infection rates, access to alcohol, and socio‐political strategies of containment. Unlike studies using transplant registries, we were able to evaluate the impact of COVID‐19 on high‐risk patients with ALD at several program levels and time periods, including those hospitalized/transferred for ALD, LT candidates, and recipients in our program of early LT for AH.( 6 )
Limitations of the study include its retrospective nature and single‐center description with small numbers of patients, but it provides a timely account from the initial U.S. epicenter of the COVID‐19 pandemic. Although it is possible that increases in hospitalized patients with ALD could be related to self‐delayed or geographic shifting of liver care from other centers, nationally representative surveys have demonstrated poorer mental health, increased stress, and increased alcohol use during the COVID‐19 pandemic.( 2 ) Throughout 2020, there were contemporaneous stressors, including the racial injustice movement and presidential election, which may have affected patients’ drinking patterns and resilience, but we found no supporting evidence for this in the medical record, nor following review with the social work team.
These preliminary findings support the predictions of rising AUD and ALD as an immediate consequence of the COVID‐19 pandemic. These aftershocks at our center particularly affected ethnically diverse patients with ALD with high inpatient mortality, reflecting the disproportionate impact of COVID‐19 on underserved and minority populations in the United States.( 8 ) In response, the hepatology community needs to anticipate and prepare for these continued aftershocks of ALD. Developing streamlined care pathways to addiction services and specialists for recovering patients with ALD, increased use of telehealth, promotion of psychosocial and pharmacologic therapies for AUD, and wider implementation of (or rapid referral to) early LT for AH programs based on evidence‐based guidelines will be critical.( 7 , 9 )
Alcohol relapse did not occur in long‐term early LT for AH recipients during the time of COVID‐19. This lends further support to AH being a viable indication for LT, with recipients able to demonstrate ongoing resilience in the face of this unprecedented universal stressor. Further studies are needed to determine the longer‐term aftershocks of COVID‐19 on patients with ALD.
Supporting information
Fig S1
Table S1
Potential conflict of interest: Nothing to report.
References
- 1. The Nielsen Company . Rebalancing the ‘COVID‐19 Effect’ on alcohol sales. May 7, 2020. https://nielseniq.com/global/en/insights/2020/rebalancing‐the‐covid‐19‐effect‐on‐alcohol‐sales/. [Google Scholar]
- 2. Pollard MS, Tucker JS, Green HD. Changes in adult alcohol use and consequences during the COVID‐19 pandemic in the US. JAMA Network Open 2020;3:e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spagnolo PA, Montemitro C, Leggio L. New challenges in addiction medicine: COVID‐19 infection in patients with alcohol and substance use disorders—the perfect storm. Am J Psychiatry 2020;177:805‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Da BL, Im GY, Schiano TD. COVID‐19 hangover: a rising tide of alcohol use disorder and alcohol‐associated liver disease. Hepatology 2020;72:1102‐1108. [DOI] [PubMed] [Google Scholar]
- 5. Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Im GY, Kim‐Schluger L, Shenoy A, Schubert E, Goel A, Friedman SL, et al. Early liver transplantation for severe alcoholic hepatitis in the United States—a single‐center experience. Am J Transplant 2016;16:841‐849. [DOI] [PubMed] [Google Scholar]
- 7. Asrani SK, Trotter J, Lake J, Ahmed A, Bonagura A, Cameron A, et al. Meeting report: the Dallas consensus conference on liver transplantation for alcohol associated hepatitis. Liver Transpl 2020;26:127‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clin Infect Dis 2021;72:703‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol‐associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306‐333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


