Abstract
The present study examined the relationship between polymerase chain reaction (PCR) test positivity and clinical outcomes of vitamin D levels measured within the 6 months before the PCR test in coronavirus disease 2019 (COVID‐19)‐positive patients. In this retrospective cohort study, COVID‐19 (227) and non‐COVID‐19 patients (260) were divided into four groups according to their vitamin D levels: Group I (0–10 ng/ml), Group II (10–20 ng/ml), Group III (20–30 ng/ml), and Group IV (vitamin D > 30 ng/ml). Laboratory test results and the radiological findings were evaluated. In addition, for comparative purposes, medical records of 1200 patients who had a hospital visit in the November 1, 2019–November 1, 2020 period for complaints due to reasons not related to COVID‐19 were investigated for the availability of vitamin D measurements. This search yielded 260 patients with tested vitamin D levels. Vitamin D levels were below 30 ng/ml in 94.27% of 227 COVID‐19‐positive patients (average age, 46.32 ± 1.24 years [range, 20–80 years] and 56.54% women) while 93.07% of 260 non‐COVID‐19 patients (average age, 44.63 ± 1.30 years [range, 18–75 years] and 59.50% women) had vitamin D levels below 30 ng/ml. Nevertheless, very severe vitamin D deficiency (<10 ng/ml) was considerably more common in COVID‐19 patients (44%) (average age, 44.15 ± 1.89 years [range, 23–80 years] and 57.57% women) than in non‐COVID‐19 ones (31%) (average age, 46.50 ± 2.21 years [range, 20–75 years] and 62.5% women). Among COVID‐19‐positive patients, the group with vitamin D levels of >30 ng/ml had significantly lower D‐dimer and C‐reactive protein (CRP) levels, number levels, number of affected lung segments and shorter hospital stays. No difference was found among the groups in terms of age and gender distribution. Elevated vitamin D levels could decrease COVID‐19 PCR positivity, D‐dime and CRP levels and the number of affected lung segments in COVID‐19‐positive patients, thereby shortening the duration of hospital stays and alleviating the intensity of COVID‐19.
Keywords: computed tomography, COVID‐19, CRP, D‐dimer, PCR testing, vitamin D
1. INTRODUCTION
The novel coronavirus (COVID‐19) has infected millions of people, and World Health Organization (WHO) has declared COVID‐19 as a pandemic in March 2020. 1 COVID‐19 is an RNA virus, which affects multiple organs including lung, liver, kidney, brain, and heart in humans, and has resulted in the death of about 1.8 million people worldwide (WHO COVID‐19 Dashboard). The etiology of COVID‐19 has not yet been fully elucidated. The main cause of death in COVID‐19 patients is inflammation, especially in the lung which leads to acute respiratory distress syndrome (ARDS). 1
No specific treatment is available for fatal and fast‐spreading COVID‐19 infection. Antimalarial (chloroquine and hydroxychloroquine), antiviral drugs (remdesivir), and dexamethasone (corticosteroid) are being used clinically for the treatment of COVID‐19 patients. 2 Considering the fact that an adequate immune response is crucial for overcoming this viral infection, it is important to identify the existing and known substances that strengthen immune system activity.
Vitamin D, a steroid and versatile hormone, plays crucial roles in phosphorus–calcium metabolism and in the immune system of both humans and animals. 3 Numerous studies have revealed a wide range of pharmacological and physiological functions of vitamin D. It has anti‐inflammatory, antioxidant, and antiviral effects. Besides this, it has crucial regulatory roles in the adaptive and innate immune systems. 4 It was shown that vitamin D deficiency is quite common among COVID‐19 patients. 5 A recent clinical study showed that COVID‐19 patients supplemented with vitamin D had less severe symptoms of the disease. 6
In the present study, we aimed to identify possible associations of vitamin D levels with laboratory test parameters, radiological findings, length of hospital stays, and the average age of the patients infected with COVID‐19.
