Abstract
Objectives
Many routine sinonasal procedures utilising powered instruments are regarded as aerosol‐generating. This study aimed to assess how different instrument settings affect detectable droplet spread and patterns of aerosolised droplet spread during simulated sinonasal surgery in order to identify mitigation strategies.
Design
Simulation series using three‐dimensional (3‐D) printed sinonasal model. Fluorescein droplet spread was assessed following microdebriding and drilling of fluorescein‐soaked grapes and bones, respectively.
Setting
University dry lab.
Participants
3‐D printed sinonasal model.
Main outcome measures
Patterns of aerosolised droplet spread.
Results and Conclusion
There were no observed fluorescein droplets or splatter in the measured surgical field after microdebridement of nasal polyps at aspecific irrigation rate and suction pressure. Activation of the microdebrider in the presence of excess fluid in the nasal cavity (reduced or blocked suction pressure, excessive irrigation fluid or bleeding) resulted in detectable droplet spread. Drilling with either coarse diamond or cutting burs resulted in detectable droplets and greater spread was observed when drilling within the anterior nasal cavity. High‐speed drilling is a high‐risk AGP but the addition of suction using a third hand technique reduces detectable droplet spread outside the nasal cavity. Using the instrument outside the nasal cavity inadvertently, or when unblocking, produces greater droplet spread and requires more caution.
Keywords: aerosol‐generating procedure, COVID‐19, droplet, nasal endoscopy, sinus surgery, skull base surgery
Key points.
Endoscopic sinus surgery is an aerosol‐generating procedure (AGP).
Understanding the relationship between irrigation rates and suction pressures when using the microdebrider can provide strategies to reduce the aerosolisation potential of powered sinus surgery.
Activation of the microdebrider when there is fluid accumulation in the nasal cavity has been demonstrated to cause droplet contamination.
Drilling with either coarse diamond or cutting bur resulted in detectable droplets. Greater droplet spread was observed when drilling within the anterior nasal cavity.
High‐speed drilling is a high‐risk AGP, but the addition of suction (two surgeon, three‐hand technique) reduces detectable droplet contamination outside the nasal cavity.
1. INTRODUCTION
The risk of transmitting respiratory viruses during aerosol‐generating procedures (AGPs) of the respiratory tract is high. Powered instruments typically used during ENT procedures, such as intranasal microdebriding or mastoid drilling, have been identified as AGPs although the actual risk of transmitting viral particles remains uncertain. 1 Prior to the Covid‐19 pandemic and subsequent reported deaths of surgeons contracting coronavirus from infected patients, the risk of aerosolised virus transmission was recognised but not considered to be as dangerous. This realisation resulted in temporary cessation of elective surgery, including all routine ENT procedures. 2 , 3 The current recommendations for personal protective equipment (PPE) required to undertake AGPs continue to evolve as new epidemiologic and scientific evidence become available, influenced by external factors such as socio‐economic pressures, supply chain issues and advice from medical professional associations. Recent studies, prompted by the Covid‐19 pandemic, have demonstrated that many powered instruments used in sinonasal surgery are aerosol‐generating with high‐speed drilling producing the greatest potential. 4 , 5 , 6
Whilst previous studies have described the patterns of aerosolised droplet spread during simulated endoscopic sinonasal surgery, the aim of this study was to assess how different instrument settings would affect detectable droplet spread. The ability to vary instrument settings mimics real‐life conditions where surgeons may have personal preferences or may choose to alter settings to better suit the clinicopathological requirement. It is envisaged that the results of this study will inform how best to mitigate droplet spread, evaluate choice of instruments and consider droplet spread as site dependent within the sinonasal cavity.
2. METHODS
2.1. Ethical considerations
The study protocol was approved by the Research Governance and Ethics Office of the Liverpool School of Tropical Medicine (Research Protocol 20‐046).
