Abstract
Introduction
COVID‐19 predisposes patients to a higher risk of venous thromboembolism (VTE), although the extent of these implications is unclear and the risk of bleeding has been poorly evaluated. To date, no studies have reported long‐term outcomes of patients with COVID‐19 and VTE.
Method
Prospective observational study to evaluate long‐term (90 days or more) outcomes of patients diagnosed with VTE (PE, DVT of the extremities, or both) in the setting of COVID‐19. The main outcome of the study was a compound of major bleeding and death.
Results
The study comprised 100 patients (mean age 65 ± 13.9 years). At the time of VTE diagnosis, 66% patients were hospitalized, 34.8% of them in the ICU. Mean follow‐up was 97.9 ± 23.3 days. During the study period, 24% patients died and median time to death was 12 (IQR: 2.25‐20.75) days, 11% patients had major bleeding and median time to event was 12 (IQR: 5‐16) days. The cause of death was PE in 5% and bleeding in 2% of patients. There were no VTE recurrences. The main study outcome occurred in 29% patients. Risk of death or major bleeding was independently associated with ICU admission (HR 12.2; 95% CI 3.0‐48.3), thrombocytopenia (HR 4.5; 95% CI 1.2‐16.5), and cancer (HR 21.6; 95% CI 1.8‐259).
Conclusion
In patients with COVID‐19 and VTE, mortality and major bleeding were high and almost a third of deaths were VTE‐related. The majority of complications occurred in the first 30 days. ICU admission, thrombocytopenia, and cancer are risk factors for poor prognosis.
Keywords: anticoagulation, bleeding, COVID‐19, SARS‐COV‐2, venous thromboembolism
Highlights.
Long‐term outcomes of patients with COVID‐19 and venous thromboembolism (VTE) are unknown.
We prospectively evaluated long‐term bleeding, recurrence and death of COVID‐19‐associated VTE.
Mortality (24%) and major bleeding (11%) were high and the majority occurred in the first 30 days.
ICU admission, thrombocytopenia, and cancer are risk factors for poor prognosis.
1. INTRODUCTION
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE). It causes more than 250 000 hospital admissions per year, with an incidence of 104‐183 cases per 100 000 inhabitants per year. It also entails an elevated risk of morbimortality, including VTE recurrence, bleeding, and early mortality, which can reach up to 40% at 10 years. 1 , 2
Since December 2019, a viral respiratory illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), COVID‐19, has spread throughout the world and was declared a pandemic on March 2020. 3 This disease has been reported to potentially predispose patients to a higher risk of VTE, although the extent of these implications is not yet clear. 4 Many possible explanations have been suggested, including generalized inflammatory response, endothelial dysfunction, 5 immobilization, and disseminated intravascular coagulation (DIC). 6
Due to these alterations, thromboprophylaxis is recommended for admitted COVID‐19 patients and appears to be associated with a lower mortality rate. 6 , 7 In patients admitted in Intensive Care Unit (ICU), a higher risk of VTE, despite proper thromboprophylaxis, has been reported. 8 Nonetheless, when VTE is diagnosed, patients should undergo anticoagulation at therapeutic doses if there are no contraindications, according to the current available guidelines. 9
The risk of bleeding should always be considered in patients under anticoagulation, especially in COVID‐19 patients, since there is still scarce available evidence. A recent study found an overall risk of bleeding of 4.8% in COVID‐19 patients. 10
To date, no studies have reported long‐term outcomes of patients with COVID‐19 and VTE. The objective of our study is to describe the long‐term outcomes of COVID‐19 patients with VTE and to analyze the risk factors of poor prognosis.
2. METHOD
2.1. Type of study
Prospective observational study performed in a tertiary hospital in Madrid to evaluate the long‐term (90 days or more) outcomes of patients diagnosed with VTE in the setting of SARS‐CoV‐2 infection.
2.2. Funding
The investigators did not receive funds for the present study.
