Abstract
Aims
To examine the association between dexamethasone use and mortality among patients hospitalized for COVID‐19.
Methods
We examined the association between dexamethasone use and mortality at AP‐HP Greater Paris University hospitals. Study baseline was defined as the date of hospital admission. The primary endpoint was time to death. We compared this endpoint between patients who received dexamethasone and those who did not in time‐to‐event analyses adjusted for patient characteristics (such as age, sex and comorbidity) and clinical and biological markers of clinical severity of COVID‐19, and stratified by the need for respiratory support, i.e. mechanical ventilation or oxygen. The primary analysis was a multivariable Cox regression model.
Results
Of 12 217 adult patients hospitalized with a positive COVID‐19 reverse transcriptase–polymerase chain reaction test, 171 (1.4%) received dexamethasone orally or by intravenous perfusion during the visit. Among patients who required respiratory support, the end‐point occurred in 10/63 (15.9%) patients who received dexamethasone and 298/1129 (26.4%) patients who did not. In this group, there was a significant association between dexamethasone use and reduced mortality in the primary analysis (hazard ratio, 0.46; 95% confidence interval 0.22–0.96, P = .039). Among patients who did not require respiratory support, there was no significant association between dexamethasone use and the endpoint.
Conclusions
In this multicentre observational study, dexamethasone use administered either orally or by intravenous injection at a cumulative dose between 60 mg and 150 mg was associated with reduced mortality among patients with COVID‐19 requiring respiratory support.
Keywords: COVID‐19, dexamethasone, efficacy, mortality, oxygen, SARS‐CoV‐2, treatment, ventilation
What is already known about this subject
The RECOVERY (Randomized Evaluation of COVid‐19 thERapY) trial supports that dexamethasone administered at 6 mg once per day for 10 days is associated with reduced mortality only in patients with COVID‐19 requiring respiratory support (i.e. oxygen or mechanical ventilation).
What this study adds
In a multicentre observational study of patients hospitalized for COVID‐19, we found that dexamethasone use administered either orally or by intravenous injection at a cumulative dose between 60 and 150 mg was associated with reduced mortality only in patients requiring respiratory support.
1. INTRODUCTION
Global spread of the novel coronavirus SARS‐CoV‐2, the causative agent of coronavirus disease 2019 (COVID‐19), has created an unprecedented infectious disease crisis worldwide. 1 , 2 , 3 , 4
The RECOVERY (Randomized Evaluation of COVid‐19 thERapY) trial, a randomized clinical trial examining a range of potential treatments for COVID‐19, indicated that low‐dose dexamethasone could reduce mortality in patients with COVID‐19 requiring oxygen or mechanical ventilation support. 5 In that study, a total of 2104 patients were randomized to receive dexamethasone 6 mg once per day for 10 days, administered either orally or by intravenous injection, and were compared with 4321 patients randomized to usual care alone. Dexamethasone was significantly associated with reduced 28‐day mortality in ventilated patients (29.3 vs. 41.4%; rate ratio [RR], 0.64; 95% confidence interval [CI], 0.51–0.81) and in patients receiving oxygen only (23.3 vs. 26.2%; RR, 0.82; 95% CI, 0.72–0.94). No benefit was observed among patients who did not require respiratory support (17.8 vs. 14.0%; RR, 1.19; 95% CI, 0.91–1.55).
These findings are of utmost importance and highlight that research into dexamethasone use in patients with COVID‐19 is a priority. 6
In this report, we present results of a multicentre retrospective observational study of patients admitted for COVID‐19 to 36 Greater Paris University hospitals. We examined whether oral or intravenous administration of dexamethasone to hospitalized adult patients with COVID‐19 was associated with reduced mortality: (i) among those who required respiratory support, i.e. mechanical ventilation or oxygen; and (ii) in those who did not. Following results of the RECOVERY trial, 5 we hypothesized that dexamethasone administration would be associated with reduced mortality in patients with COVID‐19 who required respiratory support, and not in those who did not.
Although randomized controlled trials (RCTs) are considered the gold standard for clinical research, thus having a high impact on clinical guidelines and daily patients' care, observational studies are also important because they can bring important complementary information in the interpretation of the safety, efficacy and effectiveness of a therapeutic option with greater external validity, i.e. in a population more closely resembling the target population, and in different subpopulations. 7 , 8 Similar results from RCTs and observational studies can increase the confidence in the efficacy of a treatment, 9 , 10 as suggested by a prior study that found little evidence that estimates of treatment effects in observational studies reported after 1984 are either consistently larger than or qualitatively different from those obtained in RCTs. 11 Specifically, if this observational study yielded similar results as those found in the RECOVERY trial, it would (i) increase the confidence in the efficacy of dexamethasone in patients with COVID‐19 who require respiratory support; and (ii) support the usefulness of observational studies of patients with COVID‐19 taking medications for other indications by showing that they could help decide which treatment should be prioritized for future RCTs and reduce the risk for patients of being exposed to potentially harmful and ineffective treatments in RCTs. 12 , 13 , 14 , 15 Finally, exploring the associations of different doses of dexamethasone with mortality in patients with COVID‐19 could bring useful information to help guide design future RCTs of dexamethasone in patients with COVID‐19. 14 , 15
2. METHODS
2.1. Setting
We conducted this study in 36 Assistance Publique–Hôpitaux de Paris (AP‐HP) hospitals. We included all adults aged 18 years or over who have been admitted with COVID‐19 to these medical centres from the beginning of the epidemic in France, i.e. 24 January until 20 May. For all patients, COVID‐19 was ascertained by a positive reverse transcriptase–polymerase chain reaction (RT‐PCR) test from analysis of nasopharyngeal or oropharyngeal swab specimens. This observational study using routinely collected data received approval from the Institutional Review Board of the AP‐HP clinical data warehouse (decision CSE‐20‐20_COVID19, IRB00011591). AP‐HP clinical Data Warehouse initiative ensures patients' information and consent regarding the different approved studies through a transparency portal in accordance with European Regulation on data protection and authorization n°1 980 120 from National Commission for Information Technology and Civil Liberties.
