Summary
The COVID‐19 pandemic continues to cause critical illness and deaths internationally. Up to 31 May 2020, mortality in patients admitted to intensive care units (ICU) with COVID‐19 was 41.6%. Since then, changes in therapeutics and management may have improved outcomes. Also, data from countries affected later in the pandemic are now available. We searched MEDLINE, Embase, PubMed and Cochrane databases up to 30 September 2020 for studies reporting ICU mortality among adult patients with COVID‐19 and present an updated systematic review and meta‐analysis. The primary outcome measure was death in intensive care as a proportion of completed ICU admissions, either through discharge from intensive care or death. We identified 52 observational studies including 43,128 patients, and first reports from the Middle East, South Asia and Australasia, as well as four national or regional registries. Reported mortality was lower in registries compared with other reports. In two regions, mortality differed significantly from all others, being higher in the Middle East and lower in a single registry study from Australasia. Although ICU mortality (95%CI) was lower than reported in June (35.5% (31.3–39.9%) vs. 41.6% (34.0–49.7%)), the absence of patient‐level data prevents a definitive evaluation. A lack of standardisation of reporting prevents comparison of cohorts in terms of underlying risk, severity of illness or outcomes. We found that the decrease in ICU mortality from COVID‐19 has reduced or plateaued since May 2020 and note the possibility of some geographical variation. More standardisation in reporting would improve the ability to compare outcomes from different reports.
Keywords: COVID‐19, intensive care, meta‐analysis, mortality, pandemic
Introduction
The global COVID‐19 pandemic continues to impact international health and healthcare delivery [1]. To date, the World Health Organization has recorded more than 96 million cases worldwide, with the real number likely many‐fold higher, and more than 2 million confirmed deaths [2]. Intensive care units (ICU) have an important role in managing the sickest of these patients, but mortality is high in this group. We conducted an initial systematic review and meta‐analysis of 24 observational studies published by 31 May 2020, which included 10,150 patients, finding that mortality was 41.6%, with evidence that this was decreasing as the pandemic progressed [3, 4].
In the last few months, several studies have clarified which treatments do and do not provide benefit in the ICU management of COVID‐19. Steroids (particularly dexamethasone) were shown in early June to improve survival in patients who are oxygen‐dependent or receiving mechanical respiratory support [5, 6], while other drugs including chloroquine, azithromycin, lopinavir/ritonavir and remdesivir have been shown to have no clear mortality benefit [7, 8, 9]. Management of COVID‐19 has also likely evolved over the year with changes in approaches to oxygen therapy, fluids and anticoagulation management [10, 11]. Since our first meta‐analysis, the pandemic has spread further into the southern hemisphere and there has been time for studies from more countries to be reported.
Given these developments, mortality from COVID‐19 in patients admitted to the ICU may have altered further. Here, we update the previous systematic review and meta‐analysis to include studies published up to 30 September 2020.
Methods
The review, including our intention to update the analyses and outputs as new data came to light, was prospectively registered with PROSPERO and conducted according to preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines [12]. The search strategy up to 31 May 2020 has been previously described [3]. We repeated the search of MEDLINE, Embase, PubMed and the Cochrane Library up until 30 September 2020 using the search terms “coronavirus”, “covid19”, “sars‐cov‐2” or “2019‐ncov”; and “intensive care”, “mortality” or “disease course”. The exact terms used were adapted to each database (online Supporting Information, Table S1). Manual searching was used to identify additional results. We also contacted intensive care registries run by national societies (online Supporting Information, Table S2) to locate published data not indexed by the libraries above. Preprints and articles that were not published in journals were not included.
Studies were eligible for inclusion where the study group included adult patients (18 years or older) admitted to an ICU with COVID‐19 and the outcome of ICU admission was reported (i.e. reported as died or discharged from ICU alive). Patients in ICU and high dependency units were included. Studies were excluded if the primary outcome was not reported, all patients were < 18 years old, or the report was a single case. We analysed studies by geographical region using the World Bank classification of regions [13] as used in other analyses [14], but included Australia and New Zealand (Australasia) as an independent region from others in the East Asia and Pacific grouping as they are geopolitically discrete and experienced a later first surge.
Screening of titles and abstracts was performed in Microsoft Excel (Microsoft, Inc., Redmond, CA, USA). All articles were screened independently by two authors (two of RA, AK, EK, FO) to identify studies potentially meeting inclusion criteria. The full texts of potentially eligible studies were independently assessed for eligibility with disagreements resolved by discussion with a third reviewer (TC). The pre‐specified primary outcome was the mortality rate in patients with completed ICU admission. Data were only included when this outcome was reported clearly. Other pre‐defined data items extracted included study setting and design, including information for risk of bias assessment, patient characteristics, clinical features and rates of organ support delivered. We used a modified version of the Newcastle‐Ottawa Scale (online Supporting Information, Table S3) to assess the quality of included studies, as previously described, and funnel plot asymmetry to assess heterogeneity and risk of publication bias [3, 15].
Meta‐analysis was conducted using the ‘meta’ package (Version 4.15‐1, 2020) in R (The R Foundation for Statistical Computing; Version 4.0.3, 2020). An inverse‐variance random‐effects model was used for all analyses. Between‐study heterogeneity was assessed using the I2 test. A funnel plot was produced using the Public Health England tool [15]. To further explore heterogeneity, we performed sub‐group analyses based on study methodology (single‐ or multi‐centre; number of participants; censoring of ICU outcomes) and geographical location (both region and World Bank income region [13]). We also conducted a sensitivity analysis excluding all national registries. Meta‐regression was used to explore the effects of patient characteristics and treatments (proportion ventilated; average age; proportion of male sex); geographical location; publication date; and proportion of patients with outcomes reported.
Results
The updated search found an additional 7341 articles available since our previous analysis [3], including 1359 duplicates, leaving 5982 to be screened. After exclusion by title or abstract of 5787 articles, 195 full‐text articles were reviewed, of which 28 reported the primary outcome of interest. One of these was an updated report from Lombardy [16]. Three studies from Wuhan, China were excluded to avoid data duplication due to the overlap of both the data collection period and hospital location [17, 18, 19]. A report from the European Risk Stratification in COVID‐19 patients in the ICU (RISC‐19‐ICU) cohort was included as it was not possible to determine whether patients were duplicated in other series [20]. Manual searching and direct contact yielded five additional regional or national registries, including an updated report from the UK's Intensive Care National Audit and Research Centre (ICNARC). To avoid duplication of cases, two earlier reports from the Netherlands [21, 22] and one from Germany [23] were excluded. A total of 52 reports were included in the analysis [16, 20, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73], comprising the 31 new reports and 21 of the 24 reports from our earlier review (removing the previous reports from ICNARC, Grasselli et al. and Klok et al. [21, 74, 75]; Table 1, Fig. 1).
Table 1.