2.3. Study population
From March 1 to June 15, the study included all consecutive patients diagnosed with COVID‐19 (confirmed by PCR in nasopharyngeal swab or with clinical and radiological findings very suggestive of the disease) who developed venous thromboembolism including PE, DVT of the extremities, or both (confirmed by angio‐CT of the chest or lung scintigraphy in the case of PE or doppler ultrasound in the case of DVT). The study comprised both patients diagnosed with VTE during hospital admission and outpatients. The study was approved by the Ethics Committee in our center. The objective of the study was to identify markers of poor prognosis in patients with COVID‐19 and VTE.
2.4. Follow‐up
Patients were followed up for a minimum of 90 days or until fatal outcome occurred.
2.5. Variables
The baseline data of the patients were collected including epidemiological data, characteristics of the VTE episode, diagnosis of COVID‐19, complementary tests and treatment received. The main outcome of the study was a compound of major bleeding and death. Major bleeding was classified according to the ISTH guidelines including as follows: fatal bleeding; bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome; bleeding leading to transfusion of two or more units of whole blood or red cells. Patients with PE were classified according to the ESC (European Society of Cardiology) risk score. 11
2.6. Statistical analysis
Qualitative variables are presented through the frequency distribution. Quantitative variables are presented as mean and standard deviation if they have a normal distribution or median and the 25th (P25) and 75th (P75) percentiles or Interquartile range (IQR) in case of a non‐normal distribution. The analysis of qualitative variables is carried out using the chi‐square test to verify its independence. The Mann‐Whitney and Kruskal‐Wallis tests are used for the comparison of means in 2 categories and in several independent categories, respectively, when the distribution is non‐parametric. The Kaplan‐Meier estimator is used to graphically represent the events (death, hemorrhage, and recurrence).
3. RESULTS
A total of 104 patients were diagnosed with VTE during the study period. Incidence of VTE among hospitalized patients (including ICU and non‐ICU setting) was 4.2%. Four patients were lost to follow‐up; thus, the sample comprised 100 patients with a mean age of 65 ± 13.9 years (62% males). Patients’ characteristics are detailed in Table 1. At the time of VTE diagnosis, 66 patients (66.0%) were hospitalized, 23 of them (34.8%) in the ICU. Type of VTE was distributed as follows: 36% had isolated DVT and 64% had PE (49% isolated PE and 15% PE and DVT). Of 51 DVT patients, one was located in upper extremities and the remaining 50 in lower extremities. PE anatomical location was more commonly peripheral (including subsegmental, segmental, or lobar arteries) than central (pulmonary trunk or main pulmonary arteries): 67.1% vs 22.9%. According to European Society of Cardiology (ESC) classification, 7.8% of patients had high‐risk PE (hemodynamically unstable), 40.6% had intermediate‐risk PE (hemodynamically stable with right ventricle dysfunction), and 51.6% had low‐risk PE (hemodynamically stable without right ventricle dysfunction).
TABLE 1.
Baseline characteristics, provoking factors for VTE and laboratory findings in patients with VTE and COVID‐19
| Variable (n = 100) | |
|---|---|
| Patient characteristics | |
| Severe COVID‐19 | 69 (69.0%) |
| Male | 62 (62.0%) |
| Obesity (BMI > 30) | 60 (60%) |
| Smoker | 2 (2.0%) |
| Arterial hypertension | 42 (42.0%) |
| Diabetes | 21 (21.0%) |
| Dyslipidemia | 25 (25.0%) |
| Heart failure | 5 (5.0%) |
| Atrial fibrillation | 3 (3.0%) |
| Ischemic heart disease | 6 (6.0%) |
| Cerebrovascular disease | 4 (4.0%) |
| Peripheral artery disease | 2 (2.0%) |
| Chronic lung disease | 13 (13.0%) |
| Liver disease | 3 (3.0%) |
| Dementia | 6 (6.0%) |
| Antiplatelet therapy | 13 (13.0%) |
| Major bleeding in the last month | 3 (3.0%) |
| Thromboprophylaxis | 69 (69%) |
| Provoking factors for VTE | |
| Active cancer | 7 (7.0%) |
| Recent surgery | 5 (5.0%) |
| Immobilization | 87 (87.0%) |
| <1 wk | 11 (12.6%) |
| 1‐4 wk | 66 (75.9%) |
| 5‐8 wk | 9 (10.3%) |
| >8 wk | 1 (1.1%) |
| Oral contraceptives | 1 (1.0%) |
| Prior VTE | 5 (5.0%) |
| Laboratory findings | |
| Anemia | 47 (47.0%) |
| Thrombocytopenia (<150 000/mm3) | 20 (20.0%) |
| Lymphopenia (<1200/mm3) | 67 (67.0%) |
| Elevated D‐dimer | 99 (99.0%) |
| D‐dimer, median (ng/mL) | 2989 (1846‐8748) |
| Elevated creatinine | 14 (14.0%) |
| Elevated proBNP (only in PE patients) | 57.1% |
Elevated D‐dimer: >250 ng/mL; elevated creatinine: >1.2 mg/dL; elevated pro‐BNP: >500 ng/L.