2.2. Experimental set‐up
All simulated surgical procedures were undertaken in a dry laboratory on a realistic, life‐sized model (3D LifePrints UK Ltd. Liverpool, U.K.) derived from open‐sourced CT scan data (OsiriX. Pixmeo SARL. Bernex, Switzerland). The 3‐D printed model was placed in a supine, 30° head‐up position on a medical examination bench covered by an impervious black sheet (Figure 1A). A grid pattern on the sheet followed the design described in a recently published study 4 . The model was placed at the apex of a triangle extending to the edges of the sheet at a 50° angle, with the sides of the triangle extending from the model measuring 55 cm to the edge of the sheet. Subdivisions were made, with the central portion of the first subdivisions positioned 6 cm away from the nasal aperture, and each subsequent subdivision at 12‐cm intervals. Sections closer to the nares were divided into smaller subdivisions. Each subdivision was at least 10 cm in maximum diameter.
FIGURE 1.
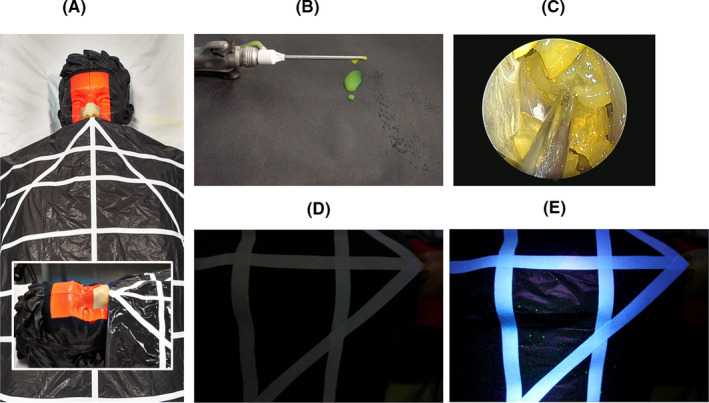
Experimental setup: A, Model of the head draped with grid detection sheet. Inset shows close‐up of the 3‐D printed nose and paranasal cavity. B, Example of dripping from microdebrider after activation. C, Endoscopic view of fluorescein stained grapes mimicking nasal polyps. D,E, Example of droplets identified on detection grip before and after UV lamp illumination, respectively
2.3. Simulated surgical procedures
The procedures include:
External activation of microdebrider and blade outside nasal cavity,
Intranasal microdebridement of nasal polyps,
High‐speed drilling of bone.
Microdebrider simulations were carried out using the Straightshot™ M5 handpiece (Medtronic Inc. Jacksonville, FL, USA) and 4 mm TriCut® blade. Fluorescein (Monument Tools, MAP UK (TA Tool Chimp) Ltd. Essex, UK) was added to the irrigation fluid; 1 g dye diluted in 250 mL irrigation fluid. Various irrigation rates, oscillation speeds and suction pressure settings were tested (Table 1). With each combination of settings, the microdebrider was activated for one minute and the presence of fluorescein‐dyed irrigation fluid drips and droplets from the instrument tip were assessed in the darkened laboratory room aided by UV lighting (Figure 1B).
TABLE 1.
Assessment of fluorescein‐dyed irrigation dripping from the microdebrider or drill bur tip during external activation for one minute. Present = Yes, not present = No. N/A = not applicable
| Handpiece type and setting | Irrigation rate (mL/min) | Suction pressure (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 (suction off) | 100 | 140 | 180 | 200 | 220 | 240 | ||
| Microdebrider. Oscillation mode, 2000 rpm | 5 | Yes | No | No | No | No | No | No |
| 15 | Yes | No | No | No | No | No | No | |
| 20 | Yes | No | No | No | No | No | No | |
| 25 | Yes | Yes | Yes | Yes | No | No | No | |
| 30 | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| 40 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Microdebrider. Oscillation mode, 5000 rpm | 25 | Yes | Yes | No | No | No | No | No |
| Microdebrider. Forward mode, 6000 rpm | 25 | Yes | No | No | No | No | No | No |
| 40 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| High‐speed drill, Diamond bur, 12 000 rpm | 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| High‐speed drill. Cutting bur, 60 000 rpm | 20 | Yes* | N/A | |||||
There is no integrated suction port in the Midas Rex Legend Stylus drill handpiece.
