Abstract
Aim
To investigate the association between routine use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors and the severity of coronavirus disease 2019 (COVID‐19) infection in patient with type 2 diabetes in a large multicentric study.
Materials and Methods
This study was a secondary analysis of the CORONADO study on 2449 patients with type 2 diabetes (T2D) hospitalized for COVID‐19 in 68 French centres. The composite primary endpoint combined tracheal intubation for mechanical ventilation and death within 7 days of admission. Stabilized weights were computed for patients based on propensity score (DPP‐4 inhibitors users vs. non‐users) and were used in multivariable logistic regression models to estimate the average treatment effect in the treated as inverse probability of treatment weighting (IPTW).
Results
Five hundred and ninety‐six participants were under DPP‐4 inhibitors before admission to hospital (24.3%). The primary outcome occurred at similar rates in users and non‐users of DPP‐4 inhibitors (27.7% vs. 28.6%; p = .68). In propensity analysis, the IPTW‐adjusted models showed no significant association between the use of DPP‐4 inhibitors and the primary outcome by Day 7 (OR [95% CI]: 0.95 [0.77–1.17]) or Day 28 (OR [95% CI]: 0.96 [0.78–1.17]). Similar neutral findings were found between use of DPP‐4 inhibitors and the risk of tracheal intubation and death.
Conclusions
These data support the safety of DPP‐4 inhibitors for diabetes management during the COVID‐19 pandemic and they should not be discontinued.
Keywords: DPP‐4 inhibitor, observational study, type 2 diabetes
1. INTRODUCTION
Diabetes has been evidenced as one of the main clinical factors associated with severity of coronavirus disease 2019 (COVID‐19). Dipeptidyl peptidase‐4 (DPP‐4 [or CD26]), a transmembrane glycoprotein, expressed in endocrine cells, immune cells, endothelial cells and pneumocytes, among many tissues, is now recognized as a coronavirus receptor protein. 1 Its functions, which are incompletely unveiled, include degradation of incretins such as glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide, but also immune regulation by activation of T cells, upregulation of CD86 expression and NF‐kappa B pathway, and cleavage of a number of cytokines, chemokines and growth factors. 2 During previous coronavirus epidemics, it was suggested that higher severity of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection in type 2 diabetes (T2D) could be associated with a DPP‐4‐mediated dysregulated immune response. The hypothesis was supported by experimental work using human‐DPP‐4–expressing transgenic obese mice, 3 and also by a genetic association study in patients, 4 and has been recently reviewed. 5 , 6 , 7 On the other hand, administration of recombinant soluble DPP‐4 attenuated lung histopathology in another preclinical study. 8 This was consistent with the observation of lower circulating levels of soluble DPP‐4 in human subjects with MERS‐CoV, relative to healthy controls. 9
People with T2D, whether they are obese or not, are commonly treated with DPP‐4 inhibitors. There is no evidence of a higher risk of respiratory tract infections associated with the use of this class of antidiabetic drugs according to randomized controlled trials 10 or observational studies, 11 , 12 although a 2011 report of the World Health Organization adverse drug reactions database showed a higher prevalence of upper respiratory tract infections among users of DPP‐4 inhibitors compared with users of other antidiabetic drugs. 13 Therefore, the effects of DPP‐4 inhibition on the immune response in patients with T2D remain unclear, and the critical role of regulation of cytokines during the course of COVID‐19, with the burst of apparently uncontrolled immune activation a few days after the onset of the symptoms in many severely affected patients, has led to calls for caution in the use of DPP‐4 inhibitors on one side, and the launch of a small clinical randomized trial to assess whether a DPP‐4 inhibitor could reduce the severity of COVID‐19 on the other (NCT04341935).
Thus, discrepant messages have been received by healthcare providers and people with diabetes. To the best of our knowledge, the clinical evidence they need to guide their decisions regarding the use of DPP‐4 inhibitors is still limited to a small neutral case control study. 14
Our purpose was to investigate the association between the use of DPP‐4 inhibitors and the early severity of illness and mortality in patients with T2D hospitalized for COVID‐19 infection, by using propensity score matching in the CORONADO (CORONAvirus and Diabetes Outcomes) study.
2. MATERIALS AND METHODS
2.1. Study design and participants
The current study was a secondary analysis of the CORONADO study (ClinicalTrials.gov NCT04324736), which aimed to describe the phenotype and prognosis of people with diabetes admitted to hospital for COVID‐19 and diabetes from 10 March to 10 April 2020. The study was sponsored by CHU Nantes and was designed in accordance with the declaration of Helsinki and conducted in accordance with French legislation, with approval obtained from the local ethics committee (Groupe Nantais d'Éthique dans le Domaine de la Santé [GNEDS]), the CEREES (Comité d'Expertise pour les Recherches, les Études et les Évaluations dans le domaine de la Santé; Institut National des Données de Santé [INDS]: no. 1544730) and the CNIL (Commission Nationale de l'Informatique et des Libertés; DR‐2020‐155/920129). A ‘non‐opposition to participate’ was orally collected after informed consent if feasible, according to the recommendation of the ethical committee for this observational study.
Inclusion criteria and the design of the CORONADO study have been reported elsewhere. 15 For the purpose of the current subanalysis, information on the routine use of DPP‐4 inhibitors (i.e. sitagliptin, vildagliptin and saxagliptin, which are commercially available in France) prior to admission was mandatory for inclusion.
Clinical and biological data have been described previously. 15 In the current analysis, HbA1c and estimated glomerular filtration rate (eGFR) values correspond to the more recent routine biological determinations in the 6 and 12 months preceding admission, respectively.