2.2. Data sources
We used data from the AP‐HP Health Data Warehouse (Entrepôt de Données de Santé [EDS]). 9 This warehouse contains all the clinical data available on all inpatient visits for COVID‐19 to Greater Paris University hospitals. The data obtained included patient demographic characteristics, vital signs, laboratory test and RT‐PCR test results, medication administration data, current medication lists, current diagnoses, oxygen and ventilator use data, and death certificates.
2.3. Variables assessed
We obtained the following data for each patient at the time of hospital admission: sex, age, 10 obesity, current smoking status, any medical condition associated with increased risk of severe COVID‐19, 10 , 16 and clinical and biological markers of severe COVID‐19 17 , 18 at admission. These variables are detailed in Supplementary Text 1.
All medical notes and prescriptions are computerized in Greater Paris University hospitals. Medications and their mode of administration (i.e. dose, frequency, date, condition of intake) were identified from unstructured databases including medication administration data or scanned hand‐written medical prescriptions through 2 deep learning models based on BERT contextual embeddings allowing for natural language processing, 19 1 for the medications and another for their mode of administration. The model was trained on the APmed corpus, 20 a previously annotated dataset for this task. Extracted medications names were then normalized to the Anatomical Therapeutic Chemical terminology using approximate string matching.
2.4. Endpoint
The endpoint was the time from study baseline to death. Patients without an end‐point event had their data censored on 20 May 2020.
2.5. Dexamethasone use
Study baseline was defined as the date of hospital admission. Dexamethasone use was defined as receiving this medication orally or by intravenous injection at any time during the follow‐up period, from study baseline to the end of the index hospitalization or death.
2.6. Dexamethasone cumulative dose
Dexamethasone cumulative dose received was calculated and considered as a categorical variable with the following categories defined a priori: (i) 60–150 mg based on usual prescribing practice for acute respiratory distress syndrome in AP‐HP hospitals (corresponding to 10 mg/d for 6 days, to 20 mg/d for 5 days followed by 10 mg/d for 5 days); (ii) other cumulative doses (i.e. >150 or <60 mg); and (iii) missing data.
2.7. Statistical analysis
All analyses were stratified by the need for respiratory support, i.e. oxygen or mechanical ventilation, at any time during the follow‐up.
We calculated frequencies of each baseline characteristic described above in patients receiving and not receiving dexamethasone, and compared them using χ2 tests.
To examine the association of dexamethasone use with the endpoint, we performed Cox proportional‐hazards regression models. To help account for the nonrandomized prescription of dexamethasone and reduce the effects of confounding, the primary analysis used a multivariable Cox regression model including as covariates sex, age, obesity, current smoking status, any medical condition associated with severe COVID‐19, and clinical and biological markers of severe COVID‐19 at admission. Weighted Cox regression models were used when the proportional hazards assumption was not met. Kaplan–Meier curves were performed and their pointwise 95% CIs were estimated using the nonparametric bootstrap method. 21
As a sensitivity analysis, we performed a univariate Cox regression model in a matched analytic sample using a 1:10 ratio, based on the same variables used for the multivariable Cox regression analysis. To reduce the effects of confounding, optimal matching was used in order to obtain the smallest average absolute distance across all clinical characteristics between exposed patient and nonexposed matched controls.
We performed additional analyses. First, we examined whether the cumulative dose of dexamethasone received during the visit was associated with the endpoint of death. Second, we performed multivariable Cox regression models including interaction terms to examine whether the association between dexamethasone use and the endpoint significantly differed across baseline characteristics.
For all associations, we performed residual analyses to assess the fit of the data, check assumptions, including proportional hazards assumptions, and examined the potential influence of outliers. To improve the quality of result reporting, we followed the recommendations of The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Initiative. 22 Statistical significance was fixed a priori at 2‐sided P‐value < .05. All analyses were conducted in R software version 2.4.3 (R Project for Statistical Computing).
3. RESULTS
3.1. Characteristics of the cohort
Of the 16 170 with a positive COVID‐19 RT‐PCR test consecutively admitted to the 36 AP‐HP hospitals from 24 January to 20 May 2020, a total of 3953 patients (24.4%) were excluded because of missing data or their age (i.e. <18 y). Of the remaining 12 217 adult inpatients, 178 patients (1.46%) received dexamethasone. Of them, 7 (3.9%) were excluded because the route of administration was either ophthalmic, through aerosol or unknown. Among the 171 remaining patients who received dexamethasone, 63 (36.8%) needed respiratory support (i.e. mechanical ventilation or oxygen) and 108 (63.2%) did not. Among the 12 039 patients who did not receive dexamethasone during the visit, 1129 (9.4%) required respiratory support and 10 910 (90.6%) did not (Figure 1). The mean cumulative dose administered was 107.8 mg (standard deviation [SD] = 63.5; median = 100 mg; range: 10.0–320.0 mg) in those who required respiratory support and 101.8 mg (SD = 89.8; median = 90 mg; range: 10.0–600.0 mg) in those who did not. This treatment was administered orally in 97.1% of patients and by intravenous injection in 2.9% of them.
