Abstract
Background
Residents of nursing homes and long‐term care facilities are at increased risk for severe coronavirus disease‐19 (COVID‐19) but may not be able to access monoclonal antibody therapies offered at outpatient infusion centers due to frailty and logistical issues. We describe a mobile monoclonal antibody infusion program for patients with COVID‐19 in skilled nursing facilities and provide descriptive data on its outcomes.
Design
Retrospective cohort study.
Setting
Collaboration between Mayo Clinic and skilled nursing facilities in Southeast Minnesota was developed to administer anti‐spike monoclonal antibodies under the FDA Emergency Use Authorization.
Participants
Seventy five residents of skilled nursing facilities at high risk of COVID‐19 complications.
Exposure
Emergency use treatment with bamlanivimab and casirivimab–imdevimab.
Measurements
Hospitalization and medically attended visits.
Results
The mobile infusion unit, staffed by Mayo Clinic Infusion Therapy registered nurses and supported by the skilled nursing facility staff, infused anti‐spike monoclonal antibodies to 45 of 75 patients (average age, 77.8 years) in December 2020. The infusions occurred at an average of 4.3 days after COVID‐19 diagnosis. Fourteen days after infusion, there were no deaths, two emergency department visits, and three hospitalizations, for a combined event rate of 11.1%. There was one reported adverse event.
Conclusion
The implementation of a mobile infusion unit embedded in a collaborative process resulted in rapid infusion of monoclonal antibodies to high‐risk COVID‐19 patients in skilled nursing facilities, who would otherwise be unable to access the novel therapies. The therapies were well tolerated and appear beneficial. Further study is warranted to explore the scalability and efficacy of this program.
Keywords: COVID‐19, monoclonal antibodies, bamlanivimab, casirivimab, SARS‐CoV‐2
Key Points
Residents in congregate settings are at increased risk of coronavirus disease‐19.
Timely initiation of monoclonal antibody therapies may potentially be lifesaving among residents of nursing home and long‐term care facilities.
Logistical limitations to infusion of monoclonal antibodies in skilled nursing facilities may be overcome by mobile infusion units.
Why Does this Paper Matter?
The timely infusion of monoclonal antibodies for treatment of COVID‐19 patients in skilled care facilities is limited by logistical challenge of transporting contagious and frail patients to off‐site infusion centers. This article reports an effective mobile unit that brings monoclonal antibodies for on‐site infusion in skilled nursing facilities.
INTRODUCTION
Coronavirus disease‐19 (COVID‐19) carries high morbidity and mortality in older people living in congregate environments, such as assisted living and skilled nursing facilities (SNF). 1 Until recently, there were no COVID‐19 directed treatments for residents of congregate living facilities. The U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for bamlanivimab and casirivimab with imdevimab for the treatment of mild to moderate COVID‐19 in non‐hospitalized high‐risk patients. The EUA was based on two separate randomized placebo‐controlled clinical trials that showed bamlanivimab was associated with reduced hospitalization from 15% to 4% in high‐risk patients, 2 while casirivimab with imdevimab was associated with reduction in hospitalization or emergency department (ED) visits from 9% to 3%. 3
The adoption of monoclonal antibody infusion had a slow uptake in the outpatient setting. 4 , 5 One reason has been the limited ability of SNF to transport their frail and contagious patients to off‐site outpatient infusion centers. Here, we share the details of our process to provide on‐site monoclonal antibody infusions at SNF and describe the early outcomes of this innovative practice.
METHODS
Patient population
After approval by the Mayo Clinic Institutional Review Board, this retrospective study was conducted on all consenting patients who received monoclonal antibodies at SNF in Southeast Minnesota on December 1–31, 2020. Patient demographics and clinical data were abstracted for all patients. Per Minnesota statute, patients were excluded if they refused research authorization.
Statistical analysis
Descriptive statistics were used to describe demographics, clinical characteristics and outcomes. The end points of death, ED visit and hospitalization were assessed at 14 days after infusion.