Included studies arranged by publication date. Values in the final two columns are number (proportion).
| Study | Centres | Country | Area | First admission | Last admission | Last follow‐up | Publication date | Patients with ICU outcome | Patients who died in ICU |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al. [24] | Single | China | Wuhan | 16 Dec 2019 | 02 Jan 2020 | 02 Jan 2020 | 24 Jan 2020 | 12/13 (92.3%) | 5/12 (41.7%) |
| Stoecklin et al. [25] | Multi | France | – | 10 Jan 2020 | 24 Jan 2020 | 12 Feb 2020 | 13 Feb 2020 | 1/1 (100%) | 0/1 (0%) |
| Young et al. [26] | Multi | Singapore | – | 23 Jan 2020 | 03 Feb 2020 | 25 Feb 2020 | 03 Mar 2020 | 2/2 (100%) | 0/2 (0%) |
| Zhou et al. [27] | Multi | China | Wuhan | 29 Dec 2019 | 31 Jan 2020 | 31 Jan 2020 | 09 Mar 2020 | 50/50 (100%) | 39/50 (78%) |
| Arentz et al. [28] | Single | USA | Washington | 20 Feb 2020 | 05 Mar 2020 | 17 Mar 2020 | 19 Mar 2020 | 13/21 (61.9%) | 11/13 (84.6%) |
| Wang et al. [29] | Single | China | Zhengzhou | 21 Jan 2020 | 05 Feb 2020 | 07 Feb 2020 | 26 Mar 2020 | 1/2 (50%) | 0/1 (0%) |
| Bhatraju et al. [30] | Multi | USA | Seattle | 24 Feb 2020 | 09 Mar 2020 | 23 Mar 2020 | 30 Mar 2020 | 21/24 (87.5%) | 12/21 (57.1%) |
| Ling et al. [31] | Multi | Hong Kong | – | 22 Jan 2020 | 11 Feb 2020 | 09 Mar 2020 | 06 Apr 2020 | 8/8 (100%) | 1/8 (12.5%) |
| Wang et al. [32] | Single | China | Tongji | 25 Jan 2020 | 25 Feb 2020 | 24 Mar 2020 | 08 Apr 2020 | 318/344 (92.4%) | 133/318 (41.8%) |
| Barrasa et al. [33] | Multi | Spain | Vitoria | 04 Mar 2020 | 31 Mar 2020 | 31 Mar 2020 | 09 Apr 2020 | 27/48 (56.2%) | 14/27 (51.9%) |
| Zhang et al. [34] | Single | China | Wuhan | 02 Jan 2020 | 10 Feb 2020 | 15 Feb 2020 | 09 Apr 2020 | 32/44 (72.7%) | 9/32 (28.1%) |
| Zhang et al. [35] | Single | China | Tongji | 16 Jan 2020 | 28 Feb 2020 | NR | 21 Apr 2020 | 19/19 (100%) | 8/19 (42.1%) |
| Zhou et al. [36] | Single | China | Hubei | 28 Jan 2020 | 02 Mar 2020 | NR | 21 Apr 2020 | 16/21 (76.2%) | 3/16 (18.8%) |
| Llitjos et al. [37] | Multi | France | – | 19 Mar 2020 | 11 Apr 2020 | NR | 22 Apr 2020 | 19/26 (73.1%) | 3/19 (15.8%) |
| Richardson et al. [38] | Multi | USA | New York | 01 Mar 2020 | 04 Apr 2020 | 04 Apr 2020 | 22 Apr 2020 | 371/371 (100%) | 291/371 (78.4%) |
| Pedersen et al. [39] | Single | Denmark | Roskilde | 11 Mar 2020 | 12 Mar 2020 | 16 Apr 2020 | 27 Apr 2020 | 11/17 (64.7%) | 7/11 (63.6%) |
| Ferguson et al. [40] | Multi | USA | San Francisco | 13 Mar 2020 | 11 Apr 2020 | 02 May 2020 | 14 May 2020 | 21/21 (100%) | 3/21 (14.3%) |
| Longchamp et al. [41] | Single | Switzerland | Sion | 08 Mar 2020 | 04 Apr 2020 | 09 May 2020 | 14 May 2020 | 23/25 (92%) | 5/23 (21.7%) |
| Zheng et al. [42] | Single | China | Hangzhou | 22 Jan 2020 | 05 Mar 2020 | 05 Mar 2020 | 20 May 2020 | 20/34 (58.8%) | 0/20 (0%) |
| Auld et al. [43] | Multi | USA | Atlanta | 06 Mar 2020 | 17 Apr 2020 | 07 May 2020 | 26 May 2020 | 209/217 (96.3%) | 62/209 (29.7%) |
| Maatman et al. [44] | Multi | USA | Indianapolis | 12 Mar 2020 | 31 Mar 2020 | 06 May 2020 | 27 May 2020 | 106/109 (97.2%) | 27/106 (25.5%) |
| Mitra et al. [45] | Single | Canada | Vancouver | 21 Feb 2020 | 14 Apr 2020 | 05 May 2020 | 27 May 2020 | 105/117 (89.7%) | 18/105 (17.1%) |
| Fraissé et al. [46] | Single | France | Argenteuil | 06 Mar 2020 | 22 Apr 2020 | 06 May 2020 | 02 Jun 2020 | 66/92 (71.7%) | 38/66 (57.6%) |
| Borobia et al. [47] | Single | Spain | Madrid | 25 Feb 2020 | 19 Apr 2020 | 19 Apr 2020 | 04 Jun 2020 | 121/237 (51.1%) | 55/121 (45.5%) |
| Rubin et al. [48] | Single | France | Bordeaux | 03 Mar 2020 | 14 Apr 2020 | 14 Apr 2020 | 06 Jun 2020 | 42/71 (59.2%) | 4/42 (9.5%) |
| Shahriarirad et al. [49] | Multi | Iran | Fars Province | 20 Feb 2020 | 20 Mar 2020 | NR | 18 Jun 2020 | 9/11 (81.8%) | 5/9 (55.6%) |
| Shukla et al. [50] | Single | India | Maharashtra | 01 Apr 2020 | 17 May 2020 | 17 May 2020 | 01 Jul 2020 | 24/24 (100%) | 4/24 (16.7%) |