FIGURE 1.
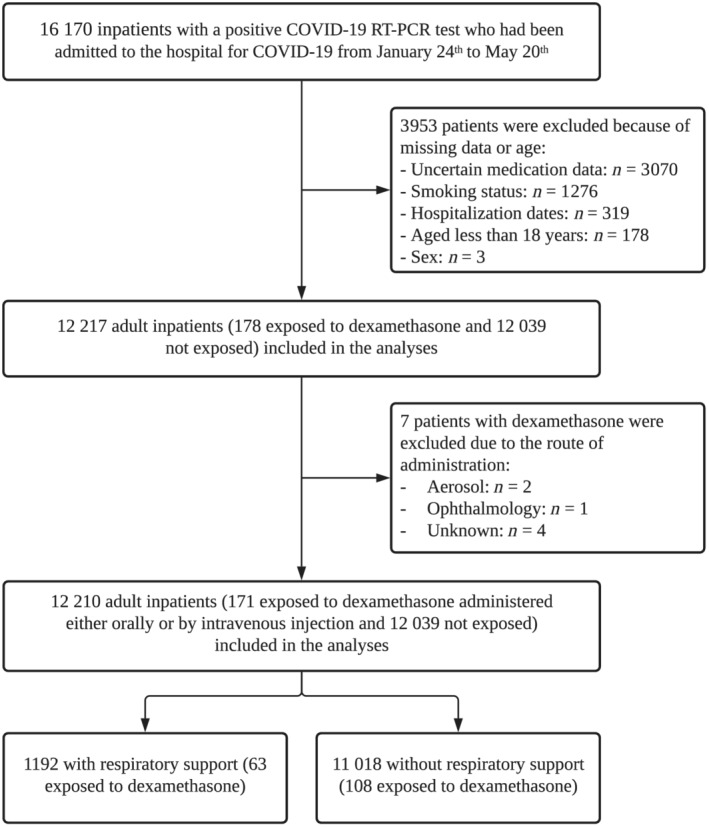
Study cohort
COVID‐19 RT‐PCR test results were obtained after a mean delay of 3.7 days (SD = 8.4, median = 0.9 days) from the date of hospital admission in patients who required respiratory support. This delay did not significantly differ between patients receiving or not receiving dexamethasone (mean delay in the exposed group = 3.0 days [SD = 6.2]; mean delay in the nonexposed group = 3.7 days (SD = 8.5); Welch's t‐test = 0.79, P = .430]). In patients who did not require respiratory support, the mean delay was 4.7 days (SD = 10.2, median = 1.0 day), and this delay did not significantly differ between patients receiving or not receiving dexamethasone [mean delay in the exposed group = 3.5 days (SD = 8.9); mean delay in the nonexposed group = 4.8 day (SD = 10.2); Welch's t‐test = 1.5, P = .163)].
Among patients who required respiratory support, the mean follow‐up was 27.9 days (SD = 20.4; median = 21 days; range: 1 day to 106 days) and 308 patients (25.8%) had an end‐point event at the time of data cut‐off on 20 May. Among those who did not require respiratory support, the mean follow‐up was 18.5 days (SD = 24.6; median = 6; range: 1–117 days), and 1100 (10.0%) patients had an end‐point event at the time of data cut‐off.
Associations between baseline characteristics and the endpoint are given in Table S1. The distribution of the patient characteristics by dexamethasone use is shown in Table 1. Dexamethasone use significantly differed in clinical and biological markers of severity at admission among patients who required respiratory support, and in sex, age, obesity and biological markers of severity at admission among those who did not. In the matched analytic samples, there were no significant differences in patient characteristics according to dexamethasone use (Table 1).
TABLE 1.
Characteristics of patients with or without respiratory support (oxygen or intubation) according to dexamethasone use
| Exposed to dexamethasone | Not exposed to dexamethasone | Nonexposed matched group | Exposed to dexamethasone vs. not exposed to dexamethasone (crude analysis) | Exposed to dexamethasone vs. nonexposed matched group | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | χ2 test (P‐value) | χ2 test (P‐value) | |
| With respiratory support | 63 (100%) | 1129 (100%) | 630 (100%) | ||
| Sex | <0.01 (>.99) | 0.09 (.770) | |||
| Female | 17 (27.0%) | 308 (27.3%) | 154 (24.4%) | ||
| Male | 46 (73.0%) | 821 (72.7%) | 476 (75.6%) | ||
| Age (y) | 2.35 (.309) | .14 (.931) | |||
| 18–50 | 10 (15.9%) | 239 (21.2%) | 102 (16.2%) | ||
| 51–70 | 40 (63.5%) | 606 (53.7%) | 386 (61.3%) | ||
| >70 | 13 (20.6%) | 284 (25.2%) | 142 (22.5%) | ||
| Obesity a | 0.25 (.621) | <0.01 (.946) | |||
| Yes | 16 (25.4%) | 329 (29.1%) | 168 (26.7%) | ||
| No | 47 (74.6%) | 800 (70.9%) | 462 (73.3%) | ||
| Smoking b | 0.11 (.736) | <0.01 (.961) | |||
| Yes | 11 (17.5%) | 170 (15.1%) | 103 (16.3%) | ||
| No | 52 (82.5%) | 959 (84.9%) | 527 (83.7%) | ||
| Any medical condition c | 2.20 (.138) | 1.83 (.176) | |||
| Yes | 36 (57.1%) | 757 (67.1%) | 419 (66.5%) | ||
| No | 27 (42.9%) | 372 (32.9%) | 211 (33.5%) | ||
| Clinical severity of Covid‐19 at admission d | 12.71 (.002*) | 2.75 (.253) | |||
| Yes | 23 (36.5%) | 529 (46.9%) | 278 (44.1%) | ||
| No | 33 (52.4%) | 354 (31.4%) | 262 (41.6%) | ||
| Missing | 7 (11.1%) | 246 (21.8%) | 90 (14.3%) | ||