Abbreviations: BMI, body‐mass index; VTE, venous thromboembolism; BNP, brain natriuretic peptide.
In 91% of patients, there was at least one provoking factor for VTE besides COVID‐19 itself, with 11% patients with more than one provoking factor (Table 1). Anemia, lymphopenia, and elevated D‐dimer were the most common laboratory findings in these patients (Table 1). During the acute phase (first 10 days), most patients received low molecular weight heparin (LMWH) or unfractionated heparin; 7% received fibrinolytics; and only 1 patient required inferior vena cava filter placement. Long‐term therapy of choice was direct oral anticoagulants (DOACs) in 52% of patients, followed by LMWH in 28% patients. Treatment strategies are detailed in Table 2.
TABLE 2.
Treatment strategies in acute and long‐term phases
| Treatment | Isolated DVT (n = 36) | PE with or without DVT (n = 64) | Total (n = 100) |
|---|---|---|---|
| Treatment in the acute phase | |||
| Cava vein filter | 0 (0.0%) | 1 (1.6%) | 1 (1.0%) |
| Fibrinolytics | 0 (0.0%) | 7 (10.9%) | 7 (7.0%) |
| ECMO | 0 (0.0%) | 1 (1.6%) | 1 (1.0%) |
| Unfractionated heparin | 3 (8.4%) | 11 (17.1%) | 14 (14.0%) |
| LMWH | 33 (91.7%) | 58 (90.6%) | 91 (91.0%) |
| Fondaparinux | 1 (2.7%) | 0 (0.0%) | 1 (1.0%) |
| DOACs | 4 (11.1%) | 5 (7.8%) | 9 (9.0%) |
| Long‐term treatment | |||
| DOACs | 18 (50.0%) | 34 (53.1%) | 52 (52.0%) |
| Apixaban | 16 (44.4%) | 23 (35.9%) | 39 (39.0%) |
| Rivaroxaban | 1 (2.7%) | 5 (7.8%) | 6 (6.0%) |
| Edoxaban | 0 (0.0%) | 5 (7.8%) | 5 (5.0%) |
| Dabigatran | 1 (2.7%) | 1 (1.5%) | 2 (2.0%) |
| VKA (acenocoumarol) | 2 (5.5%) | 3 (4.7%) | 5 (5.0%) |
| LMWH | 13 (36.1%) | 15 (23.4%) | 28 (28.0%) |
| Bemiparin | 2 (5.5%) | 4 (6.2%) | 6 (6.0%) |
| Enoxaparin | 11 (27.7%) | 11 (27.7%) | 22 (22.0%) |
| Fondaparinux | 2 (6.5%) | 0 (0.0%) | 2 (2.0%) |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; ECMO, extracorporeal membrane oxygenation; LMWH, low molecular weight heparin; DOACs, direct oral anticoagulants; VKA, vitamin K antagonists.
Median follow‐up was 97 (IQR 89, 111) days. During the study period, 24 patients died (24.0%), 20 patients bled (20%) and there were no VTE recurrences. PE was the cause of death in 5 patients (20.5%); 2 patients died due to bleeding (8.3%), and 15 patients died of respiratory failure due to COVID‐19 (62.5%) (Table 3); 20 deaths were registered during hospitalization and 4 occurred afterward. The median time from VTE diagnosis to death was 12 (IQR: 2.25‐20.75) days (Figure 1). Major bleeding occurred in 11 patients (11%). The median time from VTE diagnosis to major bleeding was 12 (IQR: 5‐16) days (Figure 1). Patients with major bleeding were receiving LMWH (9 patients) or unfractionated heparin (2 patients). Location of bleeding is detailed in Table 3.