Description of the monoclonal antibody program and Process
Monoclonal antibody allocation
The Minnesota Department of Health allocated monoclonal antibodies to medical facilities based on size, predicted incidence, and expected usage. Monoclonal antibodies were allocated directly to SNF, but these were largely declined by the facilities in our region. Barriers included inadequate SNF staffing, exacerbated by staff falling ill to COVID‐19, lack of knowledge of the monoclonal antibodies, or skepticism by SNF medical directors due to the EUA status. Moreover, SNF staff was directing their attention to COVID‐19 immunizations. The majority of SNF lacked supplies and expertise to start intravenous access, or/and were not familiar with safe infusion practices. Lastly, the complex nature of admixture preparation with fragile monoclonal proteins often exceeded the capabilities of most SNF in the region. These barriers left many high‐risk SNF residents without on‐site access to potentially life‐saving monoclonal antibodies.
Development of mobile infusion unit
Transporting patients to off‐site infusion centers that administer monoclonal antibodies was difficult due to the increased risk of spreading COVID‐19, while mitigating patient distress secondary to dementia or fragility. Some SNF suffered outbreaks of COVID‐19 which made it even more challenging to transport a large number of patients to off‐site infusion centers.
To overcome these challenges, we rapidly developed and implemented a mobile team to infuse monoclonal antibodies in SNF. The team of Infusion Therapy Center (ITC) registered nurses (RN) utilized a Mayo Clinic fleet vehicle for travel to the SNF on infusion day. The team utilized travel suitcases equipped with personal protective equipment, infusion pumps, ancillary supplies, and emergency medications.
Referral process
A standardized process for SNF to refer COVID‐19 patients to receive on‐site monoclonal antibody therapy by the mobile infusion team was developed and shared via e‐mail communication to administrators and Directors of Nursing. The e‐mail contained a Frequently Asked Questions about monoclonal antibody therapy (Appendix A) and a structured spreadsheet to facilitate transmission of encrypted clinical information to determine eligibility (Appendix B).
The SNF Director of Nursing would complete the spreadsheet with clinical information. A copy of the laboratory result confirming a positive COVID‐19 test result was required. Advanced directives and power of attorney documentation were reviewed. The spreadsheet and supporting documents were encrypted and sent electronically to an e‐mail address created specifically for this process. The e‐mail inbox was monitored 7 days per week by monoclonal antibody treatment (MATRx) team members who transcribed the information into the patient's Mayo Clinic electronic medical record to facilitate review by the physicians.
Education and consenting process
All eligible patients, or their legally authorized representatives, were contacted by MATRx providers via telehealth to provide education about monoclonal antibodies and obtain verbal consent for infusion. The standardized consenting script is in Appendix C. A unique step for the consenting process of SNF residents is the need to ensure documented consent is provided to the facility. This was accomplished by the mobile team on the day of infusion through electronic faxing from the patients' medical record to the facility.
The script acted as the documentation tool ensuring that all the requirements of consenting to a medication infusion under EUA were addressed and explained to the patient, or their representative. This process ensured that other providers were also able to see through chart review what information had been shared with the patient, in case they were contacted for a second opinion about these therapies.
A patient education video was shared to patients or decision maker through the patient's online portal, if available, to reinforce the information shared in the call. If a patient or their legal representative was unsure and needed more time to make decision, they were given 48 h to review the education resources, as long as they received the infusion within 10 days of symptom onset.
Infusion therapy appointment
After obtaining consent, a nurse‐initiated protocol for monoclonal antibody therapy was signed by the physician. Coordination of an infusion date and time was determined by the ITC nurse manager and the SNF Director of Nursing. On the night before infusion, the monoclonal antibody admixtures were prepared, labeled, and delivered to the mobile infusion team in coolers with ice packs by Mayo Clinic ITC pharmacy personnel.
Upon arrival to the facility, the mobile team met with SNF leadership to identify patient locations, emergency resources, and nurses they will be partnering with during the infusion. Before infusion, the mobile team assessed patients to ensure they met clinical eligibility criteria. Physicians were available by telephone to answer questions and concerns. Once patients were cleared for infusion, intravenous access was obtained, and the monoclonal antibody was infused over 1 h followed by an additional hour of monitoring. Following infusion and monitoring, a clear handoff was provided to SNF staff. All potential adverse effects were reported back to the MATRx Team.
RESULTS
Mayo Clinic and its facilities provide care for patients residing in 3325 beds across 51 SNFs in Southeast Minnesota. In December 2020, 75 COVID‐19 patients were referred to the MATRx Team (Figure 1).
FIGURE 1.