2. MATERIALS AND METHODS
2.1. Setting
This retrospective cohort study was conducted in the town of Tokat (39° 52' °N latitude), middle black sea region of Turkey. The average temperature in this town is 12.8°C. The study was first approved by the Turkish Ministry of Health (on May 5, 2020). Then, ethics committee approval was granted from Tokat Gaziosmanpasa University, Faculty of Medicine, Ethics of Human Diseases (approval no: 20‐KAEK‐158). The research was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Study sample and data collection
The study sample was selected retrospectively among 2600 patients over 18 years of age with COVID‐19 positivity based on real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay performed in Tokat State Hospital on March 30, 2020–November 1, 2020 period. The patients whose vitamin D levels were measured within the 6‐month period before their COVID‐19 test were identified and 227 such patients were found. COVID‐19‐positive patients whose vitamin D levels were not measured within the 6‐month before the PCR test were excluded. Data of the participants regarding daily medical knowledge, laboratory and radiological results were retrieved from the electronic medical records. The studied parameters were the laboratory tests and radiological findings at the first arrival of COVID‐19 patients to the hospital along with their age (over 18 years old), genders, and hospital stay.
The study covered patients who had PCR positivity for COVID‐19 in the March 30–November 1, 2020 period and whose vitamin D levels were measured in the 6 months before the PCR test. However, to make comparisons, non‐COVID‐19 patients whose vitamin D levels were measured in the November 1, 2019–November 1, 2020 period in Tokat State Hospital were screened retrospectively using the hospital automation system and included in the study. Age (over the 18‐year old), gender, and vitamin D levels of the patients were recorded. When more than one vitamin D measurement was available, only the first one was used. 7 , 8 The patients were divided into four groups based on their vitamin D levels:
Group I: patients with vitamin D levels less than 10 ng/ml.
Group II: patients with vitamin D levels between 10 and 20 ng/ml.
Group III: patients with vitamin D levels between 20 and 30 ng/ml.
Group IV: patients with a vitamin D level higher than 30 ng/ml.
2.3. Collection of samples
For detection of the epidemic virus "SARS‐CoV‐2 (2019‐nCoV)" causing coronavirus disease 2019 (COVID‐19), a Bio‐Speedy® Kit (Bioeksen) was used. The kit was applied to nucleic acid isolates from nasopharyngeal and oropharyngeal swab samples. Rapid diagnosis with the kit was achieved via one‐step reverse‐transcription (RT) and real‐time PCR (qPCR) (RT‐qPCR) targeting SARS‐CoV‐2 (2019‐nCoV)‐specific RdRp (RNA‐dependent RNA polymerase) gene fragment.
2.4. Radiological features
Computed tomography (CT) scans were performed using a 16‐slice CT scanner (Toshiba) for all participants who were admitted to the hospital. One or more ground‐glass opacities, consolidation, and reticular pattern were accepted as the diagnostic criteria of typical CT manifestations of COVID‐19. CT Scans were performed just after admission to the hospital. An expert specialist evaluated all CT images.
2.5. Statistical analyses
All statistical analyses were performed using SPSS software (version 15). The data were presented as mean ± SEM. Moreover, the number (n) and percentages (%) of the patients were calculated for study groups established for vitamin D level. Normality of distributions in the groups was determined. When a normal distribution was involved, ANOVA and subsequent LSD testing were performed to compare group means. When the distribution was not normal, Kruskal‐Wallis variance analysis test and Mann‐Whitney U test were used. The gender distribution among the groups were compared using the χ 2‐test with continuity correction.
3. RESULTS
3.1. Vitamin D parameters
A total of 2600 patients whose RT‐PCR tests for COVID‐19 were positive were screened, and medical records of these patients were examined to identify those who had their vitamin D levels tested anytime within the 6‐month period before the COVID‐19 test. It was revealed that 227 patients matched these criteria. Of them, 99 patients (43.61%) (average age, 44.15 ± 1.89 years [range, 23–80 years] and 57.57% women) had vitamin D levels below 10 ng/ml (mean, 6.31 ± 0.21), while 73 patients (32.15%) (average age, 45.74 ± 2.02 years [range, 21–77 years] and 53.42% women) had vitamin D levels between 10 and 20 ng/ml (mean, 14.63 ± 0.29), 42 patients (18.50%) (average age, 48.10 ± 2.28 years [range, 20–75 years] and 59.52% women) between 20 and 30 ng/ml (mean, 24.16 ± 0.49) and 13 patients (5.72%) (average age, 40.67 ± 5.45 years [range, 25–80 years] and 61.53% women) between 30 and 40 ng/ml (mean, 38.05 ± 1.53). Thus, vitamin D levels were below 30 ng/ml in 94.27% (average age, 46.32 ± 1.24 years [range, 20–80 years] and 56.54% women) of the COVID‐19‐positive patients (Figure 1, Table 1).