TABLE 3.
Outcomes during follow‐up
| Death | 24 (24.0%) |
| COVID‐19 (respiratory failure) | 15 (15.0%) |
| PE | 5 (5.0%) |
| Bleeding | 2 (2.0%) |
| Cancer | 1 (1.0%) |
| Unknown | 1 (1.0%) |
| VTE recurrence | 0 (0.0%) |
| Bleeding | 20 (20.0%) |
| Major bleeding | 11 (11.0%) |
| Location of bleeding (n = 20) | |
| Hematoma | 4 (4.0%) |
| Cerebral | 3 (3.0%) |
| Retroperitoneal | 3 (3.0%) |
| Muscular | 2 (2.0%) |
| Urinary | 2 (2.0%) |
| Gastrointestinal | 1 (1.0%) |
| Other | 5 (4.0%) |
Abbreviations: PE, pulmonary embolism; VTE, venous thromboembolism.
FIGURE 1.
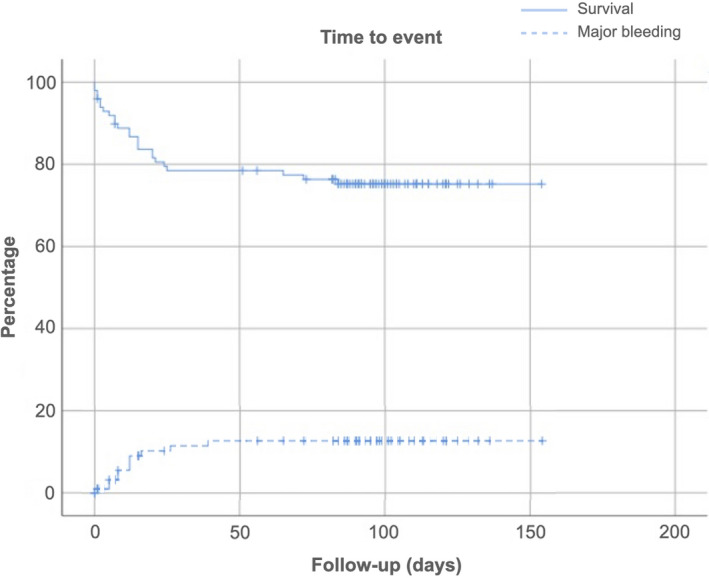
Kaplan‐Meier curve for mortality and major bleeding
We analyzed the predictive capacity of the ESC classification for mortality in PE patients. Mortality was significantly higher in patients in the high‐risk group when compared with the intermediate and low‐risk groups (100% vs 23.1% vs 15.6%, P < .001). We also studied the predictive capacity of RIETE score and HAS‐BLED score to predict major bleeding, with no significant differences between them (P = .51 for RIETE score and P = .78 for HAS‐BLED score).
The main study outcome (a compound of death and major bleeding) was observed in 29 patients (29.0%). The development of main outcome was significantly associated with ICU admission, anemia, thrombocytopenia, and cancer (Table 4). A multivariate analysis was performed including age, sex, thrombocytopenia, cancer, ICU admission, anemia, and D‐dimer levels. Risk of death or major bleeding was independently associated with ICU admission (HR 12.2; 95% CI 3.0‐48.3), thrombocytopenia (HR 4.5; 95% CI 1.2‐16.5), and cancer (HR 21.6; 95% CI 1.8‐259).
TABLE 4.