Peeled grapes soaked overnight in diluted fluorescein dye solution (1 mg in 25 mL) were used to simulate nasal polyps. Endonasal surgery was performed using a 4 mm 0° endoscope connected to monitor and camera system (Karl Storz). At the start of each experiment, pieces of grape were placed in the nasal cavity and middle meatus of the model before microdebriding for one minute (Figure 1C). The black sheet was then inspected for fluorescein droplets using the UV lamp (Figure 1D,E). The microdebrider was unblocked with the supplied cleaning brush stylet when required. Remnants of the grapes were removed from the model and replaced with fresh pieces prior to the next experiment. The model and surrounding surgical field were then cleaned and rechecked with the UV lamp before commencing the next experiment. Each was repeated four times to provide five sets of data.
For surgical drilling simulation, 1 cm × 1 cm blocks of sterilised porcine rib soaked in fluorescein dye solution (1 mg in 25 mL) were used. One piece of bone was placed on the face of sphenoid adjacent to the nasal septum to simulate drilling of the sphenoid rostrum. A second piece was tucked under the inferior turbinate to simulate drilling in the anterior nasal cavity (eg lateral nasal wall during medial maxillectomy). Drilling was undertaken with either a 5 mm 15° curved coarse diamond bur or a 4 mm 15° curved cutting bur with an activation period of one minute. The diamond bur was attached to the Straightshot™ M5 handpiece, while the cutting bur was attached to the Midas Rex Legend Stylus. An additional suction (Storz 3 mm Frazier suction tube) was introduced and placed within the surgical field to remove excess irrigation fluid (two surgeon, three‐hand technique).
2.4. Quantification of fluorescein droplets and reporting of data
The assessment of dripping from the instrument tip during external activation of the microdebrider was undertaken in binary fashion, that is present or not present (Figure 1B). Similarly, the presence of droplet deposition on the surgical field following intranasal activation of the microdebrider or drill was determined in a binary fashion (Figure 1D,E). As each experiment had a total of five data sets, the results were aggregated into a heat map to illustrate the frequency of droplet detection; 0 = black, 1‐2 = yellow, 3‐4 = orange and 5 = red.
3. RESULTS
During external activation of the microdebrider at 2000 rpm (oscillation mode), dripping from the instrument tip occurred as the irrigation rate was increased incrementally while suction pressure was fixed (Table 1). Higher irrigation rates required higher suction pressures to stop dripping. Expectantly, dripping from the microdebrider tip occurred when suction was switched off and when the irrigation rate was increased to 40 mL/min despite having maximum suction pressure (240 mmHg). With the irrigation rate fixed at 25 ml/min, no dripping was observed during oscillation at 5000 rpm with suction pressure set at 140 mmHg and above. When the microdebrider was switched to forward mode (e.g. to simulate shaving turbinate bone during turbinoplasty) and 25 mL/min irrigation maintained, no dripping was observed at all suction pressure settings. However, at 40 mL/min irrigation, dripping was observed even at the highest suction pressure setting.
Extra‐nasal microdebriding of fluorescein‐soaked grapes resulted in droplets on the detection grid (Figure 2A). When microdebriding the simulated nasal polyps within the nasal cavity, no fluorescein droplets were detected at a constant microdebrider setting of 2000 rpm oscillation (irrigation 25 mL/min, suction pressure 200 mmHg) (Figure 2B). In contrast, droplets were detected on the grid area adjacent to the nares when suction pressure was reduced to 100 mmHg (Figure 2C).
FIGURE 2.