| Biological severity of Covid‐19 at admission e | 9.59 (.008*) | 0.20 (.906) | |||
| Yes | 52 (82.5%) | 716 (63.4%) | 507 (80.5%) | ||
| No | 8 (12.7%) | 279 (24.7%) | 93 (14.8%) | ||
| Missing | 3 (4.76%) | 134 (11.9%) | 30 (4.76%) | ||
| Without respiratory support | 108 (100%) | 10 910 (100%) | 1080 (100%) | ||
| Sex | 21.70 (<.001*) | 0.05 (.820) | |||
| Women | 32 (29.6%) | 5738 (52.6%) | 337 (31.2%) | ||
| Men | 76 (70.4%) | 5172 (47.4%) | 743 (68.8%) | ||
| Age (y) | 28.86 (<.001*) | 0.17 (.918) | |||
| 18–50 | 16 (14.8%) | 4153 (38.1%) | 147 (13.6%) | ||
| 51–70 | 54 (50.0%) | 3338 (30.6%) | 559 (51.8%) | ||
| >70 | 38 (35.2%) | 3419 (31.3%) | 374 (34.6%) | ||
| Obesity a | 6.87 (.009*) | <0.01 (>.99) | |||
| Yes | 22 (20.4%) | 1279 (11.7%) | 220 (20.4%) | ||
| No | 86 (79.6%) | 9631 (88.3%) | 860 (79.6%) | ||
| Smoking b | 1.47 (.225) | <0.01 (>.99) | |||
| Yes | 13 (12.0%) | 908 (8.32%) | 130 (12.0%) | ||
| No | 95 (88.0%) | 10 002 (91.7%) | 950 (88.0%) | ||
| Any medical condition c | 1.76 (.184) | <0.01 (.992) | |||
| Yes | 33 (30.6%) | 2679 (24.6%) | 336 (31.1%) | ||
| No | 75 (69.4%) | 8231 (75.4%) | 744 (68.9%) | ||
| Clinical severity of Covid‐19 at admission d | 5.49 (.064) | <0.01 (.998) | |||
| Yes | 17 (15.7%) | 2022 (18.5%) | 170 (15.7%) | ||
| No | 38 (35.2%) | 2763 (25.3%) | 377 (34.9%) | ||
| Missing | 53 (49.1%) | 6125 (56.1%) | 533 (49.4%) | ||
| Biological severity of Covid‐19 at admission e | 61.83 (<.001*) | 0.01 (.993) | |||
| Yes | 69 (63.9%) | 3254 (29.8%) | 696 (64.4%) | ||
| No | 22 (20.4%) | 2903 (26.6%) | 216 (20.0%) | ||
| Missing | 17 (15.7%) | 4753 (43.6%) | 168 (15.6%) |
Defined as having a body mass index >30 kg/m2 or an International Statistical Classification of Diseases and Related Health Problems (ICD‐10) diagnosis code for obesity (E66.0, E66.1, E66.2, E66.8, E66.9).
Current smoking status was self‐reported.
Assessed using ICD‐10 diagnosis codes for diabetes mellitus (E11), diseases of the circulatory system (I00–I99), diseases of the respiratory system (J00–J99), neoplasms (C00–D49), and diseases of the blood and blood‐forming organs and certain disorders involving the immune mechanism (D5–D8).
Defined as having at least 1 of the following criteria: respiratory rate >24 breaths/min or <12 breaths/min, resting peripheral capillary oxygen saturation in ambient air <90%, temperature >40°C, or systolic blood pressure < 100 mmHg.
Defined as having at least 1 of the following criteria: high neutrophil‐to‐lymphocyte ratio, low lymphocyte‐to‐C‐reactive protein (both variables were dichotomized at the median of the values observed in the full sample), and plasma lactate >2 mmol/L.
P‐value is significant (P < .05).
3.2. Study endpoint
Among patients with COVID‐19 who required respiratory support, the end‐point event of death occurred in 10/63 patients (15.9%) who received dexamethasone and 298/1129 patients (26.4%) who did not (Figure 2). In this group of patients, we found a significant association between dexamethasone use and reduced mortality in both the crude, unadjusted analysis (hazard ratio [HR], 0.40; 95% CI, 0.18–0.87, P = .021) and the primary multivariable Cox regression analysis (HR, 0.46; 95% CI, 0.22–0.96, P = .039; Figures 2, 3). In the sensitivity analysis, the univariate Cox regression model in the matched analytic sample yielded a same tendency, albeit nonsignificant (HR, 0.31; 95% CI, 0.08–1.14, P = .077; Figures 2, 4).
FIGURE 2.