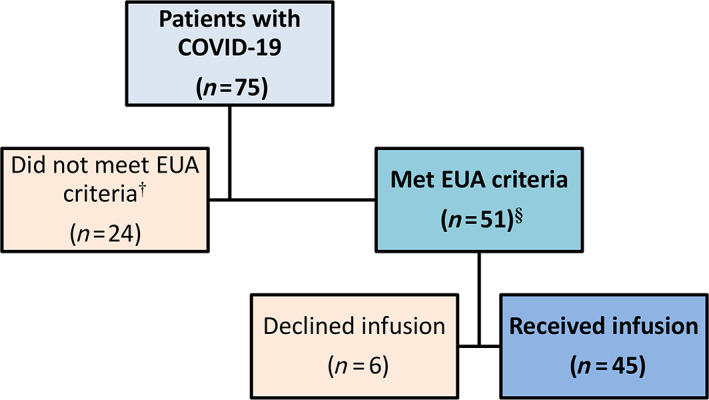
Allocation of monoclonal antibody to skilled care facility residents with coronavirus disease‐19. §Criteria for monoclonal antibody therapy under the FDA Emergency Use Authorization (EUA) include mild to moderate coronavirus disease‐19 (COVID‐19) within 10 days of onset of symptoms. Patients should be 65 years and older, have diabetes mellitus, have body mass index of 35 and higher, have chronic kidney disease, or have an immunocompromised status. Patients who are 55 years and older are also eligible if they have hypertension, chronic lung disease, or cardiovascular disease. See Table 1 for the distribution of risk profile of 45 patients who received the monoclonal antibody infusion. †Twenty‐four patients were not eligible for monoclonal antibodies because of advanced directives (hospice, comfort care, do‐not‐hospitalize status) (n = 8; 33.3%), asymptomatic infection (n = 3; 12.5%), severe COVID‐19 symptoms, as manifested by increasing oxygen requirement (n = 5; 20.8%), symptoms longer than 10 days (n = 3; 12.5%), or hospitalization for COVID‐19 (n = 5; 20.8%)
Forty‐five of 75 patients (60.0%) were infused with monoclonal antibodies. The majority (39 patients, 87%) received casirivimab and imdevimab (1200 mg/1200 mg) combination, while six (13%) received bamlanivimab (700 mg). Infusions occurred in eight of 51 SNFs, ranging from 2 to 17 patients per site. Two SNFs were located locally in Rochester, Minnesota whereas six were rural SNFs with the furthest site 86 miles from Rochester.
The average age of patients was 77.8 years (range, 52–99) (Table 1). The average time to infusion was 4.3 days (range, 1–8) after COVID‐19 diagnosis. During the 14‐day follow‐up after infusion, the combined event rate of all‐cause ED visit, hospitalization or death was 11.1% (Table 2). There were three hospitalizations and two ED visits. No patient was hospitalized due to COVID‐19 progression. There were no deaths. However, two patients were subsequently enrolled into a hospice program. One patient developed shortness of breath due to congestive heart failure during monoclonal antibody infusion.
TABLE 1.
Demographic information of 45 COVID‐19 patients who received monoclonal antibodies at congregate living facilities
| Patients (%), n = 45 | |
|---|---|
| Age | 77.8 (52–99) |
| Gender | |
| Male | 19 (42.2%) |
| Female | 26 (57.8%) |
| Risk factors | |
| Aged >65 | 40 (88.9%) |
| Cardiovascular disease | 23 (51.1%) |
| Diabetes mellitus | 22 (48.9%) |
| BMI >35 | 12 (26.7%) |
| Chronic lung disease | 9 (20.0%) |
| Chronic kidney disease | 4 (8.9%) |
| Immunocompromised status | 2 (4.4%) |
| Monoclonal antibody preparation | |
| Bamlanivimab | 6 (13.3%) |
| Casirivimab/imdevimab | 39 (86.7%) |
TABLE 2.
Clinical events following monoclonal antibody infusion for coronavirus disease‐19
| Event | Days post‐infusion | Description |
|---|---|---|
| Emergency Department visit | 11 | Retinal vein occlusion |
| Emergency Department visit | 11 | Violent behavior |
| Hospitalization | 3 | Congestive heart failure |
| Hospitalization | 8 | Urosepsis |
| Hospitalization | 13 | Congestive heart failure |
Note: No patients were hospitalized for progression of COVID‐19.