Figure 1.
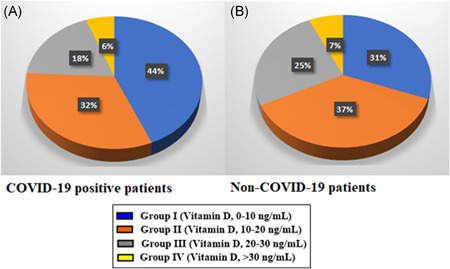
A, Vitamin D levels of coronavirus positive patients. B, Non‐COVID‐19 patients who applied to hospital for other reasons
Table 1.
Laboratory parameters and radiological examination results of COVID‐19‐positive patients with different vitamin D levels
| Group I | Group II | Group III | Group IV | The reference | |
|---|---|---|---|---|---|
| (n = 99) | (n = 73) | (n = 42) | (n = 13) | range | |
| Mean vitamin D concentration (ng/ml) | 6.31 ± 0.21 | 14.63 ± 0.29 | 24.16 ± 0.49 | 38.05 ± 1.53 | 30–60 (ng/ml) |
| WBC (109/L) | 5.84 ± 0.21 | 6.19 ± 0.18 | 6.51 ± 0.25 | 5.69 ± 0.33 | 4–10 (109/L) |
| HGB (g/dl) | 12.83 ± 0.18 | 12.91 ± 0.20 | 13.34 ± 0.30 | 12.40 ± 0.36 | 11.0–15.0 (g/dl) |
| PLT (109/L) | 219.9 ± 10.08 | 221.7 ± 7.60 | 232 ± 9.57 | 223.8 ± 6.68 | 150–400 (109/L) |
| MPV (fl) | 9.88 ± 0.11 | 10.10 ± 0.13 | 10.09 ± 0.06 | 9.89 ± 0.23 | 6.5–12 (fl) |
| Fasting glucose (mg/dl) | 126.3 ± 6.76 | 120.4 ± 3.69 | 114.2 ± 5.14 | 108.4 ± 3.53 | 74–106 (mg/dl) |
| Urea (mg/dl) | 27.50 ± 1.11 | 30.30 ± 1.95 | 32.08 ± 1.64 | 26.21 ± 1.31 | 17–43 (mg/dl) |
| Creatinine (mg/dl) | 0.72 ± 0.05 | 0.73 ± 0.08 | 0.76 ± 0.07 | 0.74 ± 0.01 | 0.51–0.95 (mg/dl) |
| ALT (U/L) | 21.86 ± 1.14 | 23.67 ± 1.62 | 21.76 ± 0.92 | 19.02 ± 1.18 | 0–50 (U/L) |
| AST (U/L) | 26.36 ± 0.94 | 27.83 ± 1.33 | 23.09 ± 1.65 | 23.20 ± 1.71 | 0–50 (U/L) |
| K (mmol/L) | 4.23 ± 0.03 | 4.17 ± 0.05 | 4.31 ± 0.08 | 4.33 ± 0.12 | 3.5–5.1 (mmol/L) |
| Na (mmol/L) | 139.9 ± 0.93 | 138.4 ± 0.54 | 138.7 ± 0.63 | 139.8 ± 0.90 | 135–145 (mmol/L) |
| Ca (mmol/L) | 9.19 ± 0.17 | 9.52 ± 0.08 | 9.28 ± 0.08 | 9.13 ± 0.12 | 8.8–10.6 (mg/dl) |
| Lymphocyte (k/µl) | 1.72 ± 0.07 | 1.68 ± 0.10 | 1.89 ± 0.11 | 2.02 ± 0.19 | 0.8–4.0 (k/µl) |
| Neutrophil (k/µl) | 3.94 ± 0.17 | 4.39 ± 0.16 | 4.53 ± 0.31 | 4.19 ± 0.45 | 2–7 (k/µl) |
| D‐dimer (ng/ml) | 0.459 ± 0.033 | 0.522 ± 0.038 | 0.367 ± 0.043 | 0.067 ± 0.001***,•••,⊗⊗⊗ | 0.1–0.5 (ng/ml) |
| CRP (mg/L) | 22.49 ± 2.88 | 20.14 ± 4.55 | 10.99 ± 4.10 | 0.39 ± 0.16***,•••,⊗⊗⊗ | 0–8 (mg/L) |
| Affected number of lung segments in CT | 2.33 ± 0.20 | 2.18 ± 0.27 | 2.02 ± 0.25 | 0.25 ± 0.13***,••, ⊗⊗⊗ | Affected number of lung segments |
| Hospitalization time for COVID‐19 patients | 3.88 ± 0.28 | 4.15 ± 0.53 | 3.26 ± 0.61 | 0.50 ± 0.19***, ••,⊗ | Days |
| Age (years) | 44.15 ± 1.89 | 45.74 ± 2.02 | 48.10 ± 2.28 | 40.67 ± 5.45 | Mean age of patients (years) |
| Gender (female/male) | 57/42 | 39/34 | 25/17 | 8/5 |
p < .01.
p < .001 for the comparison of Group II and Group IV.
p < .05.
p < .001 for the comparison of Group III and Group IV.