2.2. Study outcomes
The composite primary endpoint combined tracheal intubation for mechanical ventilation and death within 7 days of admission. Secondary outcomes included death on Day 7, tracheal intubation on Day 7, admission to intensive care units and discharge on Day 7. In the population still hospitalized on Day 7, these outcomes were reassessed until Day 28.
2.3. Statistical analyses: propensity score analysis
Quantitative data are given as mean ± SD or median (25th–75th percentile). Categorical variables are given as number (%) of participants. Patients were classified into two groups according to the use of DPP‐4 inhibitors prior to admission. For between‐group comparisons, unpaired t‐tests or Wilcoxon rank‐sum tests were used for quantitative variables, while Fisher's exact tests were used for categorical variables. For missing values, a multiple imputation by chained equation using R package mice (seven replicates with ‘predictive mean matching’ and ‘logistic regression’ methods for respectively continuous and binary variables) was performed. 16 After a careful study of the performance of imputation, replicates were pooled to obtain the complete dataset to conduct multivariable analyses.
To balance the distributions of baseline covariates between groups and then limit confounding bias in analyses, we estimated a propensity score (PS) with a logistic regression model on sex, age, body mass index (BMI), arterial hypertension, history of ischaemic heart disease, history of heart failure, active cancer, treated obstructive sleep apnoea (OSA), and the use of metformin, sulphonylurea, glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs), insulin, corticosteroids, renin‐angiotensin system blockers, statins, thiazide diuretics and antiplatelet therapy. In sensitivity analysis, the following complementary variables were added: eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation, diabetes duration and the latest HbA1c (<6 months prior to admission). For each model, these variables were selected based on their relevance in clinical practice and statistically (p < .15 in univariable association with outcome). In the PS calculation, we did not include variables that are associated with exposition status but not with the primary endpoint, because this might have had a counterproductive effect by increasing bias and variance in the estimate of treatment effect. 17 Comparability was assessed by analysing the reduction in the standardized mean difference (SMD) after PS utilization. Stabilized weights 18 were computed for patients based on an overlap‐weighting method and were used in multivariable logistic regression models to estimate the average treatment effect in the treated (ATT) as inverse probability of treatment weighting (IPTW). 19 In addition, PS was used in Cox models to estimate IPTW hazard ratios (presented in the supporting information). Proportional hazards assumption was carefully studied. Analyses were performed using R version 3.6.2, in particular the packages PSW, 19 hrIPW 20 and ggplot2 21 to estimate the treatment effect in logistic regression models, in survival models and for figures, respectively.
3. RESULTS
3.1. Baseline characteristics
The study population consisted of 2449 patients with T2D declaring the use of at least one antidiabetic drug prior to hospital admission for COVID‐19, and available information on the primary outcome at Day 7 after admission (Figure 1). Among them, 596 were under DPP‐4 inhibitors (24.3%), mainly sitagliptin (n = 424; 17.2%). The baseline characteristics of the patients according to the use of DPP‐4 inhibitors are shown in Table 1. Patients using DPP‐4 inhibitors were less frequently women (32.6% vs. 37.1% in non‐users; p = .0455), had a lower median BMI, and less frequently a history of severe diabetic retinopathy, peripheral artery disease or non‐alcoholic fatty liver disease. As expected, treatment patterns were strikingly different for antidiabetic but also cardiovascular therapies. DPP‐4 inhibitor users were more frequently under metformin and less frequently under GLP‐1 RAs or insulin therapy. In addition, they were more frequently under renin‐angiotensin‐aldosterone system blockers and less frequently under beta blockers. Upon admission, patients under DPP‐4 inhibitors appeared to have a slightly more severe form of infection, with higher plasma glucose and C‐reactive protein (CRP) concentrations, two biological markers which have been associated with a poorer COVID‐19 prognosis 15 (Table 2). The information on how DPP‐4 inhibitors were handled during hospitalization was recorded for 455 patients: 372 (81%) remained on treatment, including those who had a transitory suspension (n = 147 [32%]) or a change in dosage (n = 14 [3%]), while 84 (19%) had stopped treatment.
FIGURE 1.
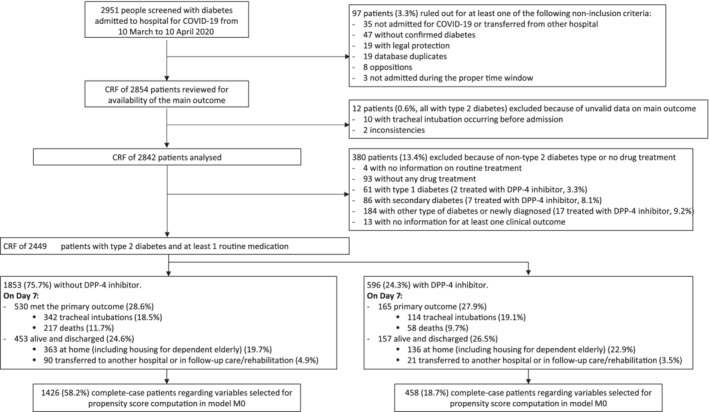
Study flowchart. CCF, case report form; DPP‐4, dipeptidyl peptidase‐4
TABLE 1.