Twenty‐four patients were ineligible for monoclonal antibody infusion (Figure 1). The most common reason for ineligibility was goal of care (do not hospitalize order, hospice or comfort care), clinically severe COVID‐19, and the need for hospitalization before the scheduled monoclonal antibody infusion. Six eligible patients declined infusion.
DISCUSSION
We demonstrate an effective process of deploying a mobile infusion team to administer monoclonal antibodies in response to COVID‐19 outbreaks at SNF. The monoclonal antibodies may have reduced the risk of progression to severe COVID‐19 that require hospitalization. 3 To our knowledge, this is the first report of this novel process, which highlights the encouraging outcomes of timely monoclonal antibody infusions to high‐risk SNF residents with mild to moderate COVID‐19.
Key elements of a successful process include an accurate communication between SNF and the MATRx team, a dedicated multi‐disciplinary mobile team (desk operation, nurse practitioner, physician, RN, pharmacy), daily infusion capabilities, early recognition of atypical COVID‐19 symptoms in older patients, and updated advanced directives/goals of care. The timely transfer of patient health information proved to be the most challenging step in the process due to multiple factors, including poor electronic health record interoperability. Patients living in SNF have limited medical record information, particularly those with primary providers outside of Mayo Clinic. There was limited documentation of comorbidities and clinical symptoms, which delayed our allocation process. We addressed this challenge by developing a HIPPA‐compliant process allowing transmitting discrete data elements, which facilitated the timely evaluation of patient's eligibility (Appendix B). An additional benefit of this approach was that it allowed for batch referral in the case of an outbreak at the facility.
Older patients have atypical symptoms in response to infections and COVID‐19 is no exception. In our experience, COVID‐19 in older patients can manifest as delirium, anorexia, lethargy, weakness, mild cough, and loose stools. 6 This is in contrast to the classic COVID‐19 symptoms of fever, dry cough, shortness of breath, headache, anosmia, diarrhea or myalgia in the general population. 7 Awareness of atypical COVID‐19 presentations in older individuals is critically important for ensuring the timely infusion of these therapies. Indeed, despite a short period of only 4 days from COVID‐19 diagnosis to infusion, there were patients who have already progressed to severe illness that warranted hospitalization before their scheduled monoclonal antibody infusions.
Aligning treatment modalities with patient goals of care is a critical underpinning of geriatric care. During the initial phases of COVID‐19 in our region, goals of care discussions were taking place with many SNF residents. Initially, monoclonal antibodies were considered as a scarce resource, and our guidance was to not infuse patients who indicated a “do not hospitalize” in their Physician Orders for Life‐Sustaining Treatment (POLST). This guidance was met with much consternation, and we encountered patients and healthcare agents wanting to change the POLST to allow for monoclonal antibody infusion. As it turned out, the supply of monoclonal antibodies had outpaced the demand, which subsequently allowed for the infusion of these therapies to consenting patients even if they had a “do not hospitalize” status on their POLST and those who are on hospice. However, the use of monoclonal antibodies should still be within the wishes and goals of care for the patient.
Monoclonal antibody therapy was well tolerated in our population, and only one adverse reaction due to fluid overload was reported. Monoclonal antibody therapy appeared beneficial with only 11.1% of patients being hospitalized or presenting to the ED within 14 days of infusion. This is below the predicted population level event rate. In one study of nursing home residents, hospitalization was as high as 54% and mortality was as high as 37%. 8 In our cohort, only two of the five events were directly attributable to COVID‐19. However, we observed that many patients had progressed to severe illness requiring hospitalization before the scheduled infusion. This finding emphasizes the need to suspect and diagnose COVID‐19 early to provide timely infusion of monoclonal antibodies to this high‐risk population.
CONCLUSION
The logistical challenges of administering monoclonal antibody therapies for the treatment of COVID‐19 in frail residents of SNF can be effectively overcome by deploying a multidisciplinary mobile infusion team. Monoclonal antibody therapies were well tolerated in this older population of SNF residents. While our early findings are encouraging, further analysis of a larger number of SNF patients, compared with a propensity‐matched control group, will be needed to better evaluate the efficacy and tolerability of monoclonal antibodies in this vulnerable population.