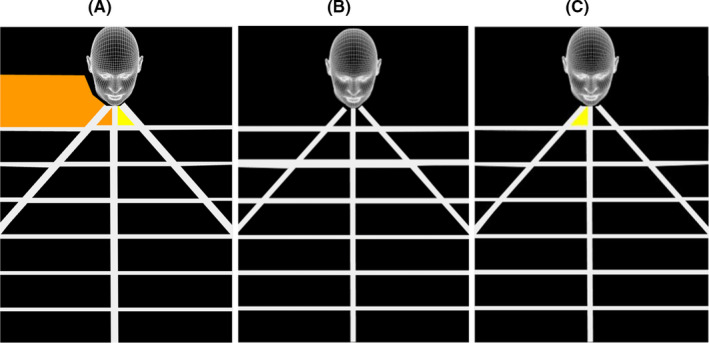
Illustration of geographic spread of aerosol droplets by 4 mm TriCut® blade: A, Extranasal microdebridement (2000 rpm oscillation, irrigation 25 mL/min, suction pressure 200 mmHg) of simulated nasal polyp. B, Intranasal microdebridement (2000 rpm oscillation, irrigation 25 mL/min, suction pressure 200 mmHg) of simulated nasal polyp and C, Extranasal microdebridement (2000 rpm oscillation, irrigation 25 mL/min, suction pressure 100 mmHg) of simulated nasal polyp
Although diamond and cutting burs have built‐in irrigation, only the former have a suction evacuation port. Drilling with the cutting burs resulted in greater and wider spread of droplets on the detection grid than with the diamond bur (Figures 3A‐F and 4A‐D). Regardless of bur type, drilling on the sphenoid rostrum resulted in less droplet detection compared to drilling within the anterior nasal cavity. The introduction of an additional suction tube resulted in no droplet detected on the grid when the sphenoid rostrum was drilled with the diamond bur (Figure 3F).
FIGURE 3.
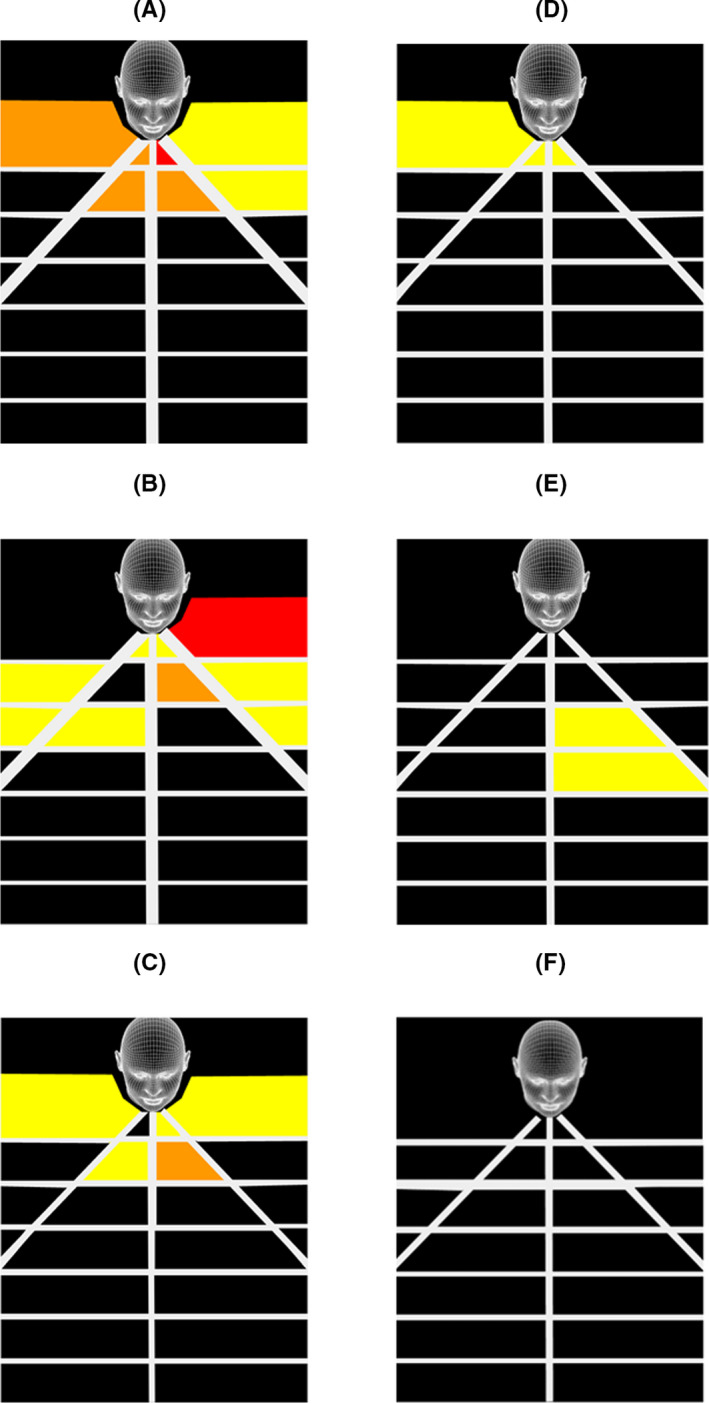
Illustration of geographic spread of aerosol droplets caused by 5 mm 15° curved diamond bur (12 000 rpm) when drilling in the anterior nasal cavity (A,B,C) and on the sphenoid rostrum (D,E,F). Built‐in suction switched off = A,D. Built‐in suction switched on = B,E. Additional Frazer suction = C,F
FIGURE 4.