Clinical characteristics prior to admission of CORONADO participants according to the use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors
| DPP‐4 inhibitor before admission | ||||||
|---|---|---|---|---|---|---|
| Clinical features | Available data | All | No (n = 1853) | Yes (n = 596) | p‐value | SMD |
| Sex (female) | 2449 | 881/2449 (36%) | 687/1853 (37.1%) | 194/596 (32.6%) | .0455 | 9.5 |
| Age (years) | 2449 | 70.9 +/− 12.5 | 71.1 +/− 12.8 | 70.3 +/− 11.5 | .2339 | 6.3 |
| Ethnicity | 2095 | .2478 | 4.1 | |||
| EU | 1229/2095 (58.7%) | 933/1587 (58.8%) | 296/508 (58.3%) | |||
| MENA | 446/2095 (21.3%) | 345/1587 (21.7%) | 101/508 (19.9%) | .5405 | ||
| AC | 339/2095 (16.2%) | 244/1587 (15.4%) | 95/508 (18.7%) | .1382 | ||
| AS | 81/2095 (3.9%) | 65/1587 (4.1%) | 16/508 (3.1%) | .3765 | ||
| BMI (kg/m 2 ) | 2150 | 28.7 [25.3; 32.7] | 28.9 [25.5; 33.1] | 28.0 [24.9; 31.6] | .0045 | 15.9 |
| Diabetes duration (years) | 1483 | 13.9 +/− 9.6 | 14.1 +/− 9.9 | 13.2 +/− 8.3 | .1274 | 9.9 |
| HbA1c (mmol/mol) | 1552 | 64.8 +/− 20.1 | 64.3 +/− 19.8 | 66.5 +/− 21.1 | .0808 | 11 |
| HbA1c (%) | 1552 | 8.1 +/− 1.8 | 8.0 +/− 1.8 | 8.2 +/− 1.9 | .0808 | 11 |
| eGFR (CKD‐EPI), mL/min.1.73m 2 | 1606 | 68.0 +/− 29.4 | 67.7 +/− 29.7 | 69.1 +/− 28.3 | .8628 | 4.8 |
| Hypertension | 2429 | 1947/2429 (80.2%) | 1472/1836 (80.2%) | 475/593 (80.1%) | .969 | 0.2 |
| Dyslipidaemia | 2375 | 1173/2375 (49.4%) | 892/1796 (49.7%) | 281/579 (48.5%) | .6351 | 2.3 |
| Tobacco use | 2005 | 113/2005 (5.6%) | 86/1532 (5.6%) | 27/473 (5.7%) | .9378 | 0.4 |
| Microvascular complications a | 1724 | 782/1724 (45.4%) | 606/1319 (45.9%) | 176/405 (43.5%) | .3793 | 5 |
| Macrovascular complications b | 2308 | 923/2308 (40.0%) | 719/1748 (41.1%) | 204/560 (36.4%) | .0482 | 9.7 |
| Co‐morbidities | ||||||
| Heart failure | 2329 | 280/2329 (12.0%) | 217/1760 (12.3%) | 63/569 (11.1%) | .4229 | 3.9 |
| NAFLD | 2078 | 158/2078 (7.6%) | 132/1577 (8.4%) | 26/501 (5.2%) | .0205 | 12.7 |
| Liver cirrhosis | 2301 | 62/2301 (2.7%) | 49/1743 (2.8%) | 13/558 (2.3%) | .5416 | 3 |
| Active cancer | 2405 | 233/2405 (9.7%) | 188/1819 (10.3%) | 45/586 (7.7%) | .0596 | 9.3 |
| COPD | 2394 | 233/2394 (9.7%) | 185/1809 (10.2%) | 48/585 (8.2%) | .1524 | 7 |
| Treated OSA | 2268 | 255/2268 (11.2%) | 194/1713 (11.3%) | 61/555 (11.0%) | .8285 | 1.1 |
| Routine treatment before admission | ||||||
| Sulphonylurea/glinide | 2449 | 754/2449 (30.8%) | 493/1853 (26.6%) | 261/596 (43.8%) | <.0001 | 36.6 |
| Metformin | 2449 | 1496/2449 (61.1%) | 1048/1853 (56.6%) | 448/596 (75.2%) | <.0001 | 40 |
| GLP‐1 RA | 2449 | 242/2449 (9.9%) | 222/1853 (12.0%) | 20/596 (3.4%) | <.0001 | 32.8 |
| Insulin therapy | 2449 | 902/2449 (36.8%) | 749/1853 (40.4%) | 153/596 (25.7%) | <.0001 | 31.7 |
| Acarbose | 2449 | 31/2449 (1.3%) | 16/1853 (0.9%) | 15/596 (2.5%) | .0048 | 12.9 |
| Thiazide diuretic c | 2449 | 494/2449 (20.2%) | 366/1853 (19.8%) | 128/596 (21.5%) | .3615 | 4.3 |
| Loop diuretic | 2449 | 495/2449 (20.2%) | 388/1853 (20.9%) | 107/596 (18.0%) | .1147 | 7.5 |
| MRA | 2449 | 113/2449 (4.6%) | 84/4853 (4.5%) | 29/596 (4.9%) | .7366 | 1.6 |
| ARB and/or ACE inhibitor | 2449 | 1422/2449 (58.1%) | 1052/1853 (56.8%) | 370/596 (62.1%) | .0225 | 10.8 |
| Beta blocker | 2449 | 919/2449 (37.5%) | 726/1853 (39.2%) | 193/596 (32.4%) | .0029 | 14.2 |
| Calcium channel blocker | 2449 | 855/2449 (34.9%) | 645/1853 (34.8%) | 210/596 (35.2%) | .8822 | 0.9 |
| Statin | 2449 | 1192/2449 (48.7%) | 882/1853 (47.6%) | 310/596 (52.0%) | .0608 | 8.8 |
| Antiplatelet agent | 2449 | 1039/2449 (42.4%) | 780/1853 (42.1%) | 259/596 (43.5%) | .5583 | 2.8 |
| Vitamin K antagonist | 2449 | 135/2449 (5.5%) | 108/1853 (5.8%) | 27/596 (4.5%) | .2567 | 2.8 |
| Oral direct factor Xa inhibitor | 2449 | 230/2449 (9.4%) | 181/1853 (9.8%) | 49/596 (8.2%) | .2940 | 5.4 |
| Corticosteroid | 2449 | 129/2449 (5.3%) | 109/1853 (5.9%) | 20/596 (3.4%) | .0178 | 12.1 |
| COPD and/or treatment of asthma | 2449 | 269/2449 (11%) | 207/1853 (11.2%) | 62/596 (10.4%) | .6019 | 2.5 |
Abbreviations: AC, African or Caribbean; ACE inhibitor, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐2 receptor blocker; AS, Asian; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation); EU, Europid; GLP‐1 RA, glucagon‐like peptide 1 receptor agonist; MENA, Middle East North Africa; MRA, mineralocorticoid receptor agonist; NAFLD, non‐alcoholic fatty liver disease; OSA, obstructive sleep apnoea; SMD, standardized mean difference.