FINANCIAL DISCLOSURE
Mayo Clinic Research grant to Raymund R. Razonable, MD.
CONFLICT OF INTEREST
No conflicts reported.
AUTHOR CONTRIBUTIONS
Study concept and design: Sidna Tulledge‐Scheitel, MD, MPH, Dennis M. Bierle, MD, Raymund R. Razonable, MD, Ravindra Ganesh, MBBS, MD.
Acquisition of subjects and/or data: Sidna Tulledge‐Scheitel, MD, MPH, Sara J. Bell, MSN, MHA, RN, Jennifer J. Larsen, MSN, RN, CRNI, Darcie E. Moehnke, MAN, RN, Molly J. Destro Borgen, MA, Donna J. Springer, APRN, CNS, MS, Karen J. Reinschmidt, MS, Lori J. Baumbach, MBA, Jennifer A. Matoush, APRN, CNS, Alexander Heyliger, PharmD, RPh, Sara N. Hanson, DO, MPH, Raymund R. Razonable, MD, Ravindra Ganesh, MBBS, MD.
Analysis and interpretation of data: Sidna Tulledge‐Scheitel, MD, MPH, Dennis M. Bierle, MD, Paul Takahashi, MD, Darcie E. Moehnke, MAN, RN, Sara N. Hanson, DO, MPH, Raymund R. Razonable, MD, Ravindra Ganesh, MBBS, MD.
Manuscript preparation: Sidna Tulledge‐Scheitel, MD, MPH, Sara J. Bell, MSN, Dennis M. Bierle, MD, Paul Takahashi, MD, Darcie E. Moehnke, MAN, RN, Sara N. Hanson, DO, MPH, Raymund R. Razonable, MD, Ravindra Ganesh, MBBS, MD.
All authors approved the final version of the manuscript for submission.
Supporting information
Data S1. Appendix A. Frequently asked questions about monoclonal antibody therapy for coronavirus disease‐19.
Appendix B. Spreadsheet of demographic and clinical information that is required for eligibility determination for monoclonal antibody therapy for coronavirus disease‐19.
Appendix C. Standardized script for education and consenting for monoclonal antibody infusion for the treatment of coronavirus disease‐19.
ACKNOWLEDGMENTS
We would like to thank our patients and their legal authorized representatives for entrusting their care to us, and to our skilled care facilities for partnering with us in providing these monoclonal therapies to the vulnerable high‐risk residents. We also thank all members of the Monoclonal Antibody Treatment (MATRx) Team for the multidisciplinary collaboration.
Tulledge‐Scheitel S, Bell SJ, Larsen JJ, et al. A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID‐19 in skilled care facilities. J Am Geriatr Soc. 2021;69:868–873. 10.1111/jgs.17090
Contributor Information
Sidna Tulledge‐Scheitel, @SidnaMarie.
Raymund R. Razonable, Email: razonable.raymund@mayo.edu, @RazonableMD.
REFERENCES
- 1. Mueller AL, McNamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people? Aging (Albany NY). 2020;12(10):9959‐9981. 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2021;384:229‐237. 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384:238‐251. 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Why Are Covid Antibody Drugs Sitting on Shelves? ‐ WSJ. https://www.wsj.com/articles/why-are-covid-antibody-drugs-sitting-on-shelves-11608499832. Accessed January 12, 2021
- 5. Slow Uptake for Promising New COVID‐19 Treatment in Minnesota ‐ StarTribune.com. https://www.startribune.com/slow-uptake-in-minnesota-for-promising-new-covid-19-therapy/600008726/. Accessed January 17, 2021.
- 6. Perrotta F, Corbi G, Mazzeo G, et al. COVID‐19 and the elderly: insights into pathogenesis and clinical decision‐making. Aging Clin Exp Res. 2020;32(8):1599‐1608. 10.1007/s40520-020-01631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pascarella G, Strumia A, Piliego C, et al. COVID‐19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192‐206. 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005‐2011. 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Appendix A. Frequently asked questions about monoclonal antibody therapy for coronavirus disease‐19.
Appendix B. Spreadsheet of demographic and clinical information that is required for eligibility determination for monoclonal antibody therapy for coronavirus disease‐19.
Appendix C. Standardized script for education and consenting for monoclonal antibody infusion for the treatment of coronavirus disease‐19.