p < .001 for the comparison of Group I and Group IV.
For comparison purposes, 1200 patients who applied to Tokat State Hospital for reasons other than COVID‐19 in November 1, 2019–November 1, 2020 period were screened for the availability of their vitamin D measurements, and 260 such patients were identified. Eighty of them (30.76%) (average age, 46.50 ± 2.21 years [range, 20–75 years] and 62.5% women) had vitamin D levels below 10 ng/ml (mean, 6.52 ± 0.19) while 96 patients (36.92%) (average age, 42.19 ± 1.75 years [range, 18–75 years] and 65.62% women) had vitamin D between 10 and 20 ng/ml (mean, 13.51 ± 0.40), 66 patients (25.38%) (average age, 44.36 ± 3.02 years [range, 22–73 years] and 46.96% women) between 20 and 30 ng/ml (mean, 22.72 ± 0.58) and 18 patients (6.92%) (average age, 43.75 ± 4.91 years [range, 26–77 years] and 61.11% women) between 30 and 40 ng/ml (mean, 37.49 ± 1.20). Thus, the vitamin D level was below 30 ng/ml in 93.07% (average age, 44.63 ± 1.30 age [range, 18–75 years] and 59.50% women) of non‐COVID‐19 patients (Figure 1, Table 2).
Table 2.
Age and gender distributions of non‐COVID‐19 patients
| <10 ng/ml | 10–20 ng/ml | 20–30 ng/ml | >30 ng/ml | |
|---|---|---|---|---|
| (n = 80) | (n = 96) | (n = 66) | (n = 18) | |
| Mean vitamin D concentration (ng/ml) | 6.52 ± 0.19 | 13.51 ± 0.40 | 22.72 ± 0.58 | 37.49 ± 1.20 |
| Age (years) | 46.50 ± 2.21 | 42.19 ± 1.75 | 44.36 ± 3.02 | 43.75 ± 4.91 |
| Gender (female/male) | 50/30 | 63/33 | 31/35 | 11/7 |
3.2. Laboratory parameters
The laboratory tests examined included hemograms, biochemical, and inflammatory markers, which were assayed when patients first arrived at the hospital (Table 1). The D‐dimer (ng/ml) level of Group IV COVID‐19‐positive patients (vitamin D level: >30 ng/ml) was significantly different from Group I (vitamin D level of <10 ng/ml) (p < .001), from Group II (vitamin D level: 10–20 ng/ml) (p < .001) and from Group III (vitamin D level: 20–30 ng/ml) (p < .001). The C‐reactive protein (CRP) level of Group IV was significantly lower than that of Group I (p < .001), Group II (p < .001) and Group III (p < .001) (Figure 2, Table 1). There were no significant differences among the four groups for WBC, HGB, PLT, MPV, fasting glucose, urea, creatinine, ALT, AST, K, Na and Ca levels, and neutrophil and lymphocyte counts. Duration of hospital stay was significantly shorter in Group IV (vitamin D level: >30 ng/ml) compared to Group I (vitamin D level: 10–20 ng/ml) (p < .001), Group II (vitamin D level: 10–20 ng/ml) (p < .01) and Group III (vitamin D level: 20–30 ng/ml) (p < .05) (Table 1).