Note: Data are presented as no. (%) and mean ± SD, or median (IQR) if not normally distributed. p‐values are calculated using the Wald test. HbA1c corresponds to the glycated haemoglobin determined in the 6 months prior to or in the first 7 days following hospital admission.
A microvascular complication was defined as history of one or more of the following: diabetic kidney disease and/or severe diabetic retinopathy and/or diabetic foot ulcer.
A macrovascular complication was defined as a history of one or more of the following co‐morbidities: acute coronary syndrome, coronary artery disease revascularization, transient ischaemic attack and/or lower limb artery revascularization.
Thiazide diuretics and potassium‐sparing diuretics.
TABLE 2.
COVID‐19‐related clinical, radiological and biological characteristics on admission of CORONADO participants according to the use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors
| Clinical features | People with available data | All | DPP‐4 inhibitor before admission | p‐value | SMD | |
|---|---|---|---|---|---|---|
| No (n = 1853) | Yes (n = 596) | |||||
| Positive SARS‐CoV‐2 PCR | 2374 | 2245/2374 (94.6%) | 1704/1802 (94.6%) | 541/572 (94.6%) | .9862 | 0.1 |
| COVID‐19 symptoms | 2448 | 2317/2448 (94.6%) | 1753/1852 (94.7%) | 564/596 (94.6%) | .9823 | 0.1 |
| Time between symptom onset and hospital admission (days) | 2399 | 5 [2; 8] | 5 [2; 8] | 6 [2; 9] | .0162 | 11.5 |
| Clinical presentation | ||||||
| Fever | 2414 | 1807/2414 (74.9%) | 1366/1827 (74.8%) | 441/587 (75.1%) | .861 | 0.8 |
| Fatigue | 2337 | 1456/2337 (62.3%) | 1075/1770 (60.7%) | 381/567 (67.2%) | .0058 | 13.5 |
| Cough | 2383 | 1606/2383 (67.4%) | 1213/1799 (67.4%) | 393/584 (67.3%) | .9529 | 0.3 |
| Cephalalgia | 2263 | 283/2263 (12.5%) | 216/1714 (12.6%) | 67/549 (12.2%) | .8061 | 1.2 |
| Dyspnoea | 2416 | 1562/2416 (64.7%) | 1183/1834 (64.5%) | 379/582 (65.1%) | .7864 | 1.3 |
| Rhinitis and/or pharyngeal signs | 2227 | 181/2227 (8.1%) | 143/1686 (8.5%) | 38/541 (7.0%) | .2811 | 5.5 |
| Ageusia and/or anosmia | 2129 | 298/2129 (14.0%) | 226/1602 (14.1%) | 72/527 (13.7%) | .7984 | 1.3 |
| Digestive disorders | 2336 | 775/2336 (33.2%) | 584/1770 (33.0%) | 191/566 (33.7%) | .7411 | 1.6 |
| Chest CT imaging | ||||||
| Abnormal chest CT | 1735 | 1675/1735 (96.5%) | 1245/1290 (96.5%) | 430/445 (96.6%) | .9068 | 0.6 |
| Ground‐glass opacity/crazy paving | 1712 | 1548/1712 (90.4%) | 1145/1270 (90.2%) | 403/442 (91.2%) | .5309 | 3.5 |
| Biological findings | ||||||
| Admission plasma glucose (mg/dL) | 1834 | 170 [127; 236] | 167 [125; 229] | 185 [131; 256] | .0063 | 12.7 |
| eGFR (CKD‐EPI) (mL/min/1.73m2) | 2287 | 67.2 [41.0; 88.5] | 67.1 [40.5; 88.3] | 67.8 [42.5; 89.3] | .1267 | 4.5 |
| ALT (% ULN) | 2056 | 0.61 [0.42; 0.98] | 0.61 [0.42; 0.98] | 0.62 [0.42; 1.00] | .9831 | 5.3 |
| AST (% ULN) | 2023 | 1.06 [0.75; 1.59] | 1.06 [0.75; 1.60] | 1.06 [0.75; 1.55] | .4104 | 6.3 |
| GGT (% ULN) | 1915 | 0.93 [0.55; 1.73] | 0.94 [0.55; 1.75] | 0.92 [0.58; 1.68] | .9234 | 0.5 |
| Haemoglobin (g/dL) | 2387 | 12.7 [11.4; 14.2] | 12.7 [11.4; 14.2] | 12.7 [11.4; 14.1] | .8144 | 0.3 |
| White cell count (103/mm3) | 2384 | 6600 [5000; 8820] | 6530 [5000; 8890] | 6685 [4985; 8718] | .5608 | 3.1 |
| Lymphocyte count (103/mm3) | 2313 | 990 [690; 1400] | 1000 [690; 1422] | 920 [690; 1300] | .1322 | 1.7 |
| Platelet count (103/mm3) | 2383 | 201 [155; 258] | 201 [155; 258] | 201 [156; 260] | .1425 | 4.8 |
| D‐dimers (μg/L) | 957 | 880 [328; 1730] | 820 [300; 1670] | 1000 [430; 1894] | .2484 | 7.8 |
| CRP (mg/L) | 2286 | 86 [40.8; 146.9] | 83.6 [38.2; 144] | 92.8 [47.5; 149.2] | .003 | 5.3 |
| LDH (UI/L) | 1253 | 350 [262; 494] | 349 [256; 485] | 351 [273; 500] | .911 | 0.4 |
| CPK (UI/L) | 1207 | 132 [66; 302] | 134 [65; 326] | 118 [67; 252] | .3342 | 8.0 |
| Fibrinogen (g/L) | 1227 | 6.2 [5.0; 7.4] | 6.2 [5.0; 7.4] | 6.2 [5.0; 7.2] | .6884 | 5.5 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; CPK, creatine phosphokinase; CRP, C‐reactive protein; CT, computed tomography; eGFR, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation); GGT, gamma‐glutamyl transferase; LDH, lactate dehydrogenase; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SMD, standardized mean difference; ULN, upper limit of normal.