| Almazeedi et al. [51] | Single | Kuwait | South Surra | 24 Feb 2020 | 20 Apr 2020 | 20 Apr 2020 | 04 Jul 2020 | 23/42 (54.8%) | 17/23 (73.9%) |
| Wendel Garcia et al. [20] | Multi | Europe | RISC‐19‐ICU registry (Switzerland, Spain, Italy, France, Germany, Others) | 13 Mar 2020 | 22 Apr 2020 | 22 Apr 2020 | 06 Jul 2020 | 398/639 (62.3%) | 97/398 (24.4%) |
| SICSAG [52] | Multi | Scotland | – | 01 Mar 2020 | 20 Jun 2020 | 20 Jun 2020 | 08 Jul 2020 | 509/521 (97.7%) | 193/509 (37.9%) |
| Giesen et al. [53] | Single | Spain | Madrid | 27 Feb 2020 | 30 Jun 2020 | 29 Jun 2020 | 11 Jul 2020 | 99/103 (96.1%) | 36/99 (36.4%) |
| Pellaud et al. [54] | Single | Switzerland | Fribourg | 01 Mar 2020 | 12 Apr 2020 | 10 May 2020 | 14 Jul 2020 | 43/49 (87.8%) | 11/43 (25.6%) |
| Grasselli et al. [16] | Multi | Italy | Lombardy | 20 Feb 2020 | 22 Apr 2020 | 30 May 2020 | 15 Jul 2020 | 3818/3988 (95.7%) | 1769/3818 (46.3%) |
| Halvatsiotis et al. [55] | Multi | Greece | – | 10 Mar 2020 | 13 Apr 2020 | 13 Apr 2020 | 17 Jul 2020 | 38/90 (42.2%) | 26/38 (68.4%) |
| Amit et al. [56] | Multi | Israel | – | 05 Mar 2020 | 27 Apr 2020 | 08 May 2020 | 18 Jul 2020 | 156/156 (100%) | 87/156 (55.8%) |
| Primmaz et al. [57] | Single | Switzerland | Geneva | 09 Mar 2020 | 19 May 2020 | 19 May 2020 | 29 Jul 2020 | 129/129 (100%) | 24/129 (18.6%) |
| Muñoz et al. [58] | Single | Spain | Madrid | 01 Mar 2020 | 11 Mar 2020 | NR | 30 Jul 2020 | 10/13 (76.9%) | 5/10 (50%) |
| Kristinsson et al. [59] | Multi | Iceland | – | 14 Mar 2020 | 13 Apr 2020 | 05 May 2020 | 11 Aug 2020 | 27/27 (100%) | 3/27 (11.1%) |
| Miller et al. [60] | Single | USA | New York | 01 Apr 2020 | 23 Apr 2020 | NR | 18 Aug 2020 | 19/19 (100%) | 5/19 (26.3%) |
| Mukherjee et al. [61] | Single | USA | New York | 10 Mar 2020 | 07 Apr 2020 | 18 May 2020 | 19 Aug 2020 | 135/137 (98.5%) | 82/135 (60.7%) |
| Zhou et al. [62] | Single | China | Hunan | 01 Jan 2020 | 28 Apr 2020 | 28 Apr 2020 | 21 Aug 2020 | 45/45 (100%) | 2/45 (4.4%) |
| Hu et al. [63] | Single | China | Wuhan | 08 Jan 2020 | 12 Mar 2020 | 12 Mar 2020 | 29 Aug 2020 | 55/55 (100%) | 16/55 (29.1%) |
| Larsson et al. [64] | Single | Sweden | Stockholm | 09 Mar 2020 | 20 Apr 2020 | 30 Apr 2020 | 06 Sep 2020 | 198/260 (76.2%) | 60/198 (30.3%) |
| Haase et al. [65] | Multi | Denmark | – | 10 Mar 2020 | 19 May 2020 | 16 Jun 2020 | 15 Sep 2020 | 319/323 (98.8%) | 108/319 (33.9%) |
| Cavayas et al. [66] | Single | Canada | Montreal | 20 Mar 2020 | 13 May 2020 | 27 Jul 2020 | 15 Sep 2020 | 75/75 (100%) | 17/75 (22.7%) |
| Lee et al. [67] | Single | Yemen | – | NR | NR | NR | 23 Sep 2020 | 47/47 (100%) | 32/47 (68.1%) |
| Kokoszka‐Bargieł et al. [68] | Single | Poland | Silesian | 10 Mar 2020 | 10 Jun 2020 | 10 Jun 2020 | 26 Sep 2020 | 27/32 (84.4%) | 18/27 (66.7%) |
| Van Aerde et al. [69] | Single | Belgium | Leuven | 13 Mar 2020 | 08 Jun 2020 | NR | 28 Sep 2020 | 111/114 (97.4%) | 11/111 (9.9%) |
| ICNARC [70] | Multi | UK | England, Wales and Northern Ireland | 01 Mar 2020 | 15 Oct 2020 | 28 May 2020 | 16 Oct 2020 | 11,480/12,133 (94.6%) | 4457/11,480 (38.8%) |
| ANZICS (Victoria) [71] | Multi | Australia | Victoria | 01 Jan 2020 | 30 Sep 2020 | 30 Sep 2020 | 22 Oct 2020 | 819/883 (92.8%) | 87/819 (10.6%) |
| Germany registry [72] | Multi | Germany | – | 01 Jan 2020 | 22 Oct 2020 | 22 Oct 2020 | 22 Oct 2020 | 19,229/20,259 (94.9%) | 4443/19,229 (23.1%) |
| Netherlands registry [73] | Multi | Netherlands | – | 01 Mar 2020 | 27 Oct 2020 | 27 Oct 2020 | 27 Oct 2020 | 3652/4161 (87.8%) | 942/3652 (25.8%) |
NR, not reported; SICSAG, Scottish Intensive Care Society Audit Group; ICNARC, Intensive Care National Audit and Research Centre; ANZICS, Australia and New Zealand Intensive Care Society.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
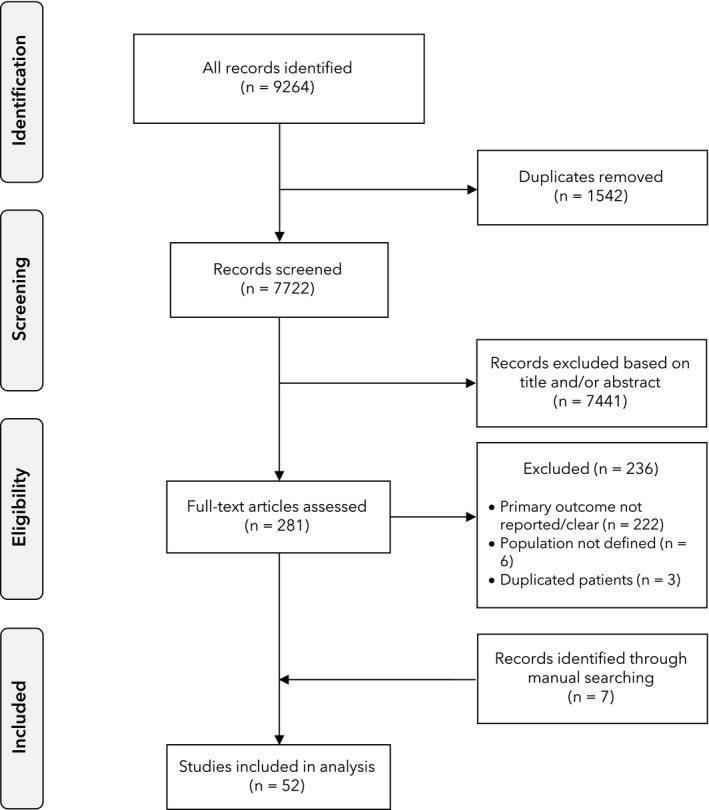
Flowchart of study inclusion.