Univariate analysis for main outcome (compound of death and major bleeding)
| Variable | Death or major bleeding | No death or major bleeding | P‐value | OR | 95% CI |
|---|---|---|---|---|---|
| Male | 20 (32.2%) | 42 (67.7%) | 0.363 | 0.652 | 0.259‐1.640 |
| >65 y | 14 (27.5%) | 37 (72.5%) | 0.729 | 0.858 | 0.360‐2.045 |
| BMI > 30 | 8 (25.0%) | 24 (75.0%) | 0.734 | 0.843 | 0.316‐2.253 |
| Hospital admission | 23 (34.8%) | 43 (65.2%) | 0.079 | 2.496 | 0.898‐6.935 |
| ICU admission | 15 (65.2%) | 8 (34.8%) | <0.001 | 8.437 | 2.981‐23.882 |
| Recent bleeding | 0 (0.0%) | 3 (100%) | – | – | |
| Anemia | 19 (40.4%) | 28 (59.6%) | 0.021 | 2.918 | 1.179‐7.221 |
| Platelets <150 000/mm3 | 10 (50.0%) | 10 (50.0%) | 0.025 | 3.211 | 1.156‐8‐919 |
| Lymphocytes <1200/mm3 | 22 (33.3%) | 44 (66.7%) | 0.141 | 2.167 | 0.774‐6.068 |
| Altered prothrombin time | 20 (27.0%) | 54 (73.0%) | 0.402 | 1.543 | 0.560‐4.254 |
| Creatinine > 1.2 mg/dL | 7 (50.0%) | 7 (50.0%) | 0.071 | 2.909 | 0.912‐9.280 |
| Creatinine > 1.5 mg/dL | 2 (40.0%) | 4 (60%) | 0.584 | 1.679 | 0.263‐10.712 |
| D‐dimer > 1000 ng/mL | 21 (25.0%) | 63 (75.0%) | 0.041 | 0.286 | 0.086‐0.951 |
| D‐dimer > 5000 ng/mL | 11 (32.4%) | 23 (67.6%) | 0.580 | 1.294 | 0.519‐3.226 |
| D‐dimer > 10 000 ng/mL | 5 (25.0%) | 15 (75.0%) | 0.671 | 0.783 | 0.253‐2.421 |
| Cancer | 5 (71.4%) | 2 (28.6%) | 0.024 | 7.187 | 1.296‐39.856 |
| Type of VTE: PE | 18 (28.6%) | 45 (71.4%) | 0.902 | 0.945 | 0.386‐2.315 |
Abbreviations: CI, confidence interval; BMI, body‐mass index; ICU, intensive care unit; VTE, venous thromboembolism; PE, pulmonary embolism.
4. DISCUSSION
The hypercoagulable state in COVID‐19 patients may lead to a high thrombotic risk, similar to the association between community‐acquired pneumonia with an increased risk of vascular disease, both arterial and venous thrombosis. 12 , 13 Two recent meta‐analyses have shown a high prevalence of VTE in COVID‐19 patients with an estimated overall VTE‐prevalence ranging from 14.1% to 30%. 14 , 15 Another retrospective study of 256 patients with COVID‐19 pneumonia and 360 patients with community acquired pneumonia (CAP) showed that COVID‐19 patients were more frequently categorized as high risk for VTE (15.6% vs 10%, P = .036). However, the overall rate of VTE was similar in both groups (2% and 3.6%, respectively). 15 Nevertheless, data examining the long‐term outcomes after the acute episode of VTE are limited. To our knowledge, this is the first prospective study to assess the long‐term outcomes of patients with VTE associated to COVID‐19 infection.