Note: Data are presented as no. (%) and mean ± SD, or median (IQR) if not normally distributed. p‐values are calculated using the Wald test. Quantitative variables were natural log‐transformed and associated ORs correspond to an increase of 1 SD after standardization, except for time between symptoms onset and hospital admission (1‐day increase).
3.2. Clinical outcomes according to the use of DPP‐4 inhibitors
The primary outcome (tracheal intubation for assisted mechanical ventilation or death on Day 7 after admission) occurred at similar rates in users and non‐users of DPP‐4 inhibitors (27.7% vs. 28.6%; p = .6765). The same was true for each component of the primary outcome taken individually (Table S1). The pattern was similar when outcomes were reassessed at Day 28, except for a trend of a non‐significant reduction in mortality in DPP‐4 inhibitor users (18.1% vs. 21.8%; p = .0561) (Table S1 ).
3.3. Propensity score analysis
Because the use of DPP‐4 inhibitors and outcomes were significantly associated with some baseline characteristics that can alter the severity of COVID‐19, we conducted a PS analysis to balance baseline distributions of age, sex, BMI, history of heart failure, arterial hypertension or ischaemic heart disease, and active cancer, and also regarding treatments for obstructive apnoea, antiplatelet therapy and the use of metformin, insulin, sulphonylurea, renin‐angiotensin system blockers, statins, corticosteroids and thiazide diuretics. We performed a multiple imputation for the missing values. As illustrated in Figure 2, the reduction in SMD after using IPTW in models illustrated the gain in comparability between groups on baseline covariates. The IPT‐weighted models at Day 7 showed no association between the use of DPP‐4 inhibitors and the primary outcome or its individual component, even after further adjustment for kidney function (i.e. eGFR values), diabetes duration and HbA1c (Table 3).
FIGURE 2.

Baseline characteristics balance between dipeptidyl peptidase‐4 (DPP‐4) inhibitor users and non‐users after propensity score use in models as inverse probability of treatment weighting. BMI, body mass index; GLP‐1, glucagon‐like peptide‐1; IPTW, inverse probability of treatment weighting
TABLE 3.
Association between the use versus no use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors and outcomes estimated in logistic regression models weighted by patients' inverse probability of treatment (n = 2449, imputed sample)
| Day 7 | Day 28 | |||||
|---|---|---|---|---|---|---|
| Model M0 baseline variables | Model M1 M0 + eGFR using CKD‐EPI | Model M2 M1 + diabetes duration + HbA1c | Model M0 baseline variables | Model M1 M0 + eGFR using CKD‐EPI | Model M2 M1 + diabetes duration + HbA1c | |
| DPP‐4 inhibitor user/population size | 596/2449 (24%) | |||||
| Primary outcome | 0.95 [0.77–1.17] | 0.94 [0.76–1.16] | 0.93 [0.75–1.15] | 0.96 [0.78–1.17] | 0.94 [0.77–1.15] | 0.93 [0.76–1.14] |
| Tracheal intubation | 0.93 [0.73–1.18] | 0.94 [0.74–1.19] | 0.93 [0.73–1.18] | 0.97 [0.77–1.22] | 0.97 [0.77–1.23] | 0.97 [0.77–1.23] |
| Death | 0.99 [0.73–1.34] | 0.96 [0.71–1.30] | 0.95 [0.70–1.29] | 0.94 [0.74–1.18] | 0.90 [0.71–1.14] | 0.89 [0.70–1.12] |
Abbreviation: eGFR, estimated glomerular filtration rate (according to the Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation). OR [95% CI] for primary outcome (tracheal intubation for assisted mechanical ventilation and death), tracheal intubation and death.
4. DISCUSSION
In the current study, we report evidence supporting the safety of the use of DPP‐4 inhibitors prior to hospitalization for COVID‐19 in people with T2D. These results, based on the largest cohort analysed to date to test the safety of this class of drugs during the course of the SARS‐Cov‐2 pandemic, thus provide reassurance in that regard.
The prevalence of the use of DPP‐4 inhibitors in patients with T2D requiring hospitalization for COVID‐19 from the CORONADO cohort was slightly lower than reported in previous observational studies in France (24.3% vs. 32% in Roussel et al., mostly sitagliptin, 22 and 27% in Overbeek et al. 23 ). This is not suggestive of an increased risk of the severe form of COVID‐19 because of the use of DPP‐4 inhibitors in the community, prior to admission to hospital. This finding was also consistent with a recent observational study from Italy. 14 In this work, similar rates of treatment with DPP‐4 inhibitors were reported in people hospitalized for COVID‐19 and in several control groups of people with diabetes in the community and requiring hospitalization for other causes of pneumonia.