These studies reported ICU outcome data for 43,128 patients admitted to ICU with a COVID‐19 diagnosis. Median (IQR [range]) number of patients in each study was 44 (20–140 [1–19,229]) patients; the smallest series were from reports of larger cohorts that included non‐ICU patients. Recruitment in these 52 studies was from 16 December 2019 to 27 October 2020 with publication dates from 24 January 2020 to 27 October 2020 (Fig. 2). The median (IQR [range]) interval from recruitment of the last patient to publication was 50 (26–82 [0–170]) days, but this was longer after 31 May 2020 than before this (40 (21–50 [9–76]) days vs. 84 (45—117 [0–170]) days, p = 0.002). [Correction added on 9 February 2021, after first online publication: In the preceding sentence, the median: (404 (21–50 [9–76]) was changed to (40 (21–50 [9–76])]. Studies reported on patients from China (n = 10) [24, 63]; USA (n = 8) [28, 30, 38, 40, 43, 44, 60, 61]; France (n = 4) [25, 37, 46, 48]; Spain (n = 4) [33, 47, 53, 58]; Switzerland (n = 3) [41, 54, 57]; Canada (n = 2) [45, 66]; Denmark (n = 2) [39, 65]; Australia [71]; Belgium [69]; Europe [20]; Germany [72]; Greece [55]; Hong Kong [31]; Iceland [59]; India [50]; Iran [49]; Israel [56]; Italy [16]; Kuwait [51]; Netherlands [73]; Poland [68]; Scotland [52]; Singapore [26]; Sweden [64]; UK [70]; and Yemen [67] (n = 1 each). Reported ICU mortality rates ranged from 0% to 84.6%, with values at both extremes arising from small case series.
Figure 2.
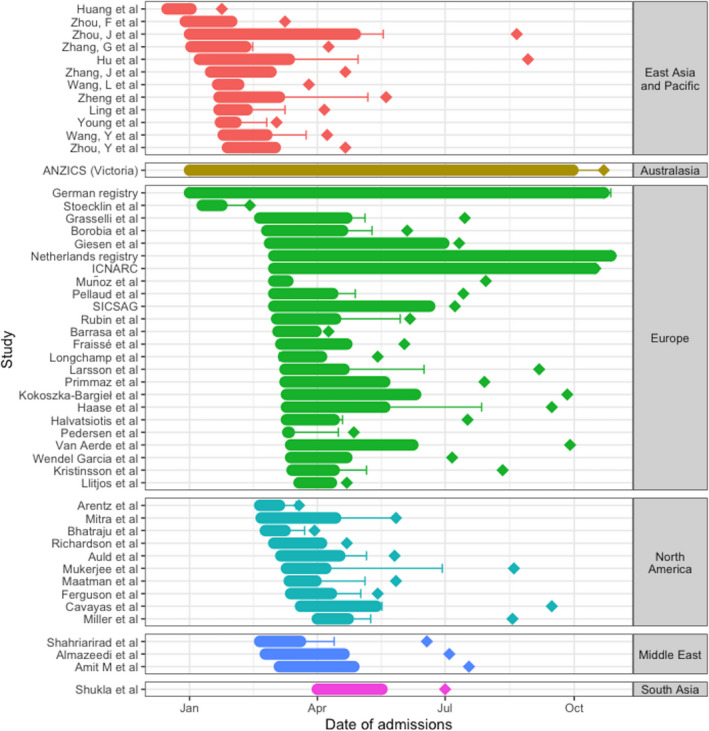
Indicative summary of study recruitment, follow‐up and reporting. Data represent study admission dates (filled bar), length of final patient follow‐up (solid line) and publication date (diamond) for all studies, grouped by continent (represented by colour). ICNARC, Intensive Care National Audit and Research Centre; SICSAG, Scottish Intensive Care Society Audit Group; ANZICS, Australia and New Zealand Intensive Care Society. [Correction added on 9 February 2021, after first online publication: Fig. 2 was updated to reflect correct analysis of data].
The proportion of included patients who had completed their ICU stay (being dead or discharged) at the point the study was reported varied between studies: 16 studies reported outcome data for all participants and in the remaining 36 studies the percentage varied from 42.2% to 98.8% (Table 1, online Supporting Information, Figure S1). All studies were observational cohort studies with varying durations of patient follow‐up. The median (IQR [range]) quality score for risk of bias was 6 (5–7 [3–8]) out of 8, indicating a low risk of bias. Only four studies were rated 8/8, with one scoring 3/8 and six scoring 4/8 (online Supporting Information, Table S4). Details of ICU treatments were variably reported making further analysis of the impact of treatment on the outcome, other than invasive mechanical ventilation, impractical (online Supporting Information, Table S5).
The ICU mortality rate (95%CI) across all studies included in the quantitative analysis was 35.5% (31.3–39.9%), I2 = 97.6% (Fig. 3). The largest patient cohorts were from national registries of Germany (19,229 patients [72]) and the UK (11,480 [70]). A sensitivity analysis removing all national and regional registries [52, 70, 71, 72, 73] did not significantly affect the mortality rate or heterogeneity (36.8% (31.6–42.4%), I2 = 91.8%) and Egger's test of funnel plot asymmetry was negative (t = 0.89, p = 0.38; Fig. 4).
Figure 3.