A recent retrospective study showed that, 30 days after hospital discharge, the rates of thrombosis are low (cumulative incidence of VTE was 0.6%) in patients with COVID‐19 infection without thromboprophylaxis, 16 suggesting that this complication is limited to the hospitalization period. In the present study, most of the patients had a “provoked” episode of VTE (91%), being hospital immobilization the most frequently associated provoking factor (76%). Similarly, a recent study of the RIETE Registry including 420 patients with VTE and COVID‐19, showed that the vast majority of patients (78%) had recent immobilization. 17 These results are consistent with previous studies showing that respiratory infection is an independent risk factor for VTE during the following 3 months, being immobilization a mediator for this association. 12 , 13 , 18
The frequency of PE in our study (64%) was markedly higher than the previously reported in real‐world VTE studies, where it ranges from 25% to 38%. 19 , 20 In the previously mentioned RIETE Registry study, 83% of COVID‐19 patients with VTE had acute PE, while only 17% had isolated DVT. 17 A meta‐analysis that included COVID‐19 or non‐COVID‐19 patients showed that the pooled percentage of PE among all VTE patients was significantly higher in COVID‐19 compared with non‐COVID‐19 patients (22.1% vs 6.4% P = .048) in studies in which DVT was systematically screened by CUS (compression ultrasonography). 21 Emerging data regarding the discrepancy between the rate of DVT and PE in COVID‐19 patients, and the fact that many of the documented cases of PE occur in the absence of DVT and are located in the more peripheral pulmonary arteries 22 have led to the hypothesis that there may be a unique PE phenotype in these patients, characterized by thrombi and not emboli—that is, immunothrombosis—is probably much more prominent than originally recognized. 23
ISTH guidelines consider that bleeding is rare in the setting of COVID‐19. 18 However, several retrospective studies have reported variable rates of bleeding complications ranging from 1.2% to 11% mainly related to therapeutic doses of anticoagulation 10 , 17 , 24 , 25 , 26 , 27 , 28 (Table 5). A retrospective study by Shah et al 24 including 187 COVID‐19 critically ill patients found 4.8% of major bleeding events. Musoke et al 25 found that COVID‐19 patients on therapeutic anticoagulation had significantly higher rates of major bleeding compared with those without anticoagulation (11% vs 2%, P = .044). A study by Pesavento et al 26 of 324 COVID‐19 hospitalized patients showed that the rate of major bleeding was higher in those who received higher doses of anticoagulants (9.5% vs 3.3%). Mattioli et al 27 retrospectively evaluated 105 hospitalized COVID‐19 patients treated with intermediate dose of LMWH and only 1.9% patients had major bleeding. Fernández‐Capitán et al 17 investigated the short‐term outcomes (the first 10‐days) of 420 patients diagnosed with VTE during hospitalization for COVID‐19 and reported major bleeding in 2.9% patients. In the present study, 11% of the sample had major bleeding, higher than the previously reported in non‐COVID VTE patients in clinical trials and real world registries. The median number of days from VTE diagnosis to major bleeding was 12. This suggests that this complication is not only related to anticoagulant treatment, but also to the severe inflammation and coagulation disturbances occurring during the acute phase of the infection.
TABLE 5.
Published studies reporting major bleeding in COVID‐19 patients
| Author | N | dose of anticoagulation | Follow‐up period (median) | Major bleeding rate |
|---|---|---|---|---|
| Mattioli et al 27 | 105 hospitalized COVID‐19 patients |
33.4% prophylactic LMWH 62.8% intermediate LMWH |
36 (IQR 24, 43) d | 1.2% |
| Shah et al 24 | 187 COVID‐19 critically ill patients |
80.7% prophylactic LMWH 16.6% therapeutic LMWH |
Not reported Median length of stay from 12 (non‐thrombotic patients) to 17 d (thrombotic patients) |
4.8% |
| Fernández‐Capitán et al 17 | 420 hospitalized COVID‐19 patients with VTE |
88% therapeutic LMWH 6.4% unfractionated heparin 6.2% other (therapeutic dose) |
10 d | 2.9% |
| Musoke et al 25 | 355 hospitalized COVID‐19 patients |
15.4% No anticoagulation 50% prophylactic LMWH 5.6% sub‐therapeutic LMWH 29% therapeutic LMWH |
Not reported |
2% 4% 5% 11% |
| Helms et al 28 | 150 critically ill COVID‐19 patients |
70% prophylactic LMWH 30% therapeutic LMWH |
length of stay 9.6 ± 4.2 d |
2.7% |
| Al‐Samkari et al 10 | 400 hospitalized COVID‐19 patients |
Non‐critically ill: −3.5% No anticoagulation −89.8% prophylactic LMWH: −6.6% Intermediate or full‐dose anticoagulation Critically ill: −1.4% No anticoagulation: −86.1% prophylactic LMWH: −12.5% Intermediate or full‐dose anticoagulation |
Not reported Mean length of stay from 6 (non‐critically ill) to 9 d (critically ill) |
2.3% 4.8% |
| Pesavento et al 26 | 324 hospitalized COVID‐19 patients |
74% prophylactic doses 25.9% sub‐therapeutic doses |
30 d |
3.3% 9.5% |
| The present study | 100 COVID‐19 patients with VTE |
During acute phase LMWH 91% Unfractionated heparin 14% DOAC 9% Long‐term treatment DOAC 52% LMWH 28% VKA 5% |
97 (IQR 89, 111) d | 11% |
Abbreviations: LMWH: low molecular weight heparin; IQR: interquartile range; VTE: venous thromboembolism.