Figure 2.
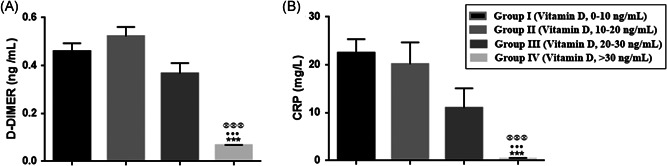
A, D‐dimer (ng/ml) and (B) C‐reactive protein (CRP) (mg/L) levels of coronavirus‐positive patients. As vitamin D levels increased, the level of D‐dimer and CRP decreased. ***p < .001 for the comparison of Group I and Group IV; ••• p < .01 for the comparison of Group II and Group IV and p < .001 for the comparison of Group III and Group IV
3.3. Radiological parameters
The mean number of affected lung segments were 2.33 ± 0.20 in Group I, 2.18 ± 0.27 in Group II, 2.02 ± 0.25 in Group III, and 0.25 ± 0.13 in Group IV. The number of affected lung segments was lowest in Group IV (>30 ng) compared to Group I (p < .001), Group II (p < .01), and Group III (p < .001). Thus, vitamin D levels of >30 ng/ml reduced the number of affected lung segments, consolidation, and reticular pattern in patients diagnosed with COVID‐19 (Figures 3 and 4) (Table 1).
Figure 3.
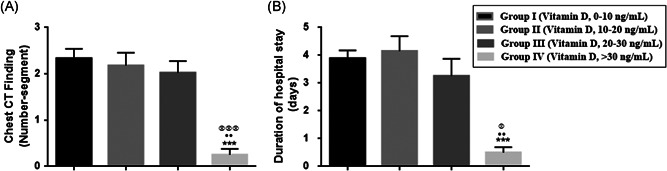
A, Number of lung segments in chest computed tomography (CT) and (B) duration of hospital stay in coronavirus positive patients. ***p < .001 for the comparison of Group I and Group IV; •• p < .05 for the comparison of Group II with Group IV; and p < .05 and p < .001 for the comparison of Group III and Group IV
Figure 4.
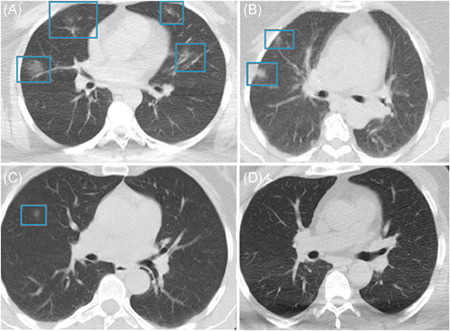
Chest computed tomography (CT) images of COVID‐19 pneumonia in (A) Group I, (B) Group II, (C) Group III, and (D) Group IV
4. DISCUSSION
The present retrospective study examined the associations of vitamin D levels measured within 6 months before the COVID‐19 test with the incidence of COVID‐19 positivity, CT findings, and laboratory parameters. It was revealed that as the vitamin D levels rose: (1) the patients’ risk of getting COVID‐19 infection decreased; (2) the number of lung segments with common ground‐glass appearance decreased; (3) the level of D‐dimer and CRP decreased considerably; in fact, the CRP level was within the normal range in Group IV, which had a mean vitamin D level of 38; and (4) duration of the hospital stay of COVID‐19 patients decreased.