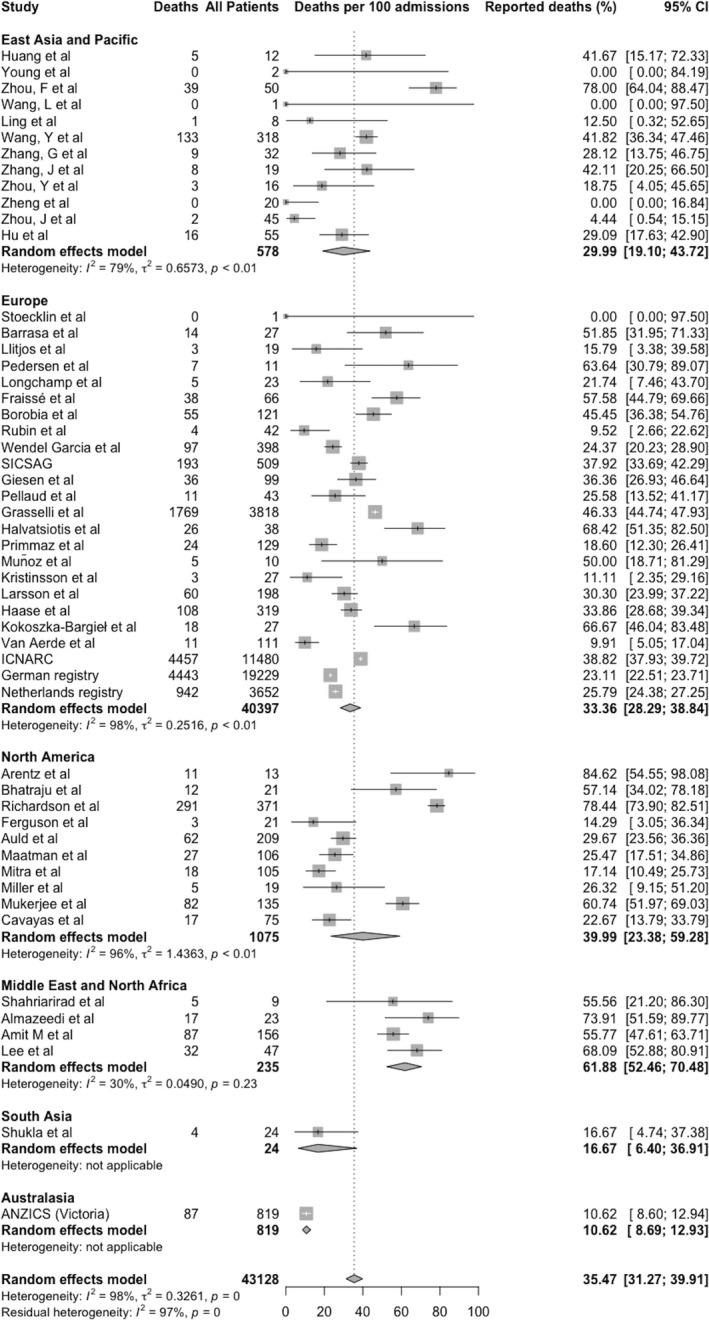
Meta‐analysis of mortality of patients admitted to ICU with COVID‐19 infection. Data represent deaths per 100 completed intensive care admissions, grouped by geography and combined. Each study is represented by a square with outcome estimate in the centre and 95%CI as a horizontal line either side. The size of the square reflects the study weight based on random effects. The diamonds represent meta‐analysis results with outcome estimate in the centre and left and right sides corresponding to lower and upper confidence limits. ICNARC, Intensive Care National Audit and Research Centre; SICSAG, Scottish Intensive Care Society Audit Group; ANZICS, Australia and New Zealand Intensive Care Society. [Correction added on 9 February 2021, after first online publication: Fig. 3 was updated to reflect correct analysis of data].
Figure 4.
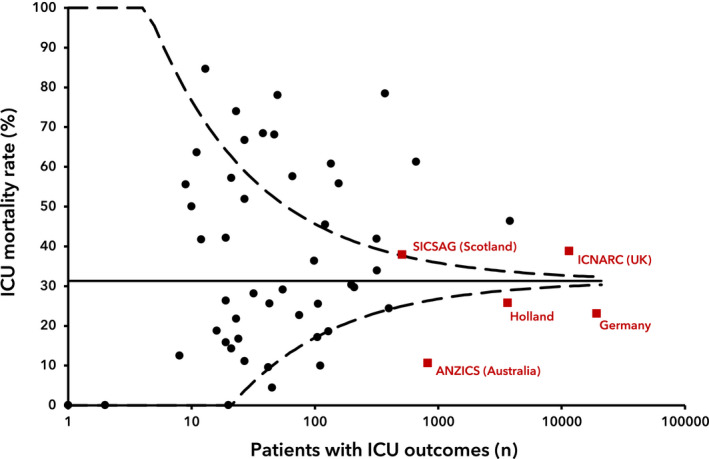
Funnel plot of the number of patients with ICU outcomes against reported ICU mortality rate (%) for 52 included studies. The solid line represents the average reported mortality. The dotted lines represent three standard deviations. [Correction added on 9 February 2021, after first online publication: The solid line representation has now been explained]
In a sub‐group analysis, the mortality reported in the registries was significantly lower than in other reports (25.7% (18.4–34.7%), I2 = 99.6% vs. 36.8% (31.6–42.4%), I2 = 96.8%, p = 0.04). Sub‐group analysis by geographical location demonstrated higher mortality in studies from the Middle East (61.9% (52.5–70.5%), I2 = 30%) and lower mortality in the single registry from Australia (10.6% (8.7–12.9%)) [71], with similar rates elsewhere (between‐group differences p < 0.001). Mortality was higher in the one low‐income country [67] but similar in other income groups (between‐group differences p < 0.001). Sub‐group analysis by month of publication demonstrated higher mortality in the earliest reported series (between‐group differences p < 0.05) (Table 2). Sub‐group analyses based on study characteristics (single or multiple centres; sample size; complete outcome reporting) showed no significant between‐group differences or substantial reductions in heterogeneity (online Supporting Information, Table S6).
Table 2.
Statistically significant sub‐group analyses showing variation in survival of intensive care unit admission after admission with COVID‐19 between registry and non‐registry reports, geographical region and month of publication.
| Studies | Mortality % (95%CI) | I2 (%) | p value | |
|---|---|---|---|---|
| Registries | ||||
| Registry reports | 5 | 25.7% (18.4–34.7%) | 99.6% | 0.037 |
| Other studies | 47 | 36.8% (31.6–42.4%) | 91.8% | |
| Geographical region | ||||
| East Asia and Pacific | 12 | 30.0% (19.1–43.7%) | 79.4% | < 0.001 |
| Europe | 24 | 33.4% (28.3–38.8%) | 98.4% | |
| North America | 10 | 40.0% (23.4–59.3%) | 96.3% | |
| Middle East and North Africa | 4 | 61.9% (52.5–70.5%) | 30.0% | |
| South Asia | 1 | 16.7% ( 6.4–36.9%) | – | |
| Oceania | 1 | 10.6% ( 8.7–12.9%) | – | |
| World Bank income region | ||||
| High‐income | 39 | 35.1% (30.4–40.0%) | 98.1% | < 0.001 |
| Upper‐middle income | 11 | 33.7% (22.1–47.8%) | 80.6% | |
| Lower‐middle income | 1 | 16.7% ( 6.4–36.9%) | – | |
| Low income | 1 | 68.1% (53.6–79.8%) | – | |
| Month of publication | ||||
| Jan–Mar | 7 | 59.5% (39.8–76.5%) | 54.1% | 0.034 |
| Apr–Jun | 19 | 32.6% (22.9–44.0%) | 93.0% | |
| Jul–Oct | 26 | 33.1% (28.1–38.4%) | 98.5% | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Multivariate meta‐regressions based on patient characteristics and treatments (age; male sex; proportion of invasively ventilated patients) and proportion of patient outcomes reported (i.e. the proportion of patients in each study with a completed ICU stay) were not significant. Univariate meta‐regression by month of publication; month of last admission; and month of last patient follow‐up, all showed apparent reductions in mortality over time (treatment effect (logit transformed proportion) ‐0.13 per 1‐month increment in publication date, p = 0.002; ‐0.12 per 1‐month increment in last admission date, p = 0.004; ‐0.16 per 1‐month increment in last patient follow‐up date, p = 0.001). In multivariate meta‐regression adjusting for patient and treatment characteristics, the proportion of outcomes reported, geographical location and income region, the reduction in mortality over time remained significant for last patient follow‐up date (‐0.30 per 1‐month increment in last patient follow‐up date, p = 0.02), but not publication or last admission date (online Supporting Information, Table S7).