Twenty‐four (24%) patients died during follow‐up and the 30‐day all‐cause mortality was 21%, considerably higher than the previously reported in VTE patients. The early (<30 days) all‐cause mortality has decreased in the last decades in both PE and DVT patients (4.9% and 2.7%, respectively). 29 High in‐hospital mortality rates of COVID‐19 patients have been reported (from 8.1% to 28%) 3 , 30 , 31 and higher among critically ill patients (62%) and those who require mechanical ventilation (81%). 32 Scarce data have been published regarding mortality rate in COVID‐19 patients with VTE. The study from RIETE Registry reported a 10‐day mortality rate of 9.1% among patients in hospital wards and 19% among those in ICUs. 17 Other studies focused on the prevalence of thrombotic complications in COVID‐19 patients and did not report mortality among this subgroup. 25 , 27 , 33 Therefore, this is the first study to report the long‐term all‐cause mortality in COVID‐19 patients with VTE. Finally, severity of PE is one of the main factors of the mortality risk in VTE patients. In our study, ESC score successfully identified those patients with a higher risk of death, suggesting that PE risk stratification is valid in VTE‐COVID‐19.
This study has several limitations. First, in the absence of systematic screening or a standardized protocol for testing, patient characteristics and outcomes may have been influenced by discretionary decisions to test and diagnose VTE. A second limitation is that in the absence of an independent adjudication committee or autopsy; this study cannot provide information on the specific contribution of VTE to the mortality rate that we observed among patients who required hospitalization for COVID‐19. Third, the absence of a control group (COVID‐19 patients without VTE) limits the conclusions that can be drawn. Finally, as stated earlier, it must be clarified that the current study did not focus on the comparative effectiveness of strategies for VTE prevention or treatment. Results from ongoing randomized trials will be much more informative for that purpose.
In conclusion, in patients with COVID‐19 and VTE, mortality, and major bleeding were high and almost a third of deaths were VTE‐related. The majority of complications occurred in the first 30 days. ICU admission, thrombocytopenia, and cancer are risk factors for poor prognosis.
CONFLICT OF INTEREST
Pablo Demelo‐Rodriguez: Consulting or Advisory Role: Boehringer, LEO Pharma, Ingelheim, Techdow; Speakers’ Bureau: Rovi, Sanofi and Aspen. Lucía Ordieres‐Ortega declares that there is no conflict of interest. Zichen Ji declares that there is no conflict of interest. Jorge del‐Toro‐Cervera: Consulting or Advisory Role: Boehringer, Ingelheim, Techdow; Speakers’ Bureau: Rovi, Sanofi and Aspen. Javier de Miguel‐Díez declare that there is no conflict of interest. Luis Antonio Álvarez‐Sala‐Walther declares that there is no conflict of interest. Francisco Galeano‐Valle: Speakers’ Bureau: Techdow, Rovi.
AUTHOR CONTRIBUTIONS
P. Demelo‐Rodríguez and F. Galeano‐Valle have contributed to concept and design; P. Demelo‐Rodríguez, L. Ordieres‐Ortega, Z. Ji, and F. Galeano‐Valle have contributed to collect, analysis, and interpretation of data and critical writing the manuscript; P. Demelo‐Rodríguez, L. Ordieres‐Ortega, Z. Ji, J. del Toro‐Cervera, J. de Miguel‐Díez, L. A. Álvarez‐Sala‐Walther, and F. Galeano‐Valle have reviewed the manuscript and final approval of the version to be published.
ACKNOWLEDGEMENTS
None to declare.