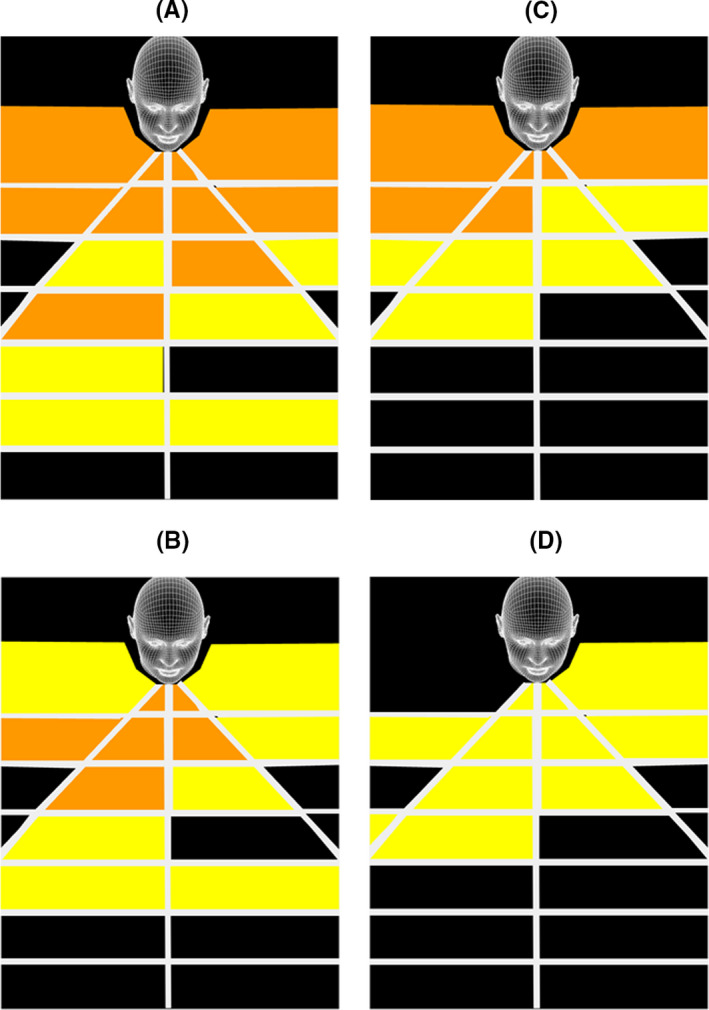
Illustration of geographic spread of aerosol droplets caused by 4 mm 15° curved cutting bur (60 000 rpm) when drilling in the anterior nasal cavity (A,B) and on the sphenoid rostrum (C,D). No suction = A,C. Additional Frazer suction = B,D
4. DISCUSSION
4.1. Synopsis of key/new findings
The paradigm shift in sinus surgery was driven by the introduction of the endoscope in the 1980s and in the following decade, adoption of powered instruments in routine clinical practice. 7 , 8 , The microdebrider, a ubiquitous tool in modern endoscopic sinus surgery, was adapted from powered instruments commonly used in orthopaedic surgery at the time. 9 Despite modifications and improvements to the microdebrider, the principles and mechanics that govern its basic functionality have stood the test of time. Adequate suction pressure is required to draw soft tissue into the rotating microdebrider blade with enough irrigation flowing through the instrument to effect removal of exenterated tissue and, equally critical, to prevent blocking the instrument. These variables are also influenced by the speed of rotation or oscillation of the microdebrider blade ‐ the higher the rate of revolution, the less time the instrument tip is open for soft tissue to be drawn in.