Discussion
In this updated systematic review and meta‐analysis of 52 studies involving 43,128 patients admitted to ICU with COVID‐19, we found an ICU mortality rate (95%CI) in those with a completed ICU stay of 35.5% (31.3–39.9%). Relative to other geographical regions, the mortality rate was higher in the Middle East and lower in a single study from Australasia. The previously identified reduction in mortality over time has become less pronounced between May and September 2020.
This updated analysis included 31 new studies and two updates of earlier reports [16, 70], with outcome data for an additional 32,978 patients. The updated search found reports from several countries and regions not represented in the previous review (Australia; Belgium; Germany; Greece; Iceland; India; Israel; Kuwait; Poland; Scotland; Sweden; Switzerland; Yemen) and several national and regional registries which had reported outcomes in the intervening months.
Overall mortality in all studies is lower to the end of September (35.5%) than when we reported this to the end of May (41.6%), and this is with the inclusion of more studies from more countries and a wider geographical area, over a longer time period, such that we now have a more complete picture of the first months of the pandemic. Before May 2020, there was a clear reduction in mortality over time. An analysis of mortality based on dates of last patient follow‐up finds mortality continues to fall, but this is complicated by the observation that the interval between data collection and publication has progressively increased (Fig. 2). Additionally, this single time‐point is only a proxy for the timeline of admissions in each study, which cannot be evaluated further due to a lack of patient‐level data. Meta‐regression also did not show clear temporal improvements within individual regions, when adjusted for other variables. Thus, the clear fall in mortality over time observed between January and May is now less evident, and while over time mortality has undoubtedly fallen, it is likely that the improvement has reduced or plateaued. We are not able to comment on whether mortality has reduced at specific time points, such as since the randomised evaluation of COVID‐19 therapy (RECOVERY) study reported reduced mortality with the use of dexamethasone [5], as this would likely require individual patient‐level data and separation of cohorts into those admitted before and after the relevant time‐point, which is not currently available.
In most geographical regions, the mortality rate is 30–40%. Two geographical regions fall outside these limits and are statistically significantly different from other geographical regions. A single registry report from Victoria State in Australia reports very low mortality of 10.6%. Conversely in the Middle East, mortality is high at 61.9%. These studies are variable in terms of the country of origin (two from high‐income countries [51, 56]; one upper‐middle [49]; and one low‐income [67]); quality (two were at high risk of bias); and one is from a critical care unit in an area of humanitarian crisis – despite this, the studies showed similar mortality rates and considerable homogeneity (I2 = 30%). There are several potential explanations for this finding, including the fact that studies from the Middle East included patients early in the pandemic when mortality was higher and those included in Australia arose later in the pandemic when mortality was lower. It is possible that variations in healthcare resource, variation in admission criteria and clinical and statistical uncertainty associated with single‐centre and small reports could have also contributed. Which of these explanations holds sway is uncertain but the variation merits further exploration and further reports from these regions would be welcome.
There remain limited reports from the southern hemisphere, where the pandemic centred later than in the north. We were unable to include any reports from South America; we are aware of a large registry from Brazil but it could not be included as the primary outcome cannot be calculated from the data reported. The African COVID‐19 Critical Care Outcomes Study (ACCCOS) has reported provisional outcomes in a pre‐print paper from 38 hospitals in 6 countries, reporting relatively high mortality (95%CI) of 54.7% (51.9–57.6%) (Biccard et al., preprint, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3707415) and further data are being added to this study.
One notable finding is that mortality was lower in registry reports than in non‐registry studies (absolute difference 11%; relative risk 0.70). Registry reports tended to have high proportions of completed episodes (mostly above 90%), included patient outcomes towards the latter stages of our data collection period and were all from high‐income countries. These factors, allied with networks that underpin the registries, may all be factors in their lower reported mortality rates. In the UK, the ICNARC group has reported a fall in mortality in the periods before and after the peak of the first surge [76]. The report is notable because mortality increased during the peak period of the surge and the characteristics of admitted patients also varied during this period, with patients being younger and sicker. That paper hints at both improvements in outcomes over time and poorer outcomes when healthcare systems are stressed. The changes in outcomes during and after periods of health system stress has implications for defining adequate health resource provision and for comparing performance between locations with differing resource and degrees of healthcare stress.
There are several limitations to this study. There remains a lack of reports from many countries and a paucity of reports from the southern hemisphere, so we are not able to provide a genuinely global picture of regional variation in outcomes. There is, as previously described [3], a notable lack of consistency of reporting with no standardisation of what constitutes intensive care, entry criteria for patients, admitted patients' underlying health characteristics and severity of critical illness or reporting on the nature or intensity of treatments. This means that the included patients' underlying risk is unknown and outcomes between studies are not directly comparable. Additional factors such as critical care provision (e.g. ICU beds per capita) may also contribute to the differences observed, though we do not have up‐to‐date data on these metrics, particularly as it is likely this has changed considerably throughout the pandemic. Indeed, it is also likely that some of these factors may even have varied during the pandemic in individual series or registries (e.g. [76]). The RISC‐19‐ICU registry (https://www.risc‐19‐icu.net) provides one approach towards the creation of a standardised minimum dataset in this patient cohort. Its website currently lists 93 participating centres from 16 countries collecting standardised data. There is an argument that unaccounted‐for differences in patient populations, definitions of ICU and marked heterogeneity of results mean the data should not be pooled, but we have decided there is value in presenting pooled data while highlighting its limitations. This analysis, therefore, likely should be a starting point for further study and analysis rather than an end in itself. Next, the vast majority of included patients have been in ICU during the first global surge and we cannot comment on whether the mortality rate has changed in the second surge, when it is likely that there will be different pressures on many healthcare systems, including through the necessity to catch up on non‐COVID‐19 healthcare demands. Our geographical analysis separates countries by geography rather than other factors which might impact outcomes, such as average national income, average population age, access to general healthcare or number of critical care beds per capita. Analyses based on such factors would be of considerable interest but are beyond the remit of this study. Our analysis includes studies published only up to the end of September and registry data up to the end of October. Since then, several variant viruses have emerged and in some countries transformed the trajectory of the pandemic through December 2020 and into January 2021. This has increased the demand on ICU in those locations and will merit further analysis in due course. To counter this, vaccination is now available in many countries and we can hope that this too will, over several months, positively impact on the pandemic trajectory and demand on ICU care.
In conclusion, this expanded meta‐analysis of survival of patients admitted to ICU with COVID‐19 has shown that any fall in mortality rate between June and September appears to have flattened or plateaued. We have identified geographical regions of both low (Australasia) and high (Middle East) mortality that merit further exploration. Mortality rate is lower in reports from registries than from non‐registry studies. Further analysis is hampered by a lack of definitions and standardisation of reporting. Standardisation of reporting would enable far more valuable comparisons of outcomes between locations and over time. Registries may be the best‐placed organisations to act on this first. Despite these limitations, this analysis provides an overview of outcomes among patients admitted to ICU with COVID‐19 in the first pandemic surge.