Demelo‐Rodríguez P, Ordieres‐Ortega L, Ji Z, et al. Long‐term follow‐up of patients with venous thromboembolism and COVID‐19: Analysis of risk factors for death and major bleeding. Eur J Haematol. 2021;106:716–723. 10.1111/ejh.13603
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Gandhi V, Hewston M, Yadam S, et al. Consequences of venous thromboembolism, including chronic thromboembolic pulmonary hypertension. Crit Care Nurs Q. 2017;40:260‐275. [DOI] [PubMed] [Google Scholar]
- 2. Winter MP, Schernthaner GH, Lang IM. Chronic complications of venous thromboembolism. J Thromb Haemost. 2017;15:1531‐1540. [DOI] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu YU, et al. China medical treatment expert group for Covid‐19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikdeli B, Madhavan MV, Jimenez D, et al.; Global COVID‐19 Thrombosis Collaborative Group . COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020;16(75):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martín‐Rojas RM, Pérez‐Rus G, Delgado‐Pinos VE, et al. COVID‐19 coagulopathy: an in‐depth analysis of the coagulation system. Eur J Hematol. 2020;105(6):741‐750.[epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019. CHEST guideline and expert panel report. Chest. 2019;2020(158):1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;23(136):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543‐603. [DOI] [PubMed] [Google Scholar]
- 12. Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12:1391‐1400. [DOI] [PubMed] [Google Scholar]
- 13. Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case‐control study through a general practice database. Int J Epidemiol. 2011;40:819‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nopp S, Moik F, Jilma B, et al. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178‐1191.[epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mei F, Fan J, Yuan J, et al. Comparison of venous thromboembolism risks between COVID‐19 pneumonia and community‐acquired pneumonia patients. Arterioscler Thromb Vasc Biol. 2020;40:2332‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID‐19. Blood. 2020;136:1342‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández‐Capitán C, Barba R, Díaz‐Pedroche MDC, et al. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID‐19. Semin Thromb Hemost. 2020. 10.1055/s-0040-1718402 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Ribeiro DD, Lijfering WM, Van Hylckama Vlieg A, et al. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012;10:1179‐1182. [DOI] [PubMed] [Google Scholar]
- 19. Bounameaux H, Haas S, Farjat AE, et al. Comparative effectiveness of oral anticoagulants in venous thromboembolism: GARFIELD‐VTE. Thromb Res. 2020;191:103‐112. [DOI] [PubMed] [Google Scholar]
- 20. Antonucci E, Migliaccio L, Abbattista M, et al. Treatment decision‐making of secondary prevention after venous thromboembolism: data from the real‐life START2‐POST‐VTE register. Clin Appl Thromb Hemost. 2020;26:1076029620945792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Birocchi S, Manzoni M, Podda GM, et al. High rates of pulmonary artery occlusions in COVID‐19. A meta‐analysis. Eur J Clin Invest. 2020. 10.1111/eci.13433 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desborough MJR, Doyle AJ, Griffiths A, et al. Image‐proven thromboembolism in patients with severe COVID‐19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhury JF, Moores LK, Connors JM. Anticoagulation in hospitalized patients with Covid‐19. N Engl J Med. 2020;383:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 24. Shah A, Donovan K, McHugh A, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID‐19: a multicentre observational study. Crit Care. 2020;24:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musoke N, Lo KB, Albano J, et al. Anticoagulation and bleeding risk in patients with COVID‐19. Thromb Res. 2020;196:227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pesavento R, Ceccato D, Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non‐critically ill patients with Covid‐19: the Padua province experience. J Thromb Haemost. 2020;18(10):2629‐2635.[epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattioli M, Benfaremo D, Mancini M, et al. Safety of intermediate dose of low molecular weight heparin in COVID‐19 patients. J Thromb Thrombolysis. 2021;51(2):286‐292.[epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morillo R, Jiménez D, Aibar MÁ, et al. DVT management and outcome trends, 2001 to 2014. Chest. 2016;150:374‐383. [DOI] [PubMed] [Google Scholar]
- 30. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Di Minno A, Ambrosino P, Calcaterra I, et al. COVID‐19 and venous thromboembolism: a meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46:763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