At an oscillating rate of 2000 rpm, the optimum point appears to be at 25 mL/min irrigation and 200 mmHg suction pressure. At this setting, no dripping was observed outside the nasal cavity (Table 1) and no detectable droplets were observed when nasal polyps were debrided (Figure 2B). However, droplets were detected when the suction pressure was reduced to 100 mmHg (Figure 2C). The latter observation may infer that greater aerosolisation of intranasal fluid occurs when the microdebrider is blocked or when there is excess fluid in the operative field. Excessive bleeding from sinonasal mucosa would also increase the volume of fluid within the nasal cavity potentially resulting in greater aerosolisation during surgery.
High‐speed drilling within the nasal cavity, regardless of revolution speed or bur type (diamond versus cutting), resulted in detectable droplet spread outside the nasal cavity (Figures 3B,E and 4A,C), corroborating observations reported by other groups. 6 , 10 The addition of suction, whether as a built‐in feature of the bur or provided by the introduction of an additional suction catheter, does not eliminate extra‐nasal droplet spread. In addition, drilling in the anterior part of the nasal cavity resulted in greater droplet spread outside the nasal cavity than drilling more posteriorly (Figure 3B,E and Figure 4C,D). Although not simulated in our study, drilling of the frontal beak also resulted in detectable splatter contamination up to 9 cm away from the nasal cavity 5 .
4.2. Strengths of the study
Unlike recent studies where cadavers were utilised in the experiments, we decided to use a realistic 3‐D printed model because we wanted to simulate common sinonasal procedures such as nasal polypectomy and be able to replicate the experiments consistently whilst observing trends in results. We also believed that fluorescein‐soaked grapes were more realistic than fluorescein‐stained mucosa as there was greater soft tissue volume for microdebriding. During the set‐up phase of our study, we concluded that 2.5 mL of diluted fluorescein (as reported by Workman et al.) was insufficient volume to completely saturate the nasal cavity, leading us to add fluorescein to the irrigation fluid. We also evaluated the technique described by another research group of filling the sinus and nasal cavity with 1 mg/mL fluorescein solution to the level of the anterior head of the inferior turbinate for 15 minutes, but realised that it was more effective to soak the simulated tissue (grapes, bone) in fluorescein 5 . We activated the powered instruments for a continuous period of one minute, while other studies described microdebriding for 10 minutes and drilling for 5 minutes. We believed that the ability to replace and reposition simulated nasal polyps and bone represented a better method of replicating each experimental condition, thus improving consistency for our observations.
4.3. Comparisons with other studies
This study has focused on instruments designed by one manufacturer (Medtronic Inc.) and therefore should not be extrapolated to other makes of microdebrider or drills. The study reported by Sharma et al utilised the Entellus Medical Shaver System SS‐100 microdebrider (Stryker Inc.) set at 5000 rpm 7 . The authors simulated endoscopic sinus surgery on cadavers, and after 10 minutes of using the microdebrider, droplets were observed up to 6 cm away from the nasal cavity. Both irrigation rate and suction pressure were not specified in their paper, and it is unclear whether blockage of the microdebrider occurred during the 10 minutes of simulated surgery. In our study, microdebriding or drilling was limited to one minute. Whilst we recognise this does not necessarily reflect rea life conditions or practice, surgeons are unlikely to have the microdebrider or drill activated for an extended period of several minutes continuously.