Supporting information
Figure S1. Proportion of included patients with outcome reported (red) and not reported (blue) for each study, grouped by geographical region.
Table S1. Search strategy.
Table S2. Registries or societies contacted directly.
Table S3. Modified Newcastle‐Ottawa Scale.
Table S4. Study quality and risk of bias assessment.
Table S5. Reported clinical features, rates of organ support and pharmacotherapy.
Table S6. Sensitivity and sub‐group analyses.
Table S7. Results of meta‐regression analyses.
Acknowledgements
This study was prospectively registered with PROSPERO (CRD42020180671). RA, AK and EK are Health Services Research Centre Clinical Research Fellows at the Royal College of Anaesthetists, UK. AK is an honorary member of the Department of Targeted Intervention, University College London, UK. No external funding or competing interests declared.
Contributor Information
R. A. Armstrong, @drrichstrong.
A. D. Kane, @adk300.
E. Kursumovic, @emirakur.
T. M. Cook, timcook007@googlemail.com, @doctimcook.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19) Pandemic, 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 (accessed 03/05/2020).
- 2. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard, 2020. https://covid19.who.int/ (accessed 10/12/2020).
- 3. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia 2020; 75: 1340–9. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong RA, Kane AD, Cook TM. Decreasing mortality rates in ICU during the COVID‐19 pandemic. Anaesthesia 2020. Epub 10 August. 10.1111/anae.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19 – Preliminary Report. New England Journal of Medicine 2020. Epub 17 July. 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 6. Angus DC, Derde L, Al‐Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. Journal of the American Medical Association 2020; 324: 1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kashour Z, Riaz M, Garbati MA, et al. Efficacy of chloroquine or hydroxychloroquine in COVID‐19 patients: a systematic review and meta‐analysis. Journal of Antimicrobial Chemotherapy 2020; 76: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCreary EK, Angus DC. Efficacy of remdesivir in COVID‐19. Journal of the American Medical Association 2020; 324: 1041–2. [DOI] [PubMed] [Google Scholar]
- 9. Horby PW, Mafham M, Bell JL, et al. Lopinavir–ritonavir in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet 2020; 396: 1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Faculty of Intensive Care Medicine and The Intensive Care Society . Clinical guide for the management of critical care for adults with COVID‐19 during the Coronavirus pandemic, 2020. https://icmanaesthesiacovid‐19.org/clinical‐guide‐for‐the‐management‐of‐critical‐care‐for‐adults‐with‐covid‐19‐during‐the‐coronavirus‐pandemic (accessed 16/12/2020).
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009; 151: 264–9. [DOI] [PubMed] [Google Scholar]
- 13. The World Bank . World Bank Country and Lending Groups, 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups (accessed 06/01/2021).
- 14. International Severe Acute Respiratory and Emerging Infection Consortium . COVID‐19 Evidence & Reports, 2021. https://isaric.org/research/covid‐19‐clinical‐research‐resources/evidence‐reports/ (accessed 15/01/2021).
- 15. Public Health England . Funnel plot tool for proportions, 2017. https://fingertips.phe.org.uk/profile/guidance (accessed 15/06/2020).
- 16. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. Journal of the American Medical Association Internal Medicine 2020; 180: 1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao J, Hu X, Cheng W, Yu L, Tu W‐J, Liu Q. Clinical features and short‐term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Medicine 2020; 46: 851–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Journal of the American Medical Association 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wendel Garcia PD, Fumeaux T, Guerci P, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID‐19 in Europe: initial report of the international RISC‐19‐ICU prospective observational cohort. EClinicalMedicine 2020; 25: 100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Research 2020; 191: 145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aleva F, van Mourik L, Broeders M, Paling A, de Jager C. COVID‐19 in critically ill patients in North Brabant, the Netherlands: patient characteristics and outcomes. Journal of Critical Care 2020; 60: 111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nachtigall I, Lenga P, Jóźwiak K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID‐19 in Germany: an observational study. Clinical Microbiology and Infection 2020; 26: 1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoecklin SB, Rolland P, Silue Y, et al. First cases of coronavirus disease 2019 (COVID‐19) in France: surveillance, investigations and control measures, January 2020. Eurosurveillance 2020; 25: 2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. Journal of the American Medical Association 2020; 323: 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. Journal of the American Medical Association 2020; 323: 1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Gao Y‐h, Zhang G‐J. The clinical dynamics of 18 cases of COVID‐19 outside of Wuhan, China. European Respiratory Journal 2020; 55: 2000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle region – case series. New England Journal of Medicine 2020; 382: 2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ling L, So C, Shum HP, et al. Critically ill patients with COVID‐19 in Hong Kong: a multicentre retrospective observational cohort study. Critical Care and Resuscitation 2020; 22: 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Lu X, Chen H, et al. Clinical course and outcomes of 344 intensive care patients with COVID‐19. American Journal of Respiratory and Critical Care Medicine 2020; 201: 1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrasa H, Rello J, Tejada S, et al. SARS‐Cov‐2 in Spanish intensive care: early experience with 15‐day survival in Vitoria. Anaesthesia Critical Care and Pain Medicine 2020; 39: 553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. Journal of Clinical Virology 2020; 127: 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Liu P, Wang M, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single‐centered, retrospective, observational study. Journal of Public Health: From Theory to Practice 2020. Epub 21 April. 10.1007/s10389-020-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases with COVID‐19. Clinical and Translational Science 2020; 13: 1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Llitjos J‐F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. Journal of Thrombosis and Haemostasis 2020; 18: 1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. Journal of the American Medical Association 2020; 323: 2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pedersen H, Hildebrandt T, Poulsen A, et al. Initial experiences from patients with COVID‐19 on ventilatory support in Denmark. Danish Medical Journal 2020; 67: A04200232. [PubMed] [Google Scholar]
- 40. Ferguson J, Rosser JI, Quintero O, et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March‐April 2020. Emerging Infectious Diseases 2020; 26: 1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Longchamp A, Longchamp J, Manzocchi‐Besson S, et al. Venous thromboembolism in critically ill patients with Covid‐19: results of a screening study for deep vein thrombosis. Research and Practice in Thrombosis and Haemostasis 2020; 4: 842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng Y, Sun L‐j, Xu M, et al. Clinical characteristics of 34 COVID‐19 patients admitted to intensive care unit in Hangzhou, China. Journal of Zhejiang University. Science. B 2020; 21: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Auld SC, Caridi‐Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Critical Care Medicine 2020; 48: e799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maatman TK, Jalali F, Feizpour C, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Critical Care Medicine 2020; 48: e783–e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitra AR, Fergusson NA, Lloyd‐Smith E, et al. Baseline characteristics and outcomes of patients with COVID‐19 admitted to intensive care units in Vancouver, Canada: a case series. Canadian Medical Association Journal 2020; 192: e694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID‐19 patients: a French monocenter retrospective study. Critical Care 2020; 24: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borobia AM, Carcas AJ, Arnalich F, et al. A cohort of patients with COVID‐19 in a major teaching hospital in Europe. Journal of Clinical Medicine 2020; 9: 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clinical Kidney Journal 2020; 13: 354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shahriarirad R, Khodamoradi Z, Erfani A, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID‐19) in the South of Iran. BMC Infectious Diseases 2020; 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shukla U, Chavali S, Mukta P, Mapari A, Vyas A. Initial experience of critically ill patients with COVID‐19 in Western India: a case series. Indian Journal of Critical Care Medicine 2020; 24: 509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Almazeedi S, Al‐Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID‐19 in Kuwait. EClinicalMedicine 2020; 24: 100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scottish Intensive Care Society Audit Group (SICSAG) . Scottish Intensive Care Society Audit Group report on COVID‐19, 2020. https://beta.isdscotland.org/find‐publications‐and‐data/population‐health/covid‐19/scottish‐intensive‐care‐society‐audit‐group‐report‐on‐covid‐19/8‐july‐2020/ (accessed 11/12/2020).