Studies performed in different countries showed significant associations between the average vitamin D levels and the number of COVID‐19 cases and especially with the mortality rate caused by this infection. 9 Vitamin D supplementation could reduce the incidence, severity, and risk of death from COVID‐19. 10 A Swiss retrospective study with 107 patients showed significantly lower vitamin D levels in COVID‐19‐positive patients based on PCR assay. 11 Another study found that 67.2% of the PCR‐positive COVID‐19 patients had vitamin D levels of less than 30 ng/ml. 12 It is a well‐known fact that vitamin D levels have a tendency to be lower in high‐latitudes. The COVID‐19 incidence rates and COVID‐19‐related mortality rates were reported to be relatively low in countries south of latitude 35 °N. 13 Turkey is situated between latitudes 36 and 42 °N, and vitamin D deficiency is common across Turkey. The European Calcified Tissue Society Working Group and Chinese Clinical Guideline has defined vitamin D deficiency as a serum 25(OH) D level of less than 30 nmol/L. 14 In a cohort study with 489 patients whose vitamin D levels were measured within a year before COVID‐19 testing, the relative risk of testing positive for COVID‐19 was 1.77 times greater in patients who were vitamin D deficient compared to the patients with sufficient vitamin D levels. 15 In the present study, 94.27% of patients with COVID‐19 positivity and 93.07% of non‐COVID‐19 patients had vitamin D levels below 30 ng/ml. Besides this, a considerably higher incidence of severe vitamin D deficiency (<10 ng/ml) was observed in COVID‐19‐positive patients (44%) than in non‐COVID‐19 patients (31%). A Turkish study reported similar severe vitamin D deficiencies (32.20%) among 35,667 individuals. 16 Thus, it could be stated that severe vitamin D deficiency was about 30% higher among COVID‐19‐positive patients compared to the general population. In other words, COVID‐19 positivity was higher among individuals with severe vitamin D deficiency. In addition, as the vitamin D levels rose, incidence of COVID‐19 positivity decreased. These findings clearly showed a strong association between vitamin D deficiency and incidence of COVID‐19 PCR positivity and were consistent with other research investigating the relationships between vitamin D deficiency and COVID‐19 infections.
D‐dimer forms as a result of production and lysis of cross‐linked fibrin, and is used as an indicator of coagulation and fibrinolysis. 17 Virus infections usually involve an aggressive pro‐inflammatory response and impaired control of anti‐inflammatory response. 18 This could lead to dysfunction of endothelial cells, resulting in excessive thrombin formation. 19 Pulmonary thrombin could even be responsible for oxygen desaturation and acute respiratory distress commonly observed in COVID‐19 patients. 20 , 21 , 22 Elevated D‐dimer levels were reported to represent a hypercoagulable state in COVID‐19 patients. 23 In the present study, the group with a vitamin D level of >30 mg/ml, D‐dimer level was significantly lower than those of other groups. Thus, vitamin D could reduce the risk of common intravascular coagulopathy, thereby improving oxygenation and alleviating the severity of COVID‐19.
CRP is an acute‐phase plasma protein synthesized in response to pro‐inflammatory cytokines, especially tumor necrosis factor (TNF), interleukin (IL‐1β), and IL‐6. 24 Therefore, CRP is an indication of cytokine storm and inflammation. The cytokine storm could result in pneumonia and ARDS, and may contribute to the rapid multiple organ damage. 25 It was shown that many COVID‐19‐positive patients had very high CRP levels. 26 , 27 , 28 Higher CRP levels associated with vitamin D deficiency were mentioned to be linked with increased risks of severe COVID‐19. 26 , 27 COVID‐19‐positive patients with serum vitamin D levels < 30 ng/ml were shown to have very high levels of CRP. 12 These findings were in line with those reported in the literature. It was also observed in the present study that COVID‐19‐positive patients with serum vitamin D levels of >30 ng/ml had very low CRP levels. Vitamin D deficiency leads to the generation of pro‐inflammatory cytokines such as TNF‐α, IL‐1β, and IL‐6, which may result in elevated CRP levels and induce inflammation. 26 , 27 , 29 Vitamin D could lower the level of inflammation marker CRP and prevent inflammation from progressing to pneumonia and ARDS. 25 This finding showed that vitamin D could reduce CRP and exert anti‐inflammatory effects. Thus, adequate vitamin D levels can prevent cytokine storms in COVID‐19 patients and reduce the severity of the disease.