The addition of a third hand suction device when drilling the sphenoid rostrum resulted in no detectable splatter of droplets (Figure 3F). The coarse diamond bur used in our experiment had a built‐in suction port at the tip of the round bur and endonasal drilling should be performed using the sides of the bur rather than the tip. Dharmajan et al also concluded that placement of an additional suction in the nasal cavity or nasopharynx whilst drilling resulted in complete elimination of all detectable aerosols by a high‐fidelity particle counter. 11 It is important to note, however, that the risk of AGP remains and in no way obviates the need for appropriate PPE. In another study which utilised an endoscopy mask connected to a suction unit operating at 200 mmHg, there was significant reduction in fine particulate production and droplet spread during simulated sinus surgery. 12
4.4. Limitations of this study
The experiments were undertaken on a life‐sized 3‐D printed model based on a normal adult CT scan. Our model lacked hair around the nasal vestibule to reduce the amount of droplet splatte, however some surgeons trim these hairs prior to surgery in order to reduce lens contamination.
In addition, anatomical variations such as septal deviation, hypertrophic inferior turbinate or concha bullosa may affect splatter patterns. This was not evaluated in our study, similar to previous AGP‐related studies on human cadavers. 4 , 5 , 6 Grapes and porcine rib may not mimic nasal polyps or sinonasal bone accurately but make an approximation as opposed to having no pathology within a cadaver specimen. These tissues could affect the splatter patterns due to different tissue characteristics and how particulates are formed during activation of the microdebrider and drill. Nevertheless, we used these human tissue substitutes as our human anatomy laboratory was closed during UK national lockdown and given the limited number of available cadavers, we would not have been able to replicate the large number of experiments in our study protocol.
The ability to detect splatter on the detection grid corroborates with previous studies that microdebriding and drilling are AGPs. The presence of airborne particulates was not assessed as we did not have an optical particle counter. The study reported by Workman et al 13 noted there were more airborne particles when dry drilling the anterior part of the nasal cavity. When suction was added during intranasal drilling, significant 1‐10 μm airborne particulate generation over baseline concentrations was not observed in either posterior or anterior drilling situations. Given that our study lacked the sophisticated droplet detection methods described in other reports, it is plausible that droplet patterns could be more intense or more widespread than what has been observed. The technique described by Workman et al had an estimated particle size detection limit of 20 μm, although the inertial impaction method described by Dharmarajan et al could detect particles <15 μm in diameter. 4 , 11 The latter study noted that placement of a suction instrument in the nasal cavity or nasopharynx led to complete elimination of all detectable aerosols.
4.5. Clinical applicability of the study
The aim of this study has not been to eliminate the AGP potential of powered instruments, but rather to provide greater understanding of an issue poorly evaluated prior to the Covid‐19 pandemic and offer mitigation strategies to optimise safer surgery. Surgeons undertaking endoscopic sinus surgery should be aware of the technical parameters of the various powered instruments they use, as well as being able to alter settings and troubleshoot when necessary. Activation of the microdebrider or drill bur should not occur outside the nasal cavity, especially after the instrument has been used in the patient.
Clear understanding of the interactions between irrigation rates and suction pressures when using a microdebrider or drill provide additional information to reduce and minimise aerosol production during sinonasal surgery. It should be noted that data presented in this study focused on instruments manufactured by one company (Medtronic Inc.) and therefore should not be extrapolated to other manufacturer's microdebriders or drills without validation. This is because bur designs and instrument performance settings differ between various manufacturers. The placement of an additional suction catheter during endonasal drilling, either held by an assistant (three hand technique) or placed in the vicinity of the surgical field, reduces droplet spread. 4 , 10 , 12 , 13
5. CONCLUSION
Understanding the relationship between irrigation rates and suction pressures whilst using a microdebrider allows a strategy to reduce droplet production and potential aerosolisation during powered sinus surgery. Activation of the microdebrider when there is fluid accumulation in the nasal cavity has been demonstrated to cause droplet contamination, similarly outside the nose when unblocking. High‐speed drilling is a high‐risk AGP, but the addition of suction (two surgeon, three‐hand technique) reduces detectable droplet contamination outside the nasal cavity.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
SCL, PA and ED conceptualised the study. SCL, DM and ED undertook the experiments. All authors contributed to the data analysis, drafting and final review of the manuscript.