In people with diabetes, prior studies suggested a lack of an association between the use of DPP‐4 inhibitors and the occurrence of community‐acquired pneumopathy from any cause, 12 , 24 but also specifically because of SARS‐CoV‐2. 14 Furthermore, recent observations reported either a reduced mortality rate 25 , 26 or a neutral effect 27 , 28 associated with the use of DPP‐4 inhibitors prior to or during hospitalization for COVID‐19, in patients with T2D. The literature already includes papers questioning a wide use of the class for preventing COVID‐19 and its complications. 29 As thoroughly discussed in a commentary paper, 30 all these studies have strong limitations (some of which were shared by our current work, first of all their observational nature). These limitations were probably balanced by the potential importance of their messages during the editorial process, because of the emergency context of the ongoing pandemic. Calls for randomized trials have been made, but they will take time to be delivered, and the community is in urgent need of more evidence in the meantime.
In the CORONADO study, participants on routine DPP‐4 inhibitors showed a few traits at admission presumed to be associated with a severe illness, such as a higher prevalence of fatigue, lower lymphocyte count, higher plasma D‐dimer, glucose and CRP concentrations. These features underline the imperative for statistical methods to control for different characteristics in participants according to the use of DPP‐4 inhibitors, with the aim of limiting residual confounding factors. Here, we used multivariable logistic regression models to estimate ATT as IPTW, elsewhere described to be less biased and associated with a lowest variance than other PS‐based methods. 31 , 32 With this approach, we observed similar rates of the primary outcome (combined tracheal intubation and/or death) as well as its individual components (i.e. tracheal intubation and death) both by 7 and 28 days after admission.
The possibility of a reduction in the severity of COVID‐19 associated with in‐hospital treatment with DPP‐4 inhibitors has raised an important issue, although it is challenged because of the limitations of the study design. 6 , 25 , 26 Indeed, COVID‐19 is a multi‐organ disease, and besides respiratory failure, which can lead eventually to tracheal intubation and supportive ventilation, other processes may cause death, like thrombotic disease. Therefore, we could speculate that DPP‐4 inhibitors could limit the damage triggered by SARS‐CoV‐2 at systemic level, beyond the lung. Unfortunately, the challenges of clinical care in hospitals at the peak of epidemics in France has meant that investigations have been less accurate than usual in critical settings, and also eventually in the reporting of deaths. Therefore, these data were not collected in the current study. However, experimental and further confirmatory epidemiological data are strongly required to validate the hypothesis raised here.
As highlighted before, our study presents some limitations that should be noted. CORONADO is an observational study that collected data from people with diabetes and COVID‐19 upon admission across a large number of hospitals in France. As such, it cannot provide insight into the outcomes of COVID‐19 in the community. Moreover, it was not feasible to reliably collect extensive data on the use of antidiabetic drugs during the hospital stay and after hospital discharge (if any). Therefore, we cannot study the relationship between in‐hospital exposure to any specific drug, including DPP‐4 inhibitors and outcomes. Even while the data are not exhaustive, it appears that a large majority (>80%) of patients remained on DPP‐4 inhibitors after admission to hospital. In addition, our experience showed that most of the patients with diabetes were switched to insulin therapy soon after their admission. Ultimately, routine prescription of drugs does not mean that they were duly taken by patients. Drug compliance was not evaluated in this study.
Consistent with recently published evidence,25‐28 the current findings did not identify any deleterious association between treatment with DPP‐4 inhibitors and severe outcomes of COVID‐19 in patients with T2D admitted to hospital. These data support the safe use of this class of drugs for treating diabetes during the COVID‐19 pandemic and they should not be discontinued.
CONFLICT OF INTEREST
RR reports grants, personal fees and non‐financial support from Sanofi, Novo Abbott, Applied Therapeutics, Astra‐Zeneca, Diabnext, Eli Lilly, Janssen, Medtronic, MSD, Mundipharma, Novo Nordisk and Servier. PD reports personal fees and non‐financial support from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Mundipharma, Novartis, Novo Nordisk and Sanofi. MP reports grants, non‐financial support or personal fees from Air Liquid, Allergan, Amgen, Elivie, Fortil, Lifescan, NHC, Novo Nordisk and Sanofi. LB reports non‐financial support or personal fees from Abbott, Astra Zeneca, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk and Sanofi. CCB reports grants, non‐financial support or personal fees from AstraZeneca, Novo Nordisk, Sanofi, Eli Lilly, Orkyn and Medtronic. EC reports non‐financial support or personal fees from Abbott, AlphaDiab, Air Liquide, Ascencia, Astra Zeneca, Bezins, BMS, Eli Lilly, LifeScan, Medtronic, MSD, Novartis, Novo‐Nordisk, Roche Diagnostics, Sanofi and YpsoMed. BF reports non‐financial support or personal fees from AstraZeneca, Eli Lilly, Isis, Merck, MSD, NHC, Novo Nordisk, Orkyn, Pfizer, Sanofi, Servier and Vitalaire. PF reports non‐financial support or personal fees from Astra Zeneca, Bayer, Eli Lilly, MSD, Novartis, Novo Nordisk and Sanofi. GP reports non‐financial support or personal fees from Abbott, AstraZeneca, Eli Lilly, MSD, Medtronic, Novo Nordisk and Sanofi. PS reports non‐financial support or personal fees from Novo Nordisk, Eli Lilly, AstraZeneca and MSD. MW reports grants, personal fees from Air Liquid, Allergan, Elivie, Fortil, Lifescan, NHC, Novo Nordisk and Sanofi. SH reports grants, non‐financial support or personal fees from Air Liquid, Allergan, Astra Zeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Eli Lilly,Elivie, Fortil, Lifescan, LVL, Merck Sharpe Dome, NHC, Novartis, Pierre Fabre Santé, Sanofi, Servier and Valbiotis. PG reports grants or personal fees from Abbott, Air Liquid, Allergan, Amgen, Astra‐Zeneca, Boehringer Ingelheim, Eli Lilly, Elivie, Fortil, Lifescan, Merck Sharp and Dohme, Mundipharma, NHC, Novo Nordisk, Sanofi and Servier. BC reports grants, non‐financial support or personal fees from Abbott, Allergan, Amgen, Akcea AstraZeneca, Pierre Fabre, Genfit, Gilead, Eli Lilly, Elivie, Fortil, Lifescan, Merck Sharpe Dome, NHC, Novo Nordisk, Regeneron and Sanofi. The other authors had nothing to disclose.