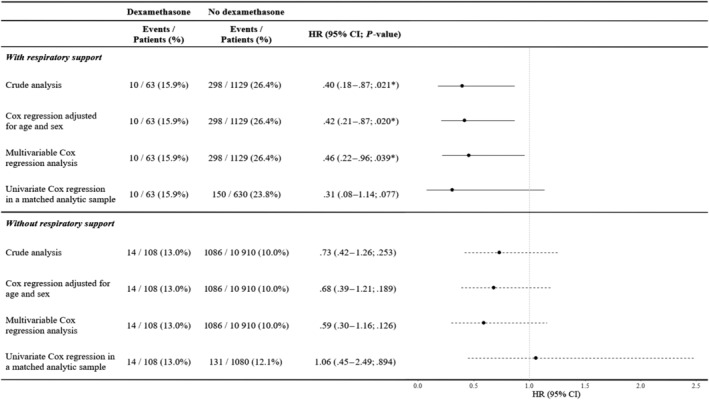
Association between dexamethasone use and time to death in the full sample and in the matched analytic sample. * P‐value is significant (P < .05). HR, hazard ratio; CI, confidence interval
FIGURE 3.
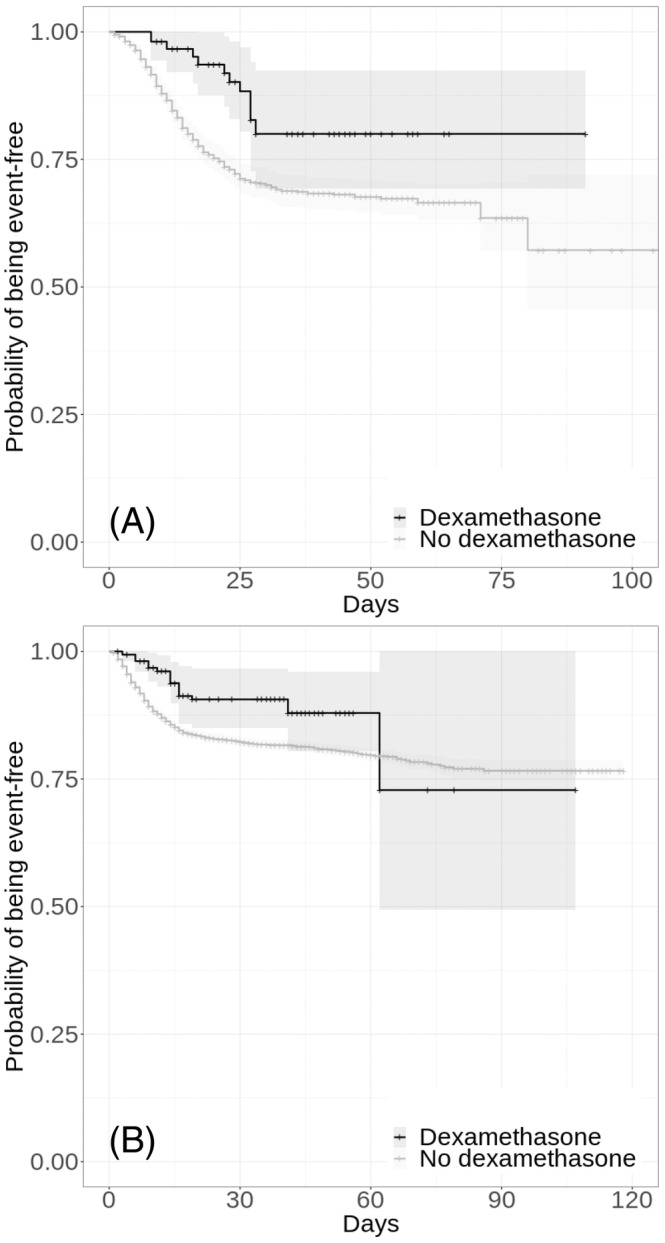
Kaplan–Meier curves for time to death in the full samples of patients hospitalized for COVID‐19 who required respiratory support (i.e. mechanical ventilation or oxygen; n = 1192; A), and of those who did not (n = 11 018; B), according to dexamethasone use. The shaded areas represent pointwise 95% confidence intervals
FIGURE 4.