ETHICAL CONSIDERATIONS
The study protocol was approved by the Research Governance and Ethics Office of the Liverpool School of Tropical Medicine (Research Protocol 20‐046).
Leong SC, Mogre D, Andrews P, Davies E. Reducing the risks of endoscopic sinonasal surgery in the Covid‐19 era. Clin Otolaryngol. 2021;46:809–815. 10.1111/coa.13743
Funding information
Equipment used in this study was supported by Karl Storz Endoscopy (UK) Ltd. and Medtronic External Research Programme Grant ERP‐2020‐12284.
DATA AVAILABILITY STATEMENT
Due to the nature of this research, data sharing is not applicable to this article.
REFERENCES
- 1. Mick P, Murphy R. Aerosol‐generating otolaryngology procedures and the need for enhanced PPE during the COVID‐19 pandemic: a literature review. J Otolaryngol Head Neck Surg. 2020;11(49):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guidance for ENT during the COVID‐19 pandemic. ENT UK. London, U.K. https://www.entuk.org/guidance‐ent‐during‐covid‐19‐pandemic‐0.
- 3. Aerosol‐generating procedures in ENT. ENT UK. London, U.K. https://www.entuk.org/aerosol‐generating‐procedures‐ent.
- 4. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10:798‐805. [DOI] [PubMed] [Google Scholar]
- 5. Sharma D, Rubel KE, Ye MJ, et al. Cadaveric simulation of endoscopic endonasal procedures: analysis of droplet splatter patterns during the COVID‐19 pandemic. Otolaryngol Head Neck Surg. 2020;163(1):145‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma D, Ye MJ, Campiti VJ, et al. Mitigation of Aerosols Generated During Rhinologic Surgery: A Pandemic‐Era Cadaveric Simulation. Otolaryngology–Head and Neck Surgery. 2021;164(2):433–442. 10.1177/0194599820951169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kennedy DW, Zinreich SJ, Rosenbaum A, et al. Functional endoscopic sinus surgery: Theory and diagnosis. Arch Otolaryngol. 1985;111(9):576‐582. [DOI] [PubMed] [Google Scholar]
- 8. Setliff RC, Parsons DS. The “hummer”: new instrumentation for functional endoscopic sinus surgery. Am J Rhinol. 1994;8(6):275‐280. [Google Scholar]
- 9. Christmas DA Jr, Krouse JH. Powered instrumentation in functional endoscopic sinus surgery. I: surgical technique. Ear Nose Throat J. 1996;75(1):33‐36, 39–40. [PubMed] [Google Scholar]
- 10. David AP, Jiam NT, Reither JM, Gurrola JG 2nd, Aghi MK, El‐Sayed IH. Endoscopic skull base and transoral surgery during COVID‐19 pandemic: minimizing droplet spread with negative‐pressure otolaryngology viral isolation drape. Head Neck. 2020;42(7):1577‐1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dharmarajan H, Freiser ME, Sim E, et al. Droplet and Aerosol Generation With Endonasal Surgery: Methods to Mitigate Risk During the COVID‐19 Pandemic. Otolaryngology–Head and Neck Surgery. 2021;164(2):285–293. 10.1177/0194599820949802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones HAS, Salib RJ, Harries PG. Reducing Aerosolized Particles and Droplet Spread in Endoscopic Sinus Surgery during COVID ‐19. The Laryngoscope. 2020. 10.1002/lary.29065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Workman AD, Xiao R, Feng A, et al. Suction mitigation of airborne particulate generated during sinonasal drilling and cautery. International Forum of Allergy & Rhinology. 2020;10(10):1136–1140. 10.1002/alr.22644 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this research, data sharing is not applicable to this article.