AUTHOR CONTRIBUTIONS
BC and RR had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: BC, PD, PG, SH, MP, RR and MW. Acquisition, analysis or interpretation of data: on behalf of the scientific committee of the study (the scientific committee list is available in the supporting information). Drafting of the manuscript: BC, PD, PG, SH, MP, RR and MW. Critical revision of the manuscript for important intellectual content: all co‐authors. Statistical analysis: MW and TG. Patient recruitment: YA, LAB, IA, DA, SB, LB, AC, NC, CCB, EC, AD, OD, PF, BF, FG, NG, AMG, EL, SL‐R, BL, LL, AM, NM, PM, IM, GP, YR, NS, PJS, PS, CV, SH, PG and BC. Fundraising: BC, PG, SH, MP and BB.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14324.
Supporting information
Appendix S1: supporting information
ACKNOWLEDGEMENTS
We thank the sponsor of the study (Délégation à la Recherche Clinique et à l'Innovation [DRCI], CHU Nantes), the Clinical Project Manager (M. Saignes) and Assistant (J. Saunier), Clinical Research Associates (S. El Andaloussi, J. Martin‐Gauthier, E. Rebouilleau) and Data Managers (B. Guyomarc'h, T. Roman). We acknowledge the head of communication department of l'institut du thorax (V. Mayoura). We acknowledge all medical and clinical research staff involved in the diagnosis and treatment of patients with COVID‐19 in participating centres. We thank all of the general practitioners, specialists, pharmacists and biological laboratories responsible for hospitalized patients for providing additional medical information to investigators. We thank the Société Francophone du Diabète (SFD) and Société Française d'Endocrinologie (SFE) for disseminating the study design and organization, and the Fédération Française des Diabétiques (FFD) for participating in the organization of the study. This study received the following funding via the fond de dotation du CHU de Nantes: the Fondation Francophone de Recherche sur le Diabète (FFRD), supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly and FFD (Fédération Française des Diabétiques) – CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) – CORONADO initiative emergency grant; Air Liquide Health Care international – CORONADO initiative emergency grant; Allergan – CORONADO initiative emergency grant; Elivie – CORONADO initiative emergency grant; Fortil – CORONADO initiative emergency grant; Lifescan – CORONADO initiative emergency grant; Nantes Métropole – CORONADO initiative emergency grant; Nestle Home Care (NHC) – CORONADO initiative emergency grant; Novo Nordisk – CORONADO initiative emergency grant; Sanofi – CORONADO emergency grant; AstraZeneca Grant – CORONADO initiative emergency grant; Nantes Metropole Grant – CORONADO initiative emergency grant.
PHRC National COVID‐19 Hospitalization and Care Organization Division (DHOS) as part of the Hospital Clinical Research Program (PHRC COVID‐19‐20‐0138).
All research facilities are acknowledged for providing research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Roussel R, Darmon P, Pichelin M, et al. Use of dipeptidyl peptidase‐4 inhibitors and prognosis of COVID‐19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study. Diabetes Obes Metab. 2021;23:1162–1172. 10.1111/dom.14324
A complete list of the CORONADO trial investigators is provided in the supporting information.
Funding information Air Liquide; Allergan; AstraZeneca; Elivie; Fondation Francophone pour la Recherche sur le Diabète; Fortil; LifeScan; Nantes Metropole; Nestle Home Care (NHC); Novo Nordisk; Sanofi; Société Francophone du Diabète; PHRC National COVID‐19 (DGOS) (PHRC COVID‐19‐20‐0138)
DATA AVAILABILITY STATEMENT
No sharing of participant data is allowed by our regulatory authorities. So far, French regulations have not validated deidentified data or avatar for data sharing. Our statement might be modified in case French law changes. We will be happy to share study protocol, SAP and information document upon request. Study protocol, SAP and information documents are available with publication. Direct requests can made on The CORONADO website (https://www.diabetes-covid.org) or be directed to PI (bertrand.cariou@univ‐nantes.fr) or Chairman of the scientific committee (samy.hadjadj@univ‐nantes.fr). Our data‐base is open for any collaborative work with priority to academic partnership. Any proposal for collaboration requires examination by the scientific committee and the sponsor (CHU Nantes). A structured application proposal for collaboration will be available on request.