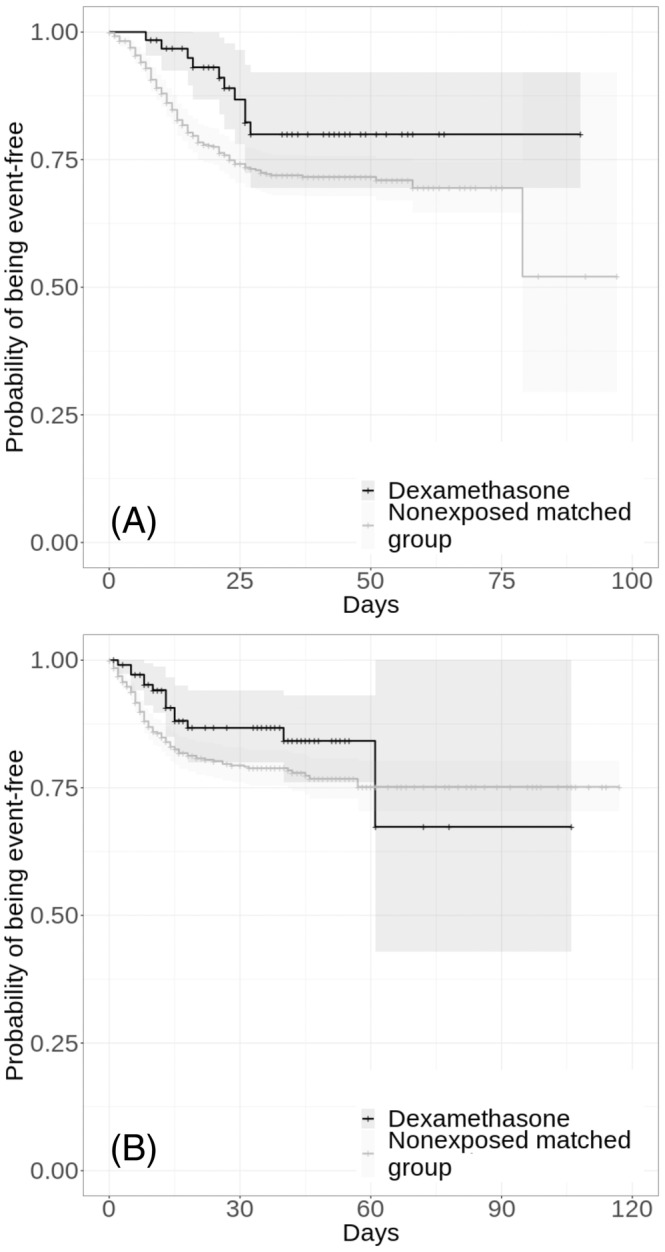
Kaplan–Meier curves for time to death in the matched analytic samples of patients hospitalized for COVID‐19 who required respiratory support (i.e. mechanical ventilation or oxygen; n = 693; A) and of those who did not (n = 1188; B), according to dexamethasone use. The shaded areas represent pointwise 95% confidence intervals. For each exposed case, 10 nonexposed controls were selected
Among patients with COVID‐19 who did not require respiratory support, the end‐point event of death occurred in 14/108 patients (13.0%) who received dexamethasone and 131/1086 patients (12.1%) who did not (Figure 2). In this group of patients, there was no significant association between dexamethasone use and the endpoint, neither in the crude, unadjusted analysis (HR, 0.73; 95% CI, 0.42–1.26, P = .253) or in the primary multivariable Cox regression analysis (HR, 0.59; 95% CI, 0.30–1.16, P = .126; Figures 2, 3). In the sensitivity analysis, the univariate Cox regression model in the matched analytic sample yielded a similar nonsignificant result (HR, 1.06; 95% CI, 0.45–2.49, P = .894; Figures 2, 4).
A posthoc analysis indicated that in the full sample of patients with respiratory support, we had 80% power to detect unadjusted hazard ratios for dexamethasone of at least 2.33/0.15 and of at least 2.37/0.14 in the matched analytic sample. In those without respiratory support, we had 80% power to detect unadjusted hazard ratios for dexamethasone of at least 1.99/0.30 in the full sample and 2.05/0.29 in the matched analytic sample using a 1:10 ratio.
When examining the association between the cumulative dose of dexamethasone received during the visit and the endpoint, we found that the administration of a cumulative dose between 60 and 150 mg among patients who required respiratory support was significantly associated with reduced mortality in the crude, unadjusted analysis (HR, 0.28; 95% CI, 0.08–0.87, P = .028), the multivariable Cox regression analysis (HR, 0.24; 95% CI, 0.07–0.87, P = .030), and in the univariate Cox regression model in the matched analytic sample using a 1:10 ratio (HR, 0.32; 95% CI, 0.07–0.95; P = .048), whereas no significant association was observed with a different dose (Table S2, Figure S1). Among patients without respiratory support, there was no significant association between the cumulative dose of dexamethasone and the endpoint in the crude, unadjusted analysis (HR, 0.37; 95% CI, 0.12–1.16, P = .089) and the adjusted multivariable analysis (HR, 0.47; 95% CI, 0.15–2.42, P = .178). However, the administration of a cumulative dose between 60 and 150 mg was significantly associated with the endpoint in the univariate Cox regression model in the matched analytic sample (HR, 0.32; 95% CI, 0.15–0.99, P = .049; Table S2, Figure S1).
Finally, the association between dexamethasone use and the endpoint did not significantly differ across subgroups defined by baseline characteristics in both groups with and without respiratory support, except for patients with obesity who did not require respiratory support, for whom dexamethasone use was significantly associated with increased mortality compared to obese patients without dexamethasone (HR, 3.90; 95% CI, 1.13–13.44, P = .031; Table S3). However, none of the 16 patients with obesity who received a cumulative dose of 60–150 mg of dexamethasone died.
4. DISCUSSION
In this multicentre retrospective observational study involving a large sample of patients hospitalized for COVID‐19, we found that dexamethasone use, administered either orally or by intravenous injection at a cumulative dose between 60 and 150 mg, was significantly and substantially associated with reduced mortality among patients with COVID‐19 requiring oxygen or mechanical ventilation support. This association did not significantly differ according to baseline clinical characteristics. No significant association between dexamethasone use and mortality was observed among patients with COVID‐19 without respiratory support, except in the univariate Cox regression model in the matched analytic sample, whereas the administration of a cumulative dose between 60 and 150 mg was significantly associated with reduced mortality.
Although these findings should be interpreted with caution due to the observational design, they are in line with the results of the RECOVERY trial, 5 which indicated that dexamethasone 6 mg once per day for 10 days, administered either orally or by intravenous injection, was significantly associated with reduced 28‐day mortality in ventilated patients and in patients receiving oxygen only.