Ground‐glass opacity is the most common CT feature of patients with COVID‐19 viral pneumonia. 30 It becomes apparent in patients a few days after the onset of the disease. CT images in the present study were obtained at the first arrival of the patients to the hospital. In the group with vitamin D level of >30 ng/ml, there were fewer lung segments with ground‐glass opacity appearance than in groups with vitamin D levels of <30 ng/ml.
Areas of ground‐glass opacity on CT scans show pathological diffuse alveolar damage. 31 Pulmonary edema is the most common cause of diffuse ground‐glass opacity. 32 Upon COVID‐19 infection, the virus attempts to enter cells through binding to ACE2 receptors, thereby preventing ACE2 receptors to perform their physiological functions. Hence, ACE2 may be downregulated by COVID‐19 infection, and Ang II accumulation could reach toxic levels, which in turn lead to pulmonary edema through elevated pulmonary vascular permeability. 25 , 33 , 34 Furthermore, elevated Ang II levels result in oxidative stress, which eventually progresses to inflammation and fibrosis. 35 Salehi et al. 36 reported that ground‐glass opacity was observed in 88.0% of COVID‐19 patients. As the virus first prevents ACE2 from functioning through binding to it, 88.0% of patients with COVID‐19 have ground‐glass opacity in the lungs. Thus, it appears that increasing the ACE2 level is very important for reducing the ground‐glass opacity and alleviating the COVID‐19 severity. 25
In an experimental study, Xu et al. 37 showed that vitamin D supplementation provides protective effects on acute lung injury through increasing ACE2 expression and decreasing renin, ACE and Ang II levels. Another study reported that 1.25‐dihydroxy vitamin D3 (calcitriol) may elevate the ACE2 expression by ACE2/Ang(1–7)/MasR pathway. 38 Vitamin D may reduce ground‐glass opacity on CT scan and prevent the formation of pulmonary edema by upregulating the ACE2 level in the lungs
5. CONCLUSION
In the present single‐centered retrospective cohort study, vitamin D deficiency was found to be associated with a higher COVID‐19 risk. The COVID‐19‐positive individuals with sufficient vitamin D levels had significantly lower blood levels of D‐dimer, inflammatory marker CRP, reduced frequencies of ground‐glass opacity appearance in chest CT scans and shorter hospital stays. The findings of the study indicated the need for randomized studies to determine whether the vitamin D level could affect COVID‐19 risk.
6. LIMITATIONS
The present study had some limitations. Vitamin D deficiency could be due to various chronic conditions or behavioral factors, which themselves might be associated with a higher COVID‐19 risk. In addition, the study covered a limited amount of data in the electronic database of Tokat State Hospital only.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Hatice Aygun conceived and designed the research. Hatice Aygun, Fadime Demir, Mustafa Demir collected, analyzed and interpreted the data. Hatice Aygun wrote the article. All authors read and approved the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26832
ETHICAL APPROVAL
The study was approved by the Turkish Ministry of Health (Date: 05.05.2020). Ethics committee approval was granted from Tokat Gaziosmanpasa University, Faculty of Medicine, Ethics of Human Diseases (Approval No: 20‐KAEK‐158).
Demir M, Demir F, Aygun H. Vitamin D deficiency is associated with COVID‐19 positivity and severity of the disease. J Med Virol. 2021;93:2992–2999. 10.1002/jmv.26832
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS‐CoV‐2 and COVID‐19: the most important research questions. Cell Biosci. 2020;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iannaccone G, Scacciavillani R, Del Buono MG, et al. Weathering the cytokine storm in COVID‐19: therapeutic implications. Cardiorenal Med. 2020;10(5):277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khoo AL, Chai L, Koenen H, Joosten I, Netea M, van der Ven A. Translating the role of vitamin D3 in infectious diseases. Crit Rev Microbiol. 2012;38(2):122‐135. [DOI] [PubMed] [Google Scholar]
- 5. Karahan S, Katkat F. Impact of Serum 25 (OH) Vitamin D level on mortality in patients with COVID‐19 in Turkey. J Nutr Health Aging. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID‐19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Çiçek Z, Kalkan İ, Bilgen Sivri B. Determination of the level of knowledge and attitudes of mothers regarding vitamin D use in Konya. 2015.