- 53. Giesen C, Diez‐Izquierdo L, Saa‐Requejo CM, et al. Epidemiological characteristics of the COVID‐19 outbreak in a secondary hospital in Spain. American Journal of Infection Control 2021; 49: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pellaud C, Grandmaison G, Hoa Phong PHT, et al. Characteristics, comorbidities, 30‐day outcome and in‐hospital mortality of patients hospitalised with COVID‐19 in a Swiss area–a retrospective cohort study. Swiss Medical Weekly 2020; 150: w20314. [DOI] [PubMed] [Google Scholar]
- 55. Halvatsiotis P, Kotanidou A, Tzannis K, et al. Demographic and clinical features of critically ill patients with COVID‐19 in Greece: the burden of diabetes and obesity. Diabetes Research and Clinical Practice 2020; 166: 108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amit M, Sorkin A, Chen J, et al. Clinical course and outcomes of severe Covid‐19: a national scale study. Journal of Clinical Medicine 2020; 9: 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Primmaz S, Le Terrier C, Suh N, et al. Preparedness and reorganization of care for coronavirus disease 2019 patients in a Swiss ICU: characteristics and outcomes of 129 patients. Critical Care Explorations 2020; 2: e0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muñoz P, Galar A, Catalán P, et al. The first 100 cases of COVID‐19 in a hospital in Madrid with a 2‐month follow‐up. Revista Espanola de Quimioterapia 2020; 33: 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kristinsson B, Kristinsdottir LB, Blondal AT, et al. Nationwide incidence and outcomes of patients with coronavirus disease 2019 requiring intensive care in Iceland. Critical Care Medicine 2020; 48: e1102–e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miller AO, Kapadia M, Kirksey MA, et al. Clinical experience with COVID‐19 at a specialty orthopedic hospital converted to a pandemic overflow field hospital. Hospital for Special Surgery Journal 2020; 16: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mukherjee V, Toth AT, Fenianos M, et al. Clinical outcomes in critically ill coronavirus disease 2019 patients: a unique New York City public hospital experience. Critical Care Explorations 2020; 2: e0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou J, Sun J‐J, Cao Z‐Q, et al. Epidemiological and clinical features of 201 COVID‐19 patients in Changsha, China. Medicine (Baltimore) 2020; 99: e21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hu H‐t, Xu S, Wang J, Rao X. Respiratory support in severely or critically ill ICU patients with COVID‐19 in Wuhan, China. Current Medical Science 2020; 40: 636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Larsson E, Brattström O, Agvald‐Öhman C, et al. Characteristics and outcomes of patients with COVID‐19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiologica Scandinavica 2021; 65: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions, and longer term outcomes of COVID‐19 ICU patients in Denmark – a nationwide, observational study. Acta Anaesthesiologica Scandinavica 2021; 65: 76–81. [DOI] [PubMed] [Google Scholar]
- 66. Cavayas YA, Noël A, Brunette V, et al. Early experience with critically ill patients with COVID‐19 in Montreal. Canadian Journal of Anesthesia 2020. Epub 15 September. 10.1007/s12630-020-01816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee JS, Godard A. Critical care for COVID‐19 during a humanitarian crisis – lessons learnt from Yemen. Critical Care 2020; 24: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kokoszka‐Bargieł I, Cyprys P, Rutkowska K, Madowicz J, Knapik P. Intensive care unit admissions during the first 3 months of the COVID‐19 pandemic in Poland: a Single‐Center, Cross‐Sectional Study. Medical Science Monitor 2020; 26: e926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Van Aerde N, Van den Berghe G, Wilmer A, et al. Intensive care unit acquired muscle weakness in COVID‐19 patients. Intensive Care Medicine 2020; 46: 2083–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Intensive Care National Audit and Research Centre I . ICNARC report on COVID‐19 in critical care: England, Wales and Northern Ireland, 16 October 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 16/12/2020).
- 71. Australian and New Zealand Intensive Care Society (ANZICS) . ANZICS CORE COVID‐19 Report – VIC 01 January to 30 September 2020. 2020. https://www.anzics.com.au/annual‐reports/ (accessed 11/12/2020).
- 72. Robert Koch Institute . Coronavirus disease 2019 (COVID‐19) daily situation report of the Robert Koch Institute, 22nd Oct 2020. 2020. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Okt_2020/2020‐10‐22‐en.pdf?__blob=publicationFile (accessed 16/12/2020).
- 73. Nationale Intensive Care Evaluatie . COVID‐19 in Dutch intensive care units; patient characteristics and outcomes compared with pneumonia patients in the ICU from 2017–2019, Report from 27th Oct 2020. 2020. https://www.stichting‐nice.nl/ (accessed 16/12/2020).
- 74. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. Journal of the American Medical Association 2020; 323: 1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Intensive Care National Audit and Research Centre . ICNARC report on COVID‐19 in critical care ‐ 29th May 2020. 2020. https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports (accessed 29/5/2020).
- 76. Doidge JC, Gould DW, Ferrando‐Vivas P, et al. Trends in intensive care for patients with COVID‐19 in England, Wales and Northern Ireland. American Journal of Respiratory and Critical Care Medicine 2020. Epub 11 December. 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Proportion of included patients with outcome reported (red) and not reported (blue) for each study, grouped by geographical region.
Table S1. Search strategy.
Table S2. Registries or societies contacted directly.
Table S3. Modified Newcastle‐Ottawa Scale.
Table S4. Study quality and risk of bias assessment.
Table S5. Reported clinical features, rates of organ support and pharmacotherapy.
Table S6. Sensitivity and sub‐group analyses.
Table S7. Results of meta‐regression analyses.