The benefits of dexamethasone for patients with COVID‐19 probably arise from its immunosuppressive properties. A prior study 23 suggests that low‐dose dexamethasone treatment could complement endogenous cortisol activity to suppress COVID‐19‐associated immunopathology, while avoiding the adverse effects of high‐dose glucocorticoid therapy. 23 , 24 Many immune‐modulating effects of glucocorticoids reflect cell type‐specific changes in the transcriptome, 23 tempering the specialized activities of different immune cell types, such as B and T cells. Inhibitory interactions between glucocorticoid receptors and the transcription factors nuclear factor‐κB and activator protein‐1 may also be important modes of glucocorticoid anti‐inflammatory action. An important observation from the RECOVERY trial and our observational study was that dexamethasone provided benefit only to severely ill patients with COVID‐19, in whom acute respiratory distress syndrome, sepsis and, eventually, organ failure may reflect hyperinflammatory state, a phase of COVID‐19 where the immunomodulatory effects of glucocorticoids are likely to be beneficial, perhaps by breaking the inflammatory feedforward loop, at least in some patients. 23 , 24
Our findings, beyond increasing the confidence in the efficacy of dexamethasone in patients with COVID‐19 who require respiratory support, also support the usefulness of observational studies of patients with COVID‐19 taking medications for other indications, by showing that they can help decide which treatment should be prioritized for future RCTs and reduce the risk for patients of being exposed to potentially harmful and ineffective treatments in RCTs. 12 , 13 , 14 , 15
Our study has several limitations. First, there are 2 possible major inherent biases in observational studies: unmeasured confounding and confounding by indication. Some amount of unmeasured confounding may remain. However, our analyses adjusted for numerous potential confounders, including sex, age, obesity, current smoking status, any medical condition associated with severe COVID‐19, and clinical and biological markers of severe COVID‐19 at admission. Second, there are missing data for some variables and potential for inaccuracies in the electronic health records, such as the possible lack of documentation of illnesses or medications, or the misidentification of treatment mode of administration (e.g. dose, frequency), especially for hand‐written medical prescriptions. Third, our study cannot establish causal relationships. Finally, despite the multicentre design, our results may not be generalizable to outpatients or other regions.
In this multicentre observational study, dexamethasone use administered either orally or by intravenous injection was associated with decreased mortality among adult patients hospitalized for COVID‐19 requiring respiratory support.
COMPETING INTERESTS
Dr Hoertel has received personal fees and nonfinancial support from Lundbeck, outside the submitted work. Dr Limosin has received speaker and consulting fees from Janssen‐Cilag outside the submitted work. Other authors declare no competing interests.
CONTRIBUTORS
N.H. designed the study, contributed to statistical analyses, and wrote the first draft of the manuscript. M.S.R. contributed to study design, performed statistical analyses and critically revised the manuscript. F.L. contributed to study design and critically revised the manuscript for scientific content. R.V. contributed to statistical analyses and critically revised the manuscript for scientific content. N.B. contributed to study design and critically revised the manuscript for scientific content. N.B., A.S.J., A.N., N.P., C.D., A.G., G.L., M.B. and A.B. contributed to database build process. A.N., N.P., C.D., A.G., G.L., M.B., E.S., A.B. and J.M.A. critically revised the manuscript for scientific content.
Supporting information
FIGURE S1 Kaplan–Meier curves for time to death in patients hospitalized for COVID‐19 who required respiratory support (i.e. mechanical ventilation or oxygen; n = 1192; A) and in those who did not (n = 11 008; B), according to dexamethasone cumulative dose. The shaded areas represent pointwise 95% confidence intervals.
TABLE S1 Associations of baseline clinical characteristics with the endpoint of death in patients with and without respiratory support (i.e. oxygen or intubation).
TABLE S2 Associations between dexamethasone cumulative dose and the endpoint of death, in the full sample and in the matched analytic sample.
TABLE S3 Interaction effect of baseline characteristics with dexamethasone use on the endpoint of death among adult inpatients with COVID‐19.
ACKNOWLEDGEMENTS
The authors thank the EDS AP‐HP COVID consortium integrating the AP‐HP Health Data Warehouse team as well as all the AP‐HP staff and volunteers who contributed to the implementation of the EDS‐COVID database and operating solutions for this database.
Collaborators of the EDS AP‐HP COVID consortium are: Pierre‐Yves Ancel, Alain Bauchet, Nathanaël Beeker, Vincent Benoit, Mélodie Bernaux, Ali Bellamine, Romain Bey, Aurélie Bourmaud, Stéphane Breant, Anita Burgun, Fabrice Carrat, Charlotte Caucheteux, Julien Champ, Sylvie Cormont, Christel Daniel, Julien Dubiel, Catherine Ducloas, Loic Esteve, Marie Frank, Nicolas Garcelon, Alexandre Gramfort, Nicolas Griffon, Olivier Grisel, Martin Guilbaud, Claire Hassen‐Khodja, François Hemery, Martin Hilka, Anne‐Sophie Jannot, Jerome Lambert, Richard Layese, Judith Leblanc, Léo Lebouter, Guillaume Lemaitre, Damien Leprovost, Ivan Lerner, Kankoe Levi Sallah, Aurélien Maire, Marie‐France Mamzer, Patricia Martel, Arthur Mensch, Thomas Moreau, Antoine Neuraz, Nina Orlova, Nicolas Paris, Bastien Rance, Hélène Ravera, Antoine Rozes, Elisa Salamanca, Arnaud Sandrin, Patricia Serre, Xavier Tannier, Jean‐Marc Treluyer, Damien Van Gysel, Gaël Varoquaux, Jill Jen Vie, Maxime Wack, Perceval Wajsburt, Demian Wassermann, Eric Zapletal. This work did not receive any external funding.