- 8. Dadaci Z, Cetinkaya S, Oncel Acir N, Oncel M, Borazan M. Serum vitamin D levels in patients with acute anterior uveitis. Ocul Immunol Inflamm. 2017;25(4):492‐496. [DOI] [PubMed] [Google Scholar]
- 9. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grant WB, Lahore H, McDonnell SL, et al. Vitamin D supplementation could prevent and treat influenza, coronavirus, and pneumonia infections. Preprint. 2020.
- 11. D'Avolio A, Avataneo V, Manca A, et al. 25‐Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS‐CoV‐2. Nutrients. 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maghbooli Z, Sahraian MA, Ebrahimi M, et al. Vitamin D sufficiency, a serum 25‐hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID‐19 infection. PLOS One. 2020;15(9):e0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Rhodes JM, Subramanian S, Laird E, Kenny RA. Low population mortality from COVID‐19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Alimentary Pharmacol Ther. 2020;51(12):1434‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D defciency: a position statement of the European Calcifed Tissue Society. Eur J Endocrinol. 2019;180:23‐54. [DOI] [PubMed] [Google Scholar]
- 15. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID‐19 test results. JAMA. 2020;3(9):e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solak I, Cihan FG, Mercan S, Kethuda T, Eryılmaz MA. Evaluation of 25‐hydroxyvitamin D levels in Central Anatolia, Turkey. BioMed Res Int. 2018;2018:4076548‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Long Y, Xiao H, Yang J, Toulon P, Zhang Z. Use of D‐dimer in oral anticoagulation therapy. Int J Lab Hematol. 2018;40:503‐507. [DOI] [PubMed] [Google Scholar]
- 18. Wong JP, Viswanathan S, Wang M, Sun LQ, Clark GC, D'Elia RV. Current and future developments in the treatment of virus‐induced hypercytokinemia. Future Med Chem. 2017;9:169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38‐44. [DOI] [PubMed] [Google Scholar]
- 20. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID‐19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oudkerk M, Büller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID‐19: report of the National Institute for public health of the Netherlands. Radiology. 2020;201629:201629‐E222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooper ID, Crofts CA, DiNicolantonio JJ, et al. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID‐19: rationale for clinical management. Open Heart. 2020;7(2):e001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with COVID‐19. J Thromb Haemost. 2020;18(6):1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salazar J, Martínez MS, Chávez‐Castillo M, et al. C‐reactive protein: an in‐depth look into structure, function, and regulation. Int Scholarly Res Notices. 2014;2014:653045‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aygun H. Vitamin D can prevent COVID‐19 infection‐induced multiple organ damage. Naunyn‐Schmiedeberg's Arch Pharmacol. 2020;393:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID‐19 patients. Aging Clinical and Exp Res. 2020;32(10):2141‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in Suppressing cytokine storm and associated mortality in COVID‐19 patients. medRxiv. 2020. [Google Scholar]
- 28. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panichi V, De Pietro S, Andreini B, et al. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. 1998;54:1463‐1469. [DOI] [PubMed] [Google Scholar]
- 30. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55(6):327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chong S, Kim TS, Cho EY. Herpes simplex virus pneumonia: high‐resolution CT findings. Br J Radiol. 2010;83(991):585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hani C, Trieu NH, Saab I, et al. COVID‐19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Maat S, De Mast Q, Danser AJ, Van De Veerdonk FL, Maas C. October Impaired breakdown of bradykinin and its metabolites as a possible cause for pulmonary edema in COVID‐19 infection. Seminar Thromb Hemostasis. 2020;46(07):835‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID‐19 mortality and the renin‐angiotensin system—a call for epidemiologic investigations. Clin Infect Dis. 2020;71(15):870‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin‐angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 Patients. Am J Roentgenol. 2020;215(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 37. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide induced acute lung injury via regulation of the renin angiotensin system. Mol Med Rep. 2017;16(5):7432‐7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cui C, Xu P, Li G, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II‐exposed microglial cells: role of renin‐angiotensin system. Redox Biol. 2019;26:101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