REFERENCES
- 1. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase‐4 inhibitors. Endocr Rev. 2014;35(6):992‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broxmeyer HE, Capitano M, Campbell TB, Hangoc G, Cooper S. Modulation of hematopoietic chemokine effects in vitro and in vivo by DPP‐4/CD26. Stem Cells Dev. 2016;25(8):575‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li K, Wohlford‐Lenane C, Perlman S, et al. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kleine‐Weber H, Schroeder S, Krüger N, et al. Polymorphisms in dipeptidyl peptidase 4 reduce host cell entry of Middle East respiratory syndrome coronavirus. Emerg Microbes Infect. 2020;9(1):155‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drucker DJ. Coronavirus infections and type 2 diabetes – shared pathways with therapeutic implications. Endocrine Rev. 2020;41(3):457‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solerte SB, Di Sabatino A, Galli M, Fiorina P. Dipeptidyl peptidase‐4 (DPP4) inhibition in COVID‐19. Acta Diabetol. 2020;57(7):779‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filardi T, Morano S. COVID‐19: Is there a link between the course of infection and pharmacological agents in diabetes? J Endocrinol Invest. 2020;43(8):1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pascal KE, Coleman CM, Mujica AO, et al. Pre‐ and postexposure efficacy of fully human antibodies against spike protein in a novel humanized mouse model of MERS‐CoV infection. Proc Natl Acad Sci U S A. 2015;112(28):8738‐8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inn KS, Kim Y, Aigerim A, et al. Reduction of soluble dipeptidyl peptidase 4 levels in plasma of patients infected with Middle East respiratory syndrome coronavirus. Virology. 2018;518:324‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang W, Cai X, Han X, Ji L. DPP‐4 inhibitors and risk of infections: a meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev. 2016;32:391‐404. [DOI] [PubMed] [Google Scholar]
- 11. Gamble JM, Donnan JR, Chibrikov E, Twells LK, Midodzi WK, Majumdar SR. Comparative safety of Dipeptidyl Peptidase‐4 inhibitors versus sulfonylureas and other glucose‐lowering therapies for three acute outcomes. Sci Rep. 2018;8(1):15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faillie JL, Filion KB, Patenaude V, Ernst P, Azoulay L. Dipeptidyl peptidase‐4 inhibitors and the risk of community‐acquired pneumonia in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(4):379‐385. [DOI] [PubMed] [Google Scholar]
- 13. Willemen MJ, Mantel‐Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG. Use of dipeptidyl peptidase‐4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care. 2011;34(2):369‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fadini GP, Morieri ML, Longato E, et al. Exposure to dipeptidyl‐peptidase 4 inhibitors and COVID‐19 among people with type 2 diabetes: a case‐control study. Diabetes Obes Metab. 2020;22(10):1946‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1‐67. [Google Scholar]
- 17. Myers JA, Rassen JA, Gagne JJ, et al. Effects of adjusting for instrumental variable on bias and precision of effect estimates. Am J Epidemiol. 2011;174:1213‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li K, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113:521. [Google Scholar]
- 19. Mao H, Li L. PSW: propensity score weighting methods for dichotomous treatments. R package version 1.1‐3. 2018. https://CRAN.R-project.org/package=PSW. Accessed July 20, 2020.
- 20. Hajage D. hrIPW: Hazard ratio Estimation using Cox Model Weighted by the Estimated Propensity Score. R package version 0.1.3. 2020. htpps://CRAN.R-project.org/package=hrIPW. Accessed July 20, 2020.
- 21. Wickham H. ggplot2: Elegant Graphics for Data Analysis. . New York, NY: Springer‐Verlag; 2016. [Google Scholar]
- 22. Roussel R, Fontaine P, Gouet D, et al. Treatment of type 2 diabetes in France: more dynamic than inert; analysis of prescription data for 847,122 patients. Méd Malad Métabol. 2018;12(4):346‐352. [Google Scholar]
- 23. Overbeek JA, Heintjes EM, Prieto‐Alhambra D, et al. Type 2 diabetes mellitus treatment patterns across Europe: a population‐based multi database study. Clin Ther. 2017;39(4):759‐770. [DOI] [PubMed] [Google Scholar]
- 24. Morieri ML, Bonora BM, Longato E, et al. Exposure to dipeptidyl‐peptidase 4 inhibitors and the risk of pneumonia among people with type 2 diabetes: retrospective cohort study and meta‐analysis. Diabetes Obes Metab. 2020;22:1925‐1934. [DOI] [PubMed] [Google Scholar]
- 25. Solerte SB, D'Addio F, Trevisan R, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID‐19: a multicentre, case‐control, retrospective, observational study. Diabetes Care. 2020;43:2999‐3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirani M, Favacchio G, Carrone F, et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID‐19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. 2020;43:3042‐3049. [DOI] [PubMed] [Google Scholar]
- 27. Kim MK, Jeon JH, Kim SW, et al. The clinical characteristics and outcomes of patients with moderate‐to‐severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44:602‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan H, Valdes AM, Vijay A, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID‐19: a large case‐control study. Clin Pharmacol Ther. 2020;108:1185‐1194. [DOI] [PubMed] [Google Scholar]
- 29. Smelcerovic A, Kocic G, Gajic M, et al. DPP‐4 inhibitors in the prevention/treatment of pulmonary fibrosis, heart and kidney injury caused by COVID‐19 – a therapeutic approach of choice in type 2 diabetic patients? Front Pharmacol. 2020;11:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nauck MA, Meier J. Reduced COVID‐19 mortality with sitagliptin treatment? Weighing the dissemination of potentially lifesaving findings against the assurance of high scientific standards. Diabetes Care. 2020;43:2906‐2909. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC. The performance of different propensity‐score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29:2137‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdia Y, Kulasekera KB, Datta S, Boakye M, Kong M. Propensity scores based methods for estimating average treatment effect and average treatment effect among treated: a comparative study. Biom J. 2017;59:967‐985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: supporting information
Data Availability Statement
No sharing of participant data is allowed by our regulatory authorities. So far, French regulations have not validated deidentified data or avatar for data sharing. Our statement might be modified in case French law changes. We will be happy to share study protocol, SAP and information document upon request. Study protocol, SAP and information documents are available with publication. Direct requests can made on The CORONADO website (https://www.diabetes-covid.org) or be directed to PI (bertrand.cariou@univ‐nantes.fr) or Chairman of the scientific committee (samy.hadjadj@univ‐nantes.fr). Our data‐base is open for any collaborative work with priority to academic partnership. Any proposal for collaboration requires examination by the scientific committee and the sponsor (CHU Nantes). A structured application proposal for collaboration will be available on request.