Hoertel N, Sánchez‐Rico M, Vernet R, et al. Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: A multicentre retrospective observational study. Brit Jnl Clinical Pharma. 2021;87(10):3766–3775. 10.1111/bcp.14784
Nicolas Hoertel and Marina Sánchez‐Rico contributed equally to this manuscript
The authors confirm that the Principal Investigators of this study are Nicolas HOERTEL and Marina Sánchez‐Rico, and that doctors from AP‐HP hospitals had direct clinical responsibility for their patients.
DATA AVAILABILITY STATEMENT
Data from the AP‐HP Health Data Warehouse can be obtained with permission at https://eds.aphp.fr/.
REFERENCES
- 1. Hoertel N, Blachier M, Blanco C, et al. A stochastic agent‐based model of the SARS‐CoV‐2 epidemic in France. Nat Med. 2020;26(9):1417‐1421. 10.1038/s41591-020-1001-6 [DOI] [PubMed] [Google Scholar]
- 2. Hoertel N, Blachier M, Blanco C, et al. Facing the COVID‐19 epidemic in NYC: a stochastic agent‐based model of various intervention strategies. medRxiv. Published online. 2020. 10.1101/2020.04.23.20076885 [DOI] [Google Scholar]
- 3. Chevance A, Gourion D, Hoertel N, et al. Ensuring mental health care during the SARS‐CoV‐2 epidemic in France: A narrative review. L'Encephale. 2020;46(3S):193‐201. 10.1016/j.encep.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoertel N, Blachier M, Sánchez‐Rico M, Limosin F, Leleu H. Impact of the timing and adherence to face mask use on the course of the COVID‐19 epidemic in France. J Travel Med. 2021;28(2):taab016. 10.1093/jtm/taab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. 10.1056/nejmoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen MP, George M, Gilroy D, Sofat R. Beyond dexamethasone, emerging immuno‐thrombotic therapies for COVID‐19. Br J Clin Pharmacol. 2020. 10.1111/bcp.14540. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7. Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol. 2016;16(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger ML, Dreyer N, Anderson F, Towse A, Sedrakyan A, Normand S‐L. Prospective observational studies to assess comparative effectiveness: the ISPOR good research practices task force report. Value Health. 2012;15(2):217‐230. [DOI] [PubMed] [Google Scholar]
- 9. AP‐HP. Entrepôt de Données de Santé . Published 2018. Available from: https://eds.aphp.fr/. Accessed 1 March 2020
- 10. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878‐1886. [DOI] [PubMed] [Google Scholar]
- 12. Hoertel N, Sánchez‐Rico M, Vernet R, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID‐19: results from an observational study. Mol Psychiatry. Published online February. 2021. 10.1038/s41380-021-01021-4 [DOI] [PubMed] [Google Scholar]
- 13. Hoertel N, Sánchez M, Vernet R, et al. Association between Hydroxyzine Use and Reduced Mortality in Patients Hospitalized for Coronavirus Disease 2019: Results from a multicenter observational study. medRxiv. Published online. 2020. 10.1101/2020.10.23.20154302 [DOI] [Google Scholar]
- 14. Hoertel N, Sánchez‐Rico M, Vernet R, et al. Observational study of haloperidol in hospitalized patients with COVID‐19. PLOS ONE. 2021;16(2):e0247122. 10.1371/journal.pone.0247122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoertel N, Sánchez‐Rico M, Vernet R, et al. Observational Study of Chlorpromazine in Hospitalized Patients with COVID‐19. Clin Drug Investig. Published online February. 2021;9:1‐13. 10.1007/s40261-021-01001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haut Conseil de la Santé Publique . Statement on the Management at Home or in a Care Facility of Suspected or Confirmed Covid‐19 Patients; 2020. https://www.hcsp.fr
- 18. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020;92(10):1733‐1734. 10.1002/jmv.25819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee J, Yoon W, Kim S, et al. BioBERT: a pre‐trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36(4):1234‐1240. 10.1093/bioinformatics/btz682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jouffroy J, Feldman SF, Lerner I, Rance B, Neuraz A, Burgun A. MedExt: combining expert knowledge and deep learning for medication extraction from French clinical texts. Published online January 23, 2020. [Google Scholar]
- 21. Efron B. Nonparametric standard errors and confidence intervals. Can J Stat. 1981;9(2):139‐158. [Google Scholar]
- 22. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. [DOI] [PubMed] [Google Scholar]
- 23. Cain DW, Cidlowski JA. After 62 years of regulating immunity, dexamethasone meets COVID‐19. Nat Rev Immunol. 2020;20(10):587‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Hu Z, Song X. High‐dose but not low‐dose corticosteroids potentially delay viral shedding of patients with COVID‐19. Clin Infect Dis. 2020:ciaa829. 10.1093/cid/ciaa829. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Kaplan–Meier curves for time to death in patients hospitalized for COVID‐19 who required respiratory support (i.e. mechanical ventilation or oxygen; n = 1192; A) and in those who did not (n = 11 008; B), according to dexamethasone cumulative dose. The shaded areas represent pointwise 95% confidence intervals.
TABLE S1 Associations of baseline clinical characteristics with the endpoint of death in patients with and without respiratory support (i.e. oxygen or intubation).
TABLE S2 Associations between dexamethasone cumulative dose and the endpoint of death, in the full sample and in the matched analytic sample.
TABLE S3 Interaction effect of baseline characteristics with dexamethasone use on the endpoint of death among adult inpatients with COVID‐19.
Data Availability Statement
Data from the AP‐HP Health Data Warehouse can be obtained with permission at https://eds.aphp.fr/.


